Supplemental Digital Content is available in the text
Keywords: Clostridium difficile infection, Crohn disease, inflammatory bowel disease, ulcerative colitis
Abstract
To evaluate the frequency, possible risk factors, and outcome of Clostridium difficile infection (CDI) in inflammatory bowel disease (IBD) patients.
There has been an upsurge of CDI in patients with IBD who has been associated with increased morbidity and mortality. Various risk factors have been found to predispose IBD patients to CDI.
A retrospective case–control study on IBD patients admitted with exacerbation and tested for CDI at the Tel Aviv Medical Center in 2008 to 2013. Epidemiologic, laboratory, and prognostic data were retrieved from electronic files and compared between patients who tested positive (CDI+) or negative (CDI−) for CDI.
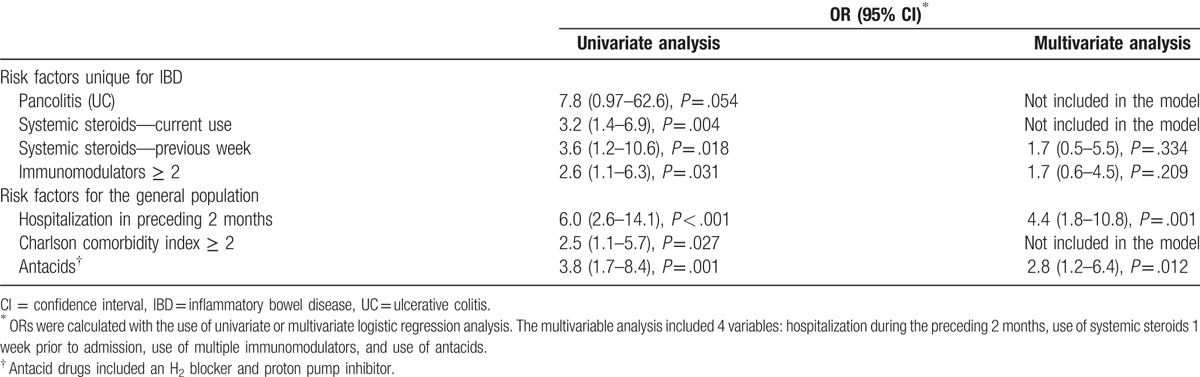
CDI was identified in 28 of 311 (7.31%) IBD patients hospitalized with diarrhea. IBD-specific risk factors (univariate analysis) for CDI included: use of systemic steroids therapy (odds ratio [OR] = 3.6, 95% confidence interval [CI] 1.2–10.6) and combinations of ≥2 immunomodulator medications (OR = 2.6, 95% CI 1.1–6.3). Additional risk factors for CDI that are common in the general population were hospitalization in the preceding 2 months (OR = 6.0, 95% CI 2.6–14.1), use of antacids (OR = 3.8, 95% CI 1.7–8.4), and high Charlson comorbidity score (OR = 2.5, 95% CI 1.1–5.7). A multivariate analysis confirmed that only hospitalization within the preceding 2 months and use of antacids were significant risk factors for CDI. The prognosis of CDI+ patients was similar to that of CDI− patients.
Hospitalized IBD patients with exacerbation treated with antacids or recently hospitalized are at increased risk for CDI and should be tested and empirically treated until confirmation or exclusion of the infection.
