Abstract
Background:
Tumor necrosis factor-alpha (TNF-α) is an important cytokine and has been reported to be associated with the pathogenesis of many autoimmune and inflammatory diseases. TNF-α gene is located on a region that has been found to be associated with obsessive-compulsive disorder (OCD). We performed this meta-analysis to assess the relationship between susceptibility to OCD and the TNF-α-238G/A gene polymorphism.
Methods:
An extensive search of the available literature on the association between the susceptibility to OCD and the TNF gene polymorphism was conducted by searching PubMed, Web of Knowledge, Embase, Chinese Web of Knowledge, Wanfang, and Chongqing VIP database. The database was searched up to December 2016 and includes language of English and/or Chinese with the keywords of “obsessive-compulsive disorder” or “OCD,” polymorphism or variant or mutation, “tumor necrosis factor” or “TNF” or “cytokine.” The association between TNF-α-238G/A gene polymorphism and the susceptibility of OCD was anticipated by odds ratio (OR) with the corresponding 95% confidence interval (95% CI).
Results:
Four studies including 435 cases and 1073 controls were incorporated in our meta-analysis. In general, TNF-α-238G/A gene polymorphism might lead to a decreased risk of OCD susceptibility (G vs A genotype model: OR = 1.01, 95% CI = 0.37–2.77, P = .981; GG vs AA+AG model: OR = 0.93, 95% CI = 0.37–2.36, P = .879; GG+AG vs AA model: OR = 0.22, 95% CI = 0.06–0.73, P = .014; GG vs AA model: OR = 0.21, 95% CI = 0.06–0.71, P = .012; AG vs AA model: OR = 0.29, 95% CI = 0.07–1.16, P = .081; GG+AA vs AG model: OR = 1.17, 95% CI = 0.55–2.51, P = .683).
Conclusion:
TNF-α-238G/A gene polymorphism might lead to a decreased risk of OCD susceptibility.
Keywords: meta-analysis, OCD, polymorphism, susceptibility, TNF
1. Introduction
Obsessive-compulsive disorder (OCD) is a severe and disabling clinical condition[1] characterized by obsessions and/or compulsions that are distressing, time-consuming, or significantly impairing[2] with a lifetime prevalence of 1.6% to 2.3% in the general adult population.[3–5] Although pathogenesis of OCD has being studied, it is still completely unclear. A lot of studies have showed that OCD had aggregation in families, and the prevalence rate of OCD and subclinical OCD in patients’ families was 10% to 20% higher than that in general population.[6]
Many reports have documented that immune dysregulation may play an important role in the development and path physiology of OCD.[7] While some authors reported that OCD is characterized by decreased immune activation with low levels of circulating cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and natural killer (NK) activities,[8–11] others found increase of TNF-α, IL-6, and NK cells in OCD patients.[12,13]TNF-α is an important cytokine and has been reported to be associated with the pathogenesis of many autoimmune and inflammatory diseases.[14,15]TNF-α gene is located on chromosome 6 (band p21.3), a region that has been found to be associated with OCD.[16] However, although many population studies on the relationship between susceptibility to OCD and the TNF-α-238G/A gene polymorphism have been conducted, the results are inconsistent and inconclusive.[17–20] Hence, for this reason, a meta-analysis was carried out to explore if there is any relationship between the TNF-α-238G/A gene polymorphism and susceptibility to OCD in the population.
2. Materials and methods
2.1. Ethical approval
The ethical approval was not necessary in the review articles.
2.2. Strategy for literature search
An extensive search of the available literature on the association between the susceptibility to OCD and the TNF gene polymorphism was conducted by searching PubMed, Web of Knowledge, Embase, Chinese Web of Knowledge, Wanfang, and Chongqing VIP database. The database was searched up to December 2016 and includes language of English and/or Chinese with the keywords of “obsessive-compulsive disorder” or “OCD,” polymorphism or variant or mutation, “tumor necrosis factor” or “TNF” or “cytokine.”
2.3. Criteria for inclusion and exclusion
A strict criterion for inclusion and exclusion was used in this meta-analysis and all selected studies must meet the following inclusion criteria: evaluation of the association between the TNF gene polymorphism and OCD susceptibility; case–control study; and detailed genotype data for estimating the odds ratio (OR) and 95% confidence intervals (95% CIs).