1. Introduction
Clostridium difficile infection (CDI) is the most common cause of nosocomial infections in developed countries[1] and has emerged as a major cause of morbidity and mortality in hospitalized patients.[2] Its clinical manifestations range from asymptomatic carriage to severe forms of fulminant colitis and death.[3,4] There has been a dramatic worldwide increase in the incidence and severity of CDI over the past 2 decades,[1] despite the prevention programs implemented in many countries.[2–4]
The doubling of the CDI rate between 1996 and 2003[5] was partially attributed to the emergence of the hypervirulent strain, NAP1/B1/027, which is associated with increased disease severity and transmissibility.[6–8]
Inflammatory bowel disease (IBD) patients, including both Crohn disease (CD) and ulcerative colitis (UC), are also predisposed to CDI, probably due to dysbiosis and immunomodulators usage.[9–22] It has been suggested that up to 20%[23] of IBD flares were associated with testing positive for C difficile.[6] Retrospective studies demonstrated doubling of the CDI incidence among patients with CD, and a 3-fold increase among those with UC.[24] They have also shown that the CDI incidence among IBD patients is estimated as being 3-fold higher than that in the general population.[22,24]
Major risk factors for CDI in the general population are well known and they include exposure to antibiotics,[25] usage of proton pump inhibitors (PPIs),[6,26,27] previous and prolonged hospitalizations,[27,28] chemotherapy, immunocompromised states, advanced age,[29] multiple comorbidities, hypoalbuminemia, renal insufficiency, use of nasogastric tubes, and gastrointestinal surgeries.[26,30,31] However, it is less clear to what extent the risk attributed to these factors is altered in IBD patients. It has been suggested that risk factors for CDI in IBD patients may be less often related to prior hospitalizations[32,33] and more frequently related to immunomodulators usage; however, this issue remain controversial.[34] Additional risk factors include disease type (i.e., higher prevalence in UC), extent, and location.[34–38]
Diagnosing and treating CDI in the IBD population is challenging due to the similar clinical and endoscopic presentations of IBD flare and CDI.[38] Current evidence indicates that CDI in IBD patients is associated with worse outcomes, as reflected by the need for colectomy,[39,40] escalation of treatment, recurrent hospitalizations,[15] a prominent increase in recurrent infection rates,[41–43] and a higher mortality rate,[33,44] all of which warrant further evaluation of the prevalence, potential risk factors, and impact of CDI on IBD patients’ outcome.
The aim of this retrospective case–control study was to investigate whether treatment by immunomodulators poses a risk factor for CDI in IBD patients hospitalized with diarrhea. In addition, we evaluated the rate of CDI, additional risk factors for CDI, and outcomes of CDI in this population. We hypothesized that immunomodulators treatment would be one of the risk factors for CDI in IBD.
2. Methods
2.1. Study population and design
IBD patients, ICD-9 codes 555 and 556, hospitalized at the Tel Aviv Sourasky Medical Center (TLVMC) in Israel, who were >18 years old were eligible for study participation. Those who presented with diarrhea and were tested for the presence of C difficile were recruited retrospectively into this case–control study between July 21, 2008 and August 26, 2013.
Medical records and laboratory tests were thoroughly reviewed to verify IBD diagnosis and to retrieve the patients’ medical history. All patients who tested positive for fecal C difficile toxin during their hospital stay were allocated into the CDI+ group (cases). Those who tested negative for fecal C difficile toxin (CDI−) served as controls. The exclusion criteria were hospitalized IBD patients who had not been tested for CDI, IBD patients hospitalized for etiologies other than IBD exacerbation, and IBD patients who had undergone colectomy prior to hospitalization (ileostomy or pouch). The Institutional Review Board of the TLVMC approved this study (No. 0622-13-TLV).
Information on demographics, diagnosis, clinical data, medical treatments, laboratory test results, and prognostic data were obtained for all patients from their digital medical files. The Charlson comorbidity index (CCI) was used for the evaluation of comorbidity severity and prediction of mortality risk during the 12 months following the index hospitalization.
2.2. CDI diagnosis
CDI was diagnosed by testing nonformed stool samples in a 2-step algorithm. The initial assay was a combined glutamate dehydrogenase antigen and toxin A/B immunochromatographic rapid test (C. DIFF QUIK CHEK COMPLETE, Techlab, Orlando, FL). Clostridium difficile toxin PCR (Xpert C. difficile, Cepheid, Sunnyvale, CA) was performed only if the results of those 2 tests were inconsistent.
2.3. Statistical analysis
Continuous variables were summarized using the mean ± standard deviation for normally distributed variables, or the median and interquartile range (IQR) for non-normally distributed variables. Categorical variables were summarized using frequency distributions. The one-sample Kolmogorov–Smirnov test was used to assess normal distribution of continuous variables.
The study sample size was calculated to answer the primary endpoint of immunomodulator treatment as a risk factor of CDI in IBD. Assuming that 74% to 90% of cases[11,33] and 56% of controls[33] were treated with immunomodulator, at least 26 to 110 patients were needed in each study group to reach a power of 80% and a significance cutoff of 5%.
Comparisons between the groups regarding demographic information, clinical data, disease characteristics, laboratory test results, risk factors, and prognosis variables were performed using the Chi-squared test for categorical variables, the Mann–Whitney test for non-normally distributed continuous variables, or the T-test for normally distributed continuous variables.