Exclusion criteria included repeated study; animal studies; comment, review studies or abstracts; and study with no genotype frequencies.
2.4. Data extraction
The data of the eligible studies were extracted by 2 investigators using a standardized data extraction method form from each article, and if a dissent existed between the 2 researchers, discussion would ensue to reach consensus. The following information was extracted: name of the first author, year of publication, country, background, sample size, selection criteria of controls, number of cases and controls, genotype frequency of cases and controls.
2.5. Quality assessment
The Newcastle–Ottawa Scale for assessing quality of observational and nonrandomized studies was adopted for quality assessment.
2.6. Statistical analysis
The association between TNF-α-238G/A gene polymorphism and the susceptibility of OCD was anticipated by OR with the corresponding 95% CI under an allele model (G vs A), a dominant model (GG vs AA+AG), a recessive model (GG+AG vs AA), and a codominant model (GG vs AA), and an over-dominant model (GG+AA vs AG). The implication of the pooled OR was determined by Z test. The numerical significance of OR was estimated using Z test, and P < .05 was measured as statistically important. In order to assess the stability of results, sensitivity analysis was done by eliminating 1 single study each time.[21] Similarly, Hardy–Weinberg equilibrium was estimated by the χ2 test in the controls.[22] In order to find publication bias, Begg funnel plot and Egger test was examined.[23,24] Also, Egger linear regression test was conducted to estimate funnel plot asymmetry (P < .05 was estimated significant publication bias), and other similar study analyses were done by using STATA version 12.0 software (Stata Corporation, College Station, TX). It was analyzed that all tests were 2-sided and the significance levels were found to be 0.05.
3. Results
3.1. Search results and characteristics of the included studies
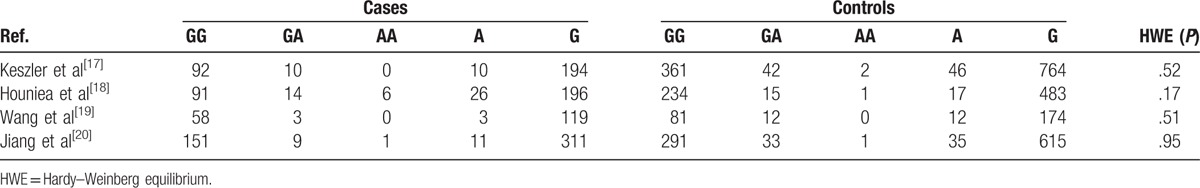
A database was used to shortlist 691 potentially relevant articles, from which a total of 676 papers were identified for further evaluation. Afterwards, a more exhaustive reading and analysis helped to remove 653 potentially relevant articles, which were rejected because of their obvious irrelevance to the purpose of this study. Ultimately, 4 studies including 435 cases and 1073 controls were incorporated in our meta-analysis. The flow chart of the search method is shown in Fig. 1, while the individuality and characteristics of these articles are listed in Table 1. All 4 studies provide the numbers of alleles in both cases and controls. In order to test all the polymorphism in the control group, the Hardy–Weinberg equilibrium model was used (Table 2).
Figure 1.