To study the relationship between potential risk factors and CDI in IBD patients, we used univariate and multivariate logistic regression models and calculated the odds ratio (OR) (95% confidence interval [CI]) for CDI in IBD. Since only 28 patients were diagnosed as having CDI, the multivariable model was limited to no more than 4 variables. Therefore, we chose the most significant risk factors for the final model (i.e., hospitalization in the preceding 2 months, use of systemic steroids 1 week prior to hospitalization, use of >2 immunomodulator drugs, and use of antacids). Each variable that was entered to the model was treated as a dichotomous covariate and all were included into the model using the enter method.
The level of significance used for all analyses was 2-tailed and set at P < .05. The SPSS statistical package (Version 23, IBM Inc., Chicago, IL) was used for all statistical analyses.
3. Results
3.1. Study population
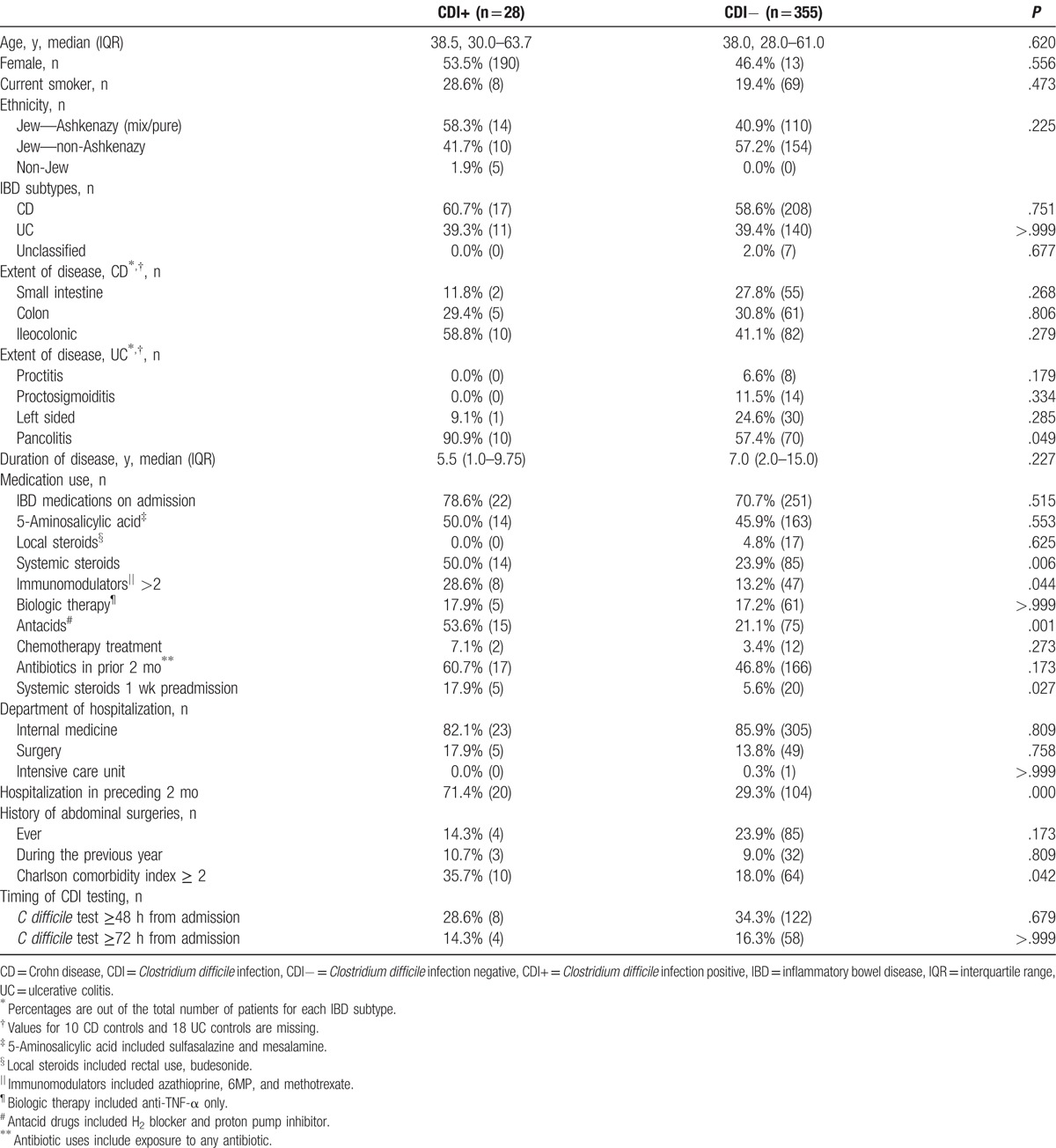
A total of 383 hospitalizations of 311 IBD patients met study inclusion criteria out of 498 hospitalizations. Clostridium difficile was identified in 28 (7.31%, CDI+) patients, of whom 11 had UC (39.3%) and 17 had CD (60.7%). Clostridium difficile toxin tested negative in 140 (39.4%) UC patients, 208 (58.6%) CD patients, and 7 (2.0%) unclassified IBD patients (Fig. 1). Baseline demographic information, disease characteristics, and clinical data of both CDI groups are provided in Table 1. Compared to the controls, the CDI+ patients were hospitalized more recently during the 2 months before the index admission, used more antacids, systemic steroids, and combinations of multiple immunomodulator treatments, and suffered from more severe comorbidities (as assessed by a CCI score of ≥2). In addition, UC patients with CDI suffered from more extensive disease. The clinical and laboratory data were not significantly different between the 2 groups (Supplementary Table 1).