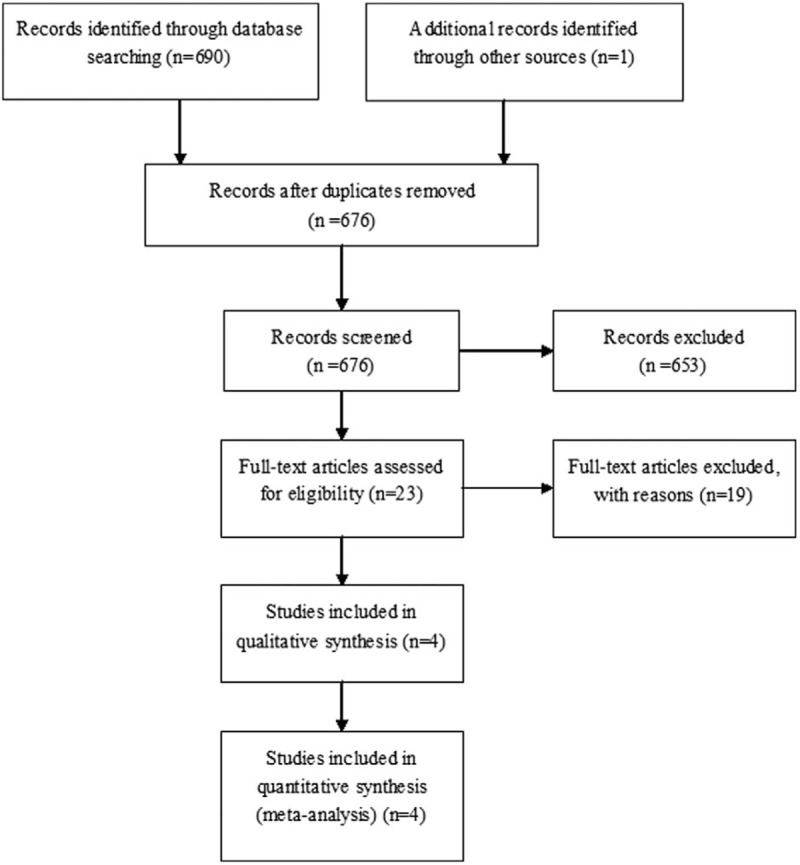
Flow diagram of included/excluded studies.
Table 1.
Characteristics of included studies.
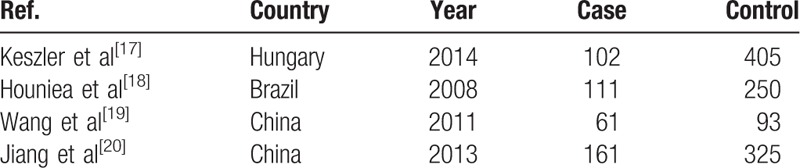
Table 2.
Genotype and allele distributions in cases and controls.
3.2. Risk of bias assessment
The quality of the studies included in the meta-analysis was assessed with the Newcastle–Ottawa Scale, and higher scores reflect better quality of the study methodology. The average score of all studies was above 6 (these results are not shown).
3.3. Pooled analyses
3.3.1. Association between the TNF-α-238G/A gene polymorphism and OCD susceptibility
Fixed-effects model was used in the dominant model and the codominant model, and random-effects model was used in the other models. In general, TNF-α-238G/A gene polymorphism might lead to a decreased risk of OCD susceptibility (G vs A genotype model: OR = 1.01, 95% CI = 0.37–2.77, P = .981; GG vs AA+AG model: OR = 0.93, 95% CI = 0.37–2.36, P = .879; GG+AG vs AA model: OR = 0.22, 95% CI = 0.06–0.73, P = .014; GG vs AA model: OR = 0.21, 95% CI = 0.06–0.71, P = .012; AG vs AA model: OR = 0.29, 95% CI = 0.07–1.16, P = .081; GG+AA vs AG model: OR = 1.17, 95% CI = 0.55–2.51, P = .683) (Table 3, Fig. 2).
Table 3.
Main results of the pooled ORs in the meta-analysis for the association between the TNF-α-238G/A polymorphism and OCD.
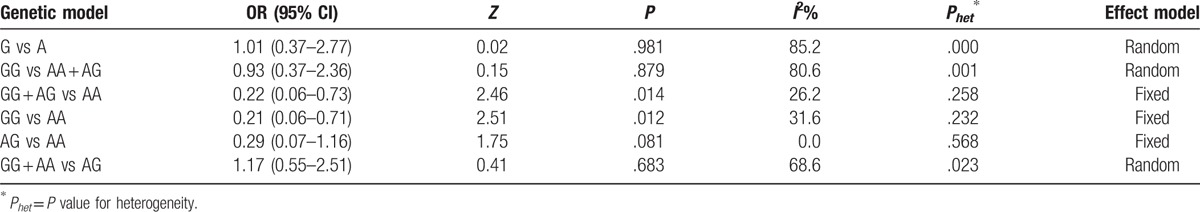
Figure 2.
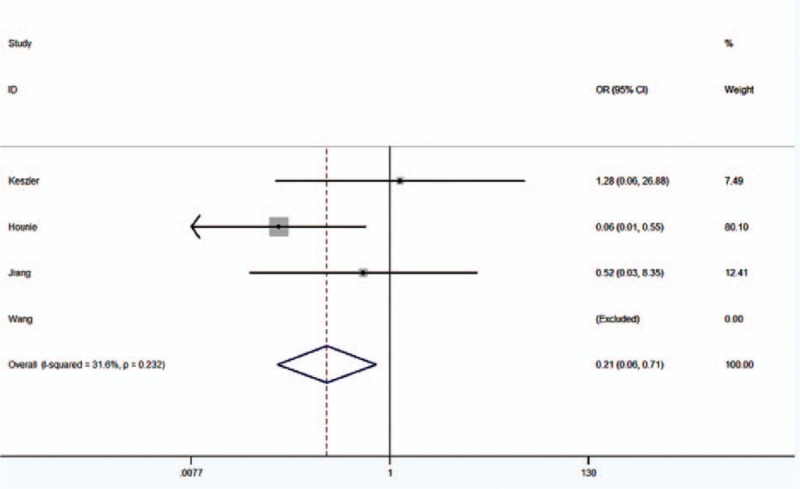
Forest plot of the susceptibility of OCD associated with TNF-α-238G/A (GG vs AA).
3.3.2. Publication bias
The Begg funnel plot and the Egger test were used to evaluate the publication bias. The customized Begg linear regression test and the Egger test detected no major publication bias in the studies included (P = .308; P = .483 for GG vs AA+ AG, Fig. 3).
Figure 3.
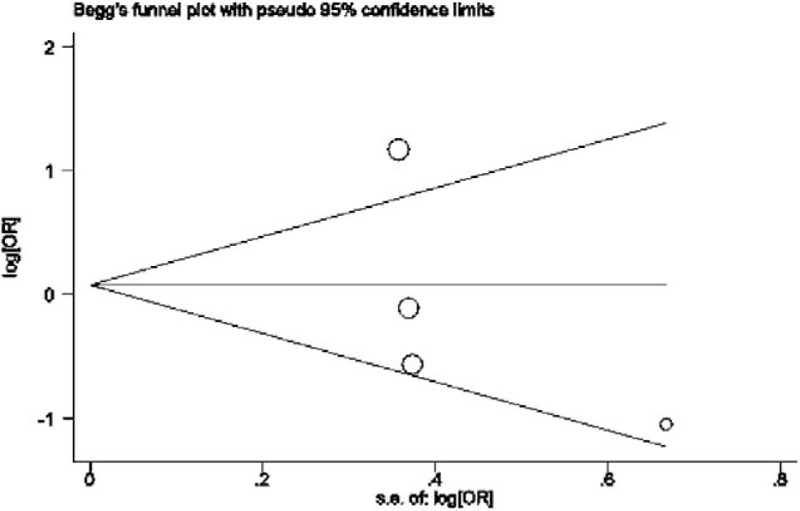
Begg funnel plot for publication bias test (GG vs AA/AG).
4. Discussion
On the basis of most of the included studies, TNF-α gene polymorphisms was found to be associated with OCD.[16] Our meta-analysis indicates a significant relationship between TNF-α-238G/A gene polymorphism and OCD risk under the dominant genetic model for 0.22 (95% CI = 0.06–0.73, P = .014) and codominant genetic model for 0.21 (95% CI = 0.06–0.71, P = .012). Thereby, the TNF-α-238G/A gene polymorphism might lead to a decreased risk of OCD susceptibility.
TNF was initially reported to induce programmed cell death or apoptosis.[25] The abnormal activation of glutamate receptors leading to the uncontrolled Ca2+ influx through N-Methyl-D-Aspartate (NMDA) receptor channels with the final result of excitotoxicity and impaired neuroplasticity may be enhanced by the abnormally elevated concentrations of inflammatory cytokines together with neuroinflammation. TNF-α is a proinflammatory cytokine with an important role in the pathogenesis of many autoimmune and inflammatory diseases.[26] TNF-α is produced by various types of cells, including macrophages, monocytes, neutrophils, T cells, and NK-cells. The coding region of TNF-α gene is located in the major histocompatibility complex (MHC) class III region on chromosome 6 (band p21.3) between the HLA-B and HLA-DR genes,[27] a region that has been found to be associated with OCD.[16]
Peripheral cytokines can tap into the brain through carrier-mediated transport across the blood–brain barrier and influence complex brain functions.[28] Leckman et al[29] found that TNF-α and interleukin (IL)-12 were elevated in patients with TD and/or OCD compared with control subjects. The study conducted by Cappi et al[30] indicates that the A allele of the TNFA rs361525 polymorphism was significantly associated with OCD subjects. The presence of the A allele may lead to increased transcription of TNF-α. TNF-α was found to be associated with the expression of serotonin transporter involved in the pathogenesis of OCD, as well other psychiatric disorders.