Figure 1.
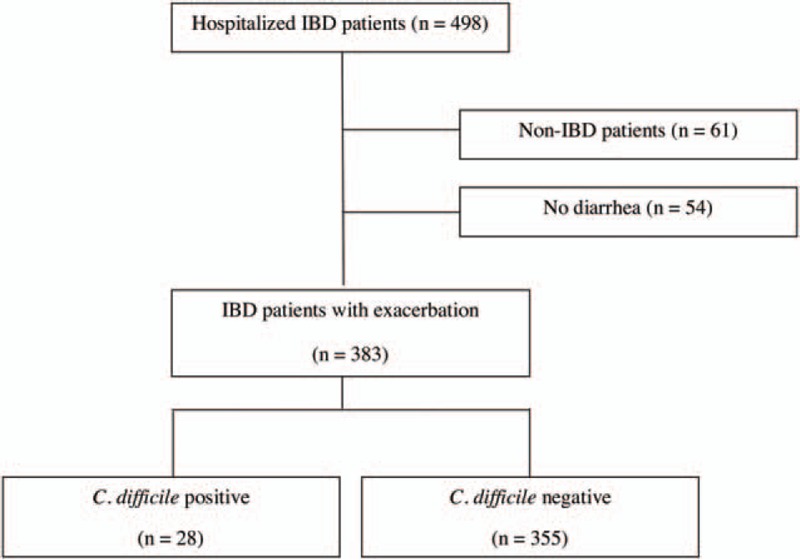
Flow chart of case definitions in all hospitalized patients for inflammatory bowel disease flares from 2008 to 2013. IBD = inflammatory bowel disease.
Table 1.
Baseline demographic, disease, and clinical characteristics of the study population.
3.2. Risk factors for CDI
Evaluation of risk factors considered to be unique for IBD patients revealed that systemic steroid treatment (currently or during the previous week), combinations of more than 1 immunomodulator treatment and extensive disease among UC patients were associated with an increased risk for CDI among the UC patients (Table 1). Three additional risk factors that were reported in the general population (i.e., hospitalization in the preceding 2 months, severe comorbidities, and use of antacids) were also found to be associated with CDI in the IBD patients (Table 2). Subsequently, we built a multivariate model and calculated the adjusted OR (95% CI) for the most significant risk factors (as described in the univariate analysis in Table 2). That analysis confirmed that only recent hospitalizations in the preceding 2 months and the use of antacids were significant risk factors, whereas all of the risk factors for CDI in IBD were not.
Table 2.
Odds ratio (OR) for Clostridium difficile infection according to significant disease characteristics and risk factors among hospitalized patients with inflammatory bowel disease (univariate and multivariate analyses).
3.3. Prognosis
CDI did not affect the prognosis of the IBD patients (Supplementary Table 2). The length of hospitalization and the colectomy rate up to 1-year post-hospitalization were similar for both groups. Two CDI+ patients (7.1%) died during the hospitalization period compared with 5 CDI− patients (1.4%) (P = .09). The CDI risk factors for the 2 patients who died were advanced age (79 and 86 years), severe comorbidities (CCI = 4), use of PPIs, and recent hospitalizations. In terms of IBD-related risk factors, both had pancolitis: one patient was in remission and the other experienced severe UC exacerbation and received systemic steroid therapy. None of the CDI+ patients died during the 2 months post-hospitalization in contrast to 7 CDI− patients (2%) (P = .45).
Previous studies have demonstrated that CDI in IBD is associated with a poor prognosis.[15] However, most of those studies also included patients who were infected with C difficile during their hospitalization. In order to examine whether the inclusion of IBD patients who had been admitted for non-IBD indications and were then infected during hospitalization would alter our results, we reassessed all the patients who were diagnosed with CDI during their hospitalization (36/437 patients). The mortality rate of the CDI+ IBD patients was significantly higher than that of the CDI− patients (13.9% vs. 2%, respectively, P = .002), while the duration of hospitalization remained similar (8.3 ± 8.7 vs. 7.92 ± 12.4 days, respectively, P = .86).
4. Discussion
This case–control study was designed to evaluate the rate, the potential risk factors (especially the use of immunomodulator drugs which was the primary endpoint), and the outcome of CDI in IBD patients who were hospitalized due to disease exacerbation. The main finding was that, in contrast to current thinking, CDI in IBD patients was not associated with worse prognosis when compared with CDI− patients. In addition, some risk factors for CDI in IBD patients were similar to those reported for the general population (e.g., previous hospitalizations or the use of antacids), whereas other risk factors unique to IBD (e.g., prehospitalization treatment with multiple immunomodulator drugs, use of systemic steroids and colon involvement) were not identified as independent risk factors for CDI. Thus, our primary endpoint of immunomodulator treatment as a risk factor of CDI in IBD was not met.
CDI is now the leading healthcare-associated infection in the United States and a major cause of morbidity and mortality.[45] Diagnosing CDI in IBD patients is of particular importance due to the difference in therapeutic approaches for these conditions.[46,47] According to the latest European Crohn and Colitis Organization guidelines if C difficile is detected in IBD patients, oral vancomycin should be administered, fecal microbial transplant considered, and immunosuppressive therapy should be stopped if possible, although this may not always be warranted.[48] Infection with C difficile may intensify the severity of IBD or even trigger IBD flare,[15] underscoring the importance of antibiotic therapy.[46] By contrast, intensifying IBD treatment may worsen the outcome of IBD patients with concomitant CDI.[49]
The rate of CDI among our IBD patients was 7.31%, which is consistent with the reported rates in the literature.[29,50] Interestingly, there was no clear trend of an increase in the rate of CDI in IBD patients during the study period, in contrast with the steep increase in the incidence of CDI documented in North America and Europe between 2000 to 2010.[19] It is, however, consistent with the stable CDI rate in Israel.[51] As previously reported, IBD patients with C difficile tend to be younger (median 38.5 years of age, IQR 30.0–63.7 years) than patients with C difficile in the general population (>65 years).[34] This may be attributed to the different profile of risk factors for CDI in IBD patients, such as exposure to immunomodulators, antibiotics, and steroids at an earlier age.
In the current study, the use of a single immunomodulator drug in the IBD patients was not associated with CDI; however, combinations of multiple immunomodulators were associated with an increased risk for CDI in the univariate analysis, but not in the multivariate analysis. Issa et al[33] identified immunomodulator maintenance therapy to be associated with a 2-fold risk of CDI in IBD patients (OR 2.56, 95% CI 1.28–5.12), although the use of anti-TNF-α agents did not correlate with increased risk. These results were confirmed by a study from Belgium.[11] Systemic steroid treatment was also associated with an increased risk for CDI in IBD patients in the univariate analysis, although this association was not confirmed in the multivariate analysis. This probably results from the nature of the study population, in which all the patients suffered from diarrhea and most of them had been previously hospitalized and/or received an outpatient empiric therapeutic intervention, probably as an intention to treat what seemed to be a worsening of the underlying disease.[11] Our results are consistent with recent studies that did not find any significant association between CDI and immunomodulators (thiopurines) or steroids in IBD patients.[23,52,53]
In contrast to our findings, Schneeweiss et al[37] found that the initiation of corticosteroids tripled the risk of CDI among IBD patients (relative risk 3.4, 95% CI 1.9–6.1), while no such association was found with immunomodulators or biologics (infliximab and adalimumab). These findings suggest that specific regimens of immunomodulators might carry different levels of risk for CDI in IBD patients.