Glutamate has important roles in many normal and abnormal physiological processes. Glutamate systems have been directly or indirectly implicated in mood and anxiety disorders, schizophrenia, substance abuse, and various neurodegenerative disorders. Serafini et al[31] recently reported that some glutamate antagonists such as ketamine may act neutralizing these abnormally elevated inflammatory cytokines levels. Most studies demonstrated that the NMDA antagonist ketamine has rapid antidepressant effects in TRD patients, confirming the active role of glutamate in the pathophysiology of this complex condition. Ketamine has been demonstrated to be rapidly effective and was associated with a significant clinical improvement in depressive symptoms within hours after administration. Also, ketamine was also found to be effective in reducing suicidality in TRD samples.
The current study presents a detailed analysis of the association between the TNF-α gene polymorphism and OCD susceptibility, but this present meta-analysis has certain limitations, which could influence the results. First, this meta-analysis was based on a relatively small number of studies, and only 4 studies were incorporated in our meta-analysis. Second, we confined our studies to published studies in English and Chinese, and thus did not include the unpublished researches; as a result, related articles published in other language or unpublished studies were left out and this method could lead to the oversight of some related studies concerning the relationship between the TNF-α-238G/A gene polymorphism and OCD susceptibility. Third, we could not test for the gene and environment interactions due to the lack of sufficient studies. Fourth, the detailed genetic data in different age and gender were insufficient in the studies of this meta-analysis, so there is no analysis about different age and gender. In the future, we will continue to collect the detailed genetic data in different age and gender for further analysis.
Apart from these drawbacks, this is the first meta-analysis that has been performed to examine the relationship between the TNF-α-238G/A gene polymorphism and OCD. In addition, the relationship between the TNF-α-238G/A gene polymorphism and OCD susceptibility is statistically more persuasive than any single study. But this issue must be further investigated in order to ascertain the relationship between other related variables with the susceptibility to OCD.
5. Conclusion
This study evaluated the association between TNF-α-238G/A gene polymorphism and OCD susceptibility. TNF-α-238G/A gene polymorphism might lead to a decreased risk of OCD susceptibility.
Acknowledgment
We thank all our colleagues working in Hebei Center for Disease Control and Prevention.
Footnotes
Abbreviations: CI = confidential interval, OCD = obsessive-compulsive disorder, OR = odds ratio.
Authorship: Designed the experiments: CJ, XM. Performed the experiments: CJ, XM, SQ. Analyzed the data: CJ, XM, SQ, GH. Contributed reagents/materials/analysis tools: CJ, XM, SQ, GH, YL, YFL, LL. Wrote the paper: CJ, XM.
Funding/support: The authors have no funding or support to report.
The author(s) declare no competing financial interests.