“Classic” risk factors[13,22,26,30,31,34,35,49] for CDI in the general population, such as advanced age, chemotherapy, and prior gastrointestinal surgeries, were not associated with a higher risk of CDI in our study population. Prior exposure to antibiotics was also not a risk factor for CDI, probably due to the high exposure rate in both the CDI+ (60.7%) and CDI− patients (46.8%). These rates are similar to those reported in other studies, in which 40% to 60% of IBD patients had prior antibiotic exposure.[11,33] However, 2 additional “classic” risk factors for CDI (i.e., the use of antacid medications and hospitalizations during the 2 months prior to the index hospitalization) were independent risk factors for CDI. The use of antacids is a well-documented risk factor for CDI,[27] as well as for recurrent infection[16] in the general population and among IBD patients in particular,[54] probably due to their impact on the enteric microbiota, resulting in dysbiosis that increases the risk for C difficile colonization.[55] The high percentage of antacid use can be at least partially attributed to the common practice of combining them with steroid therapy as “gastric protectors” and to the upper gastrointestinal involvement in CD patients. Recent hospitalizations prior to the index admission quadrupled the risk for CDI in IBD (adjusted OR = 4.4 [95% CI 1.8–10.8]) and were much more common among CDI+ patients compared with CDI− patients (71.4% vs. 29.3%, respectively, P < .001). Berg et al[22] reported that only 39% of their IBD patients were hospitalized prior to their being diagnosed as having CDI. This difference can be explained by our study design that included only IBD patients who were hospitalized for diarrhea and who had acquired CDI before admission, as opposed to the inclusion in other studies of patients who were hospitalized due to various diagnoses, sometimes not related to IBD, and who developed diarrhea during hospitalization. Our findings are consistent with those of previous studies that have been conducted on other immunosuppressed populations, such as solid-organ transplanted patients, in which recent hospitalizations were similarly associated with an increased risk for CDI[5,18,36] and for recurrent CDI.[16,56]
The clinical outcomes of IBD patients with or without CDI were not significantly different, although the CDI+ patients had a higher colectomy rate up to 1 year post-admission (14.3% vs. 7.9% for the CDI− patients, P = .74) and a higher mortality rate during hospitalization (7.1% vs. 1.4%, respectively, P = .09). It may be argued that mortality did not reach a level of significance due to the small number of CDI+ patients. However, this may also support the opposite contention: due to the small sample size, each death, even if unrelated to IBD, had a large impact on the mortality rate. Both of the CDI+ patients who died had multiple risk factors for CDI that are mostly unrelated to IBD, including advanced age, comorbidities (CCI = 4), prior hospitalizations and exposure to antibiotics, supporting the possibility that their death was more related to those factors and less to their IBD background, at least in 1 of the patients whose UC was not active. Indeed, the rate of mortality in the 2 months post-hospitalization was nil in the CDI+ group and 2% (n = 7) in the CDI− group. These results are consistent with a recent large-scale work that found that IBD patients with CDI have similar outcomes to those with IBD alone.[57] By contrast, most previous studies demonstrated an association between CDI and a poor prognosis,[15] with higher mortality rates among IBD patients with CDI compared with those who were not infected (5.7–18% vs. 1.4–2.1%, respectively).[58]
The discrepancy between our results and those of others may be explained by the early detection and the early initiation of treatment for CDI at our institution due to a common practice of test-and-treat for CDI in patients who are hospitalized with an exacerbation of IBD. In addition, the studies by other investigators were carried out solely on IBD patients, including those hospitalized for etiologies unrelated to IBD, such as cancer, pneumonia, or other major diseases that can bias the results in terms of poor prognosis and increased death rate. Some of their patients developed diarrhea during hospitalization and were diagnosed with CDI at a later stage of their hospital stay.[10,24,49,59,60] Indeed, when all hospitalized IBD patients who were tested for CDI were included in our analysis, the death rate was significantly increased among the CDI+ patients as compared with CDI− patients (13.9% vs. 2%, P = .002).
Our study has several strengths. One is a reduced information bias due to the good documentation of patients’ data in the digitalized files at the TLVMC specific for IBD patients who are routinely examined by gastroenterologists from the IBD Center upon hospitalization. In addition, we believe that most CDI+ patients were identified due to the protocol followed at our institution according to which every IBD patient hospitalized as a result of an exacerbation of disease is tested for CDI. Importantly, the study design enabled us to focus upon risk factors and prognosis specific to IBD.
We acknowledge certain limitations of this study. As in other retrospective studies, insufficient or inaccurate documentation of the patients’ data, ascertaining that all CDI patients were identified, and possible continuation of treatment in other facilities may have affected the results and caused information bias. Moreover, we did not document the timing of anti-CDI therapy initiation and the type of antibiotics (metronidazole/vancomycin) that were prescribed, both of which are factors that could affect prognosis and risk factors. However, the usual practice at our institution with regard to IBD patients hospitalized with diarrhea is to commence antibiotic therapy (that includes metronidazole) after obtaining stool for cultures and CDI testing. In addition, the relatively small number of CDI+ patients did not have a sufficient statistical power to enable comparisons between IBD subtypes and may cause a potential selection bias. Due to that limited external validity, the potential CDI risk factors for IBD patients should be interpreted with caution. Finally, there may be additional factors that we did not adjust for that could potentially bias the results, such as specific concomitant diseases or disorders, nasogastric feeding,[34] recurrent CDI,[54,56] travels prior to the infection, and specific regimens of immunomodulators that might have different levels of risk for CDI in IBD patients.[33]
In summary, the rate of CDI in IBD patients hospitalized due to an exacerbation of their IBD was 7.31%, which is higher than the CDI rates in the general population. Although the risk factors for IBD were similar to those reported for the general population, no IBD-specific risk factors were identified. Encountering these risk factors in IBD patients hospitalized due to diarrhea should alert the clinician to test-and-treat for CDI. Unlike most other studies, we did not find CDI to affect IBD patients’ prognosis. This may be partially attributed to our institution's test-and-treat for CDI, and perhaps support the adoption of this policy at other medical facilities. Whether specific IBD subgroups or clinical factors pose a higher risk or imply a worse prognosis when there is a coexisting CDI awaits prospective multicenter studies. Such large-scale studies might help us understand the relation between CDI and IBD and the effect of CDI on these patients’ prognosis, with implications for targeted therapeutic interventions.
Acknowledgments
The authors thank Esther Eshkol, MA, Tel Aviv Medical Center's institutional copyeditor, for editorial assistance. This work was performed as part of the requirements for an MD degree for Idan Barzilay.
Supplementary Material
Footnotes
Abbreviations: CCI = Charlson comorbidity index, CD = Crohn disease, CDI = Clostridium difficile infection, CI = confidence interval, IBD = inflammatory bowel disease, IQR = interquartile range, OR = odds ratio, PPIs = proton pump inhibitors, TLVMC = Tel Aviv Medical Center, UC = ulcerative colitis.
The study was reviewed and approved by the local Medical Ethics Committee of the Tel Aviv Medical Center (No. 0622-13-TLV).