References
- [1].Abramowitz JS, Taylor S, McKay D. Obsessive-compulsive disorder. Lancet 2009;374:491–9. [DOI] [PubMed] [Google Scholar]
- [2].Stewart SE, Yu D, Scharf JM, et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry 2013;18:788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- [4].Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DuPont RL, Rice DP, Shiraki S, et al. Economic costs of obsessive-compulsive disorder. Med Interface 1995;8:102–9. [PubMed] [Google Scholar]
- [6].Jonnal AH, Gardner CO, Prescott CA, et al. Obsessive-compulsive symptoms in a general population sample of female twins. Am J Med Genet 2000;96:791–6. [DOI] [PubMed] [Google Scholar]
- [7].Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep 2012;14:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weizman R, Laor N, Barber Y, et al. Cytokine production in obsessive-compulsive disorder. Biol Psychiatry 1996;40:908–12. [DOI] [PubMed] [Google Scholar]
- [9].Brambilla F, Perna G, Bellodi L, et al. Plasma interleukin-1β and tumor necrosis factor concentrations in obsessive compulsive-disorders. Biol Psychiatry 1997;42:976–81. [DOI] [PubMed] [Google Scholar]
- [10].Denys D, Fluitman S, Kavelaars A, et al. Decreased TNF-α and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology 2004;29:945–52. [DOI] [PubMed] [Google Scholar]
- [11].Monteleone P, Catapano F, Fabrazzo M, et al. Decreased blood levels of tumor necrosis factor-alpha in patients with obsessive-compulsive disorder. Neuropsychobiology 1998;37:182–5. [DOI] [PubMed] [Google Scholar]
- [12].Konuk N, Tekin IO, Ozturk U, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 in obsessive compulsive disorder. Mediators Inflamm 2006;2007:309–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ravindran AV, Griffiths J, Merali Z, et al. Circulating lymphocyte subsets in obsessive compulsive disorder, major depression and normal controls. J Affect Disord 1999;52:1–0. [DOI] [PubMed] [Google Scholar]
- [14].Lüleyap H, Onatoğlu D, Tahiroğlu A, et al. Association between obsessive compulsive disorder and tumor necrosis factor-α gene -308 (G > A) and -850 (C > T) polymorphisms in Turkish children. Balkan J Med Genet 2016;15:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jiang C, Li Z, Chen P, et al. Association between the tumor necrosis factor-α-308G/A gene polymorphism and HIV-1 susceptibility: a meta-analysis. AIDS Res Hum Retroviruses 2015;31:859–65. [DOI] [PubMed] [Google Scholar]
- [16].Hanna GL, Veenstra-VanderWeele J, Cox NJ, et al. Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet 2002;114:541–52. [DOI] [PubMed] [Google Scholar]
- [17].Keszler G, Kruk E, Kenezloi E, et al. Association of the tumor necrosis factor-308 A/G promoter polymorphism with Tourette syndrome. Int J Immunogenet 2014;41:493–8. [DOI] [PubMed] [Google Scholar]
- [18].Houniea AG, Cappia C, Cordeiroa Q, et al. TNF-alpha polymorphisms are associated with obsessive-compulsive disorder. Neurosci Lett 2008;442:86–90. [DOI] [PubMed] [Google Scholar]
- [19].Wang XM, Xiao ZP, Yu SY, et al. No association between tumor necrosis factor alpha and obsessive compulsive disorder in Chinese Han population. Med Bull Shanghai Jiaotong Univ 2011;23:1–9. [Google Scholar]
- [20].Jiang WH, Zhang XH, Tian B, et al. Association between obsessive-compulsive disorder (OCD) and polymorphisms of -238G/A and -308G/A in tumor necrosis factor-alpha (TNF-α) gene in Chinese Han population. Progr Modern Biomed 2013;13:2415–9. [Google Scholar]
- [21].Chootrakool H, Shi JQ, Yue R. Meta-analysis and sensitivity analysis for multi-arm trials with selection bias. Stat Med 2011;30:1183–98. [DOI] [PubMed] [Google Scholar]
- [22].Munafo‘ MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res 2004;129:39–44. [DOI] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1995;50:1088–101. [PubMed] [Google Scholar]
- [24].Egger M, Smiyh GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].O’Malley WE, Achinstein B, Shear MJ. Action of bacterial polysaccharide on tumors. II. Damage of sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. Nutrition Reviews 1988;46:389–91. [DOI] [PubMed] [Google Scholar]
- [26].Zhang BB, Liu XZ, Sun J, et al. Association between TNF α gene polymorphisms and the risk of duodenal ulcer: a meta-analysis. PLoS One 2013;8:e57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].EI-Tahan RR, Ghoneim AM, Noha EM. TNF-α gene polymorphisms and expression. Springerplus 2016;5:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry 2000;157:683–94. [DOI] [PubMed] [Google Scholar]
- [29].Leckman JF, Katsovich L, Kawikova I, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette's syndrome. Biol Psychiatry 2005;57:667–73. [DOI] [PubMed] [Google Scholar]
- [30].Cappi C, Muniz RK, Sampaio AS, et al. Association study between functional polymorphisms in the TNF-alpha gene and obsessive-compulsive disorder. Arq Neuropsiquiatr 2012;70:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Serafini G, Howland RH, Rovedi F, et al. The role of Ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 2014;12:444–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