KH and ID have contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol 2010;4:409–16. [DOI] [PubMed] [Google Scholar]
- [2].Davis BM, Yin J, Blomberg D, et al. Impact of a prevention bundle on Clostridium difficile infection rates in a hospital in the Southeastern United States. Am J Infect Control 2016;44:1729–31. [DOI] [PubMed] [Google Scholar]
- [3].DiDiodato G, McArthur L. Evaluating the effectiveness of an antimicrobial stewardship program on reducing the incidence rate of healthcare-associated Clostridium difficile infection: a non-randomized, stepped wedge, single-site, observational study. PLoS ONE 2016;11:e0157671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brkic S, Pellicano R, Turkulov V, et al. Prevention program for Clostridium difficile infection: a single-centre Serbian experience. Minerva Med 2016;107:131–9. [PubMed] [Google Scholar]
- [5].McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis 2006;12:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353:2433–41. [DOI] [PubMed] [Google Scholar]
- [7].Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 2004;171:466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009;7:526–36. [DOI] [PubMed] [Google Scholar]
- [9].Trifan A, Stanciu C, Stoica O, et al. Impact of Clostridium difficile infection on inflammatory bowel disease outcome: a review. World J Gastroenterol 2014;20:11736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jen MH, Saxena S, Bottle A, et al. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:1322–31. [DOI] [PubMed] [Google Scholar]
- [11].Bossuyt P, Verhaegen J, Van Assche G, et al. Increasing incidence of Clostridium difficile-associated diarrhea in inflammatory bowel disease. J Crohns Colitis 2009;3:4–7. [DOI] [PubMed] [Google Scholar]
- [12].Ricciardi R, Ogilvie JW, Jr, Roberts PL, et al. Epidemiology of Clostridium difficile colitis in hospitalized patients with inflammatory bowel diseases. Dis Colon Rectum 2009;52:40–5. [DOI] [PubMed] [Google Scholar]
- [13].Nguyen GC, Kaplan GG, Harris ML, et al. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol 2008;103:1443–50. [DOI] [PubMed] [Google Scholar]
- [14].LaMont JT, Trnka YM. Therapeutic implications of Clostridium difficile toxin during relapse of chronic inflammatory bowel disease. Lancet 1980;1:381–3. [DOI] [PubMed] [Google Scholar]
- [15].Navaneethan U, Venkatesh PG, Shen B. Clostridium difficile infection and inflammatory bowel disease: understanding the evolving relationship. World J Gastroenterol 2010;16:4892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ananthakrishnan AN, Binion DG. Impact of Clostridium difficile on inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2010;4:589–600. [DOI] [PubMed] [Google Scholar]
- [17].Saidel-Odes L, Borer A, Odes S. Clostridium difficile infection in patients with inflammatory bowel disease. Ann Gastroenterol 2011;24:263–70. [PMC free article] [PubMed] [Google Scholar]
- [18].Goodhand JR, Alazawi W, Rampton DS. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment Pharmacol Ther 2011;33:428–41. [DOI] [PubMed] [Google Scholar]
- [19].Sinh P, Barrett TA, Yun L. Clostridium difficile infection and inflammatory bowel disease: a review. Gastroenterol Res Pract 2011;2011:136064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reddy SS, Brandt LJ. Clostridium difficile infection and inflammatory bowel disease. J Clin Gastroenterol 2013;47:666–71. [DOI] [PubMed] [Google Scholar]
- [21].Nitzan O, Elias M, Chazan B, et al. Clostridium difficile and inflammatory bowel disease: role in pathogenesis and implications in treatment. World J Gastroenterol 2013;19:7577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis 2013;19:194–204. [DOI] [PubMed] [Google Scholar]
- [23].Gellad ZF, Alexander BD, Liu JK, et al. Severity of Clostridium difficile-associated diarrhea in solid organ transplant patients. Transpl Infect Dis 2007;9:276–80. [DOI] [PubMed] [Google Scholar]
- [24].Rodemann JF, Dubberke ER, Reske KA, et al. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol 2007;5:339–44. [DOI] [PubMed] [Google Scholar]
- [25].O’Donoghue C, Kyne L. Update on Clostridium difficile infection. Curr Opin Gastroenterol 2011;27:38–47. [DOI] [PubMed] [Google Scholar]
- [26].Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011;365:1693–703. [DOI] [PubMed] [Google Scholar]
- [27].Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012;107:1011–9. [DOI] [PubMed] [Google Scholar]
- [28].Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10. [DOI] [PubMed] [Google Scholar]
- [29].Meyer AM, Ramzan NN, Loftus EV, Jr, et al. The diagnostic yield of stool pathogen studies during relapses of inflammatory bowel disease. J Clin Gastroenterol 2004;38:772–5. [DOI] [PubMed] [Google Scholar]
- [30].McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis 1990;162:678–84. [DOI] [PubMed] [Google Scholar]
- [31].Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90. [DOI] [PubMed] [Google Scholar]
- [32].Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol 2009;104:1162–9. [DOI] [PubMed] [Google Scholar]
- [33].Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol 2007;5:345–51. [DOI] [PubMed] [Google Scholar]
- [34].Fu N, Wong T. Clostridium difficile infection in patients with inflammatory bowel disease. Curr Infect Dis Rep 2016;18:19. [DOI] [PubMed] [Google Scholar]
- [35].Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 2008;57:205–10. [DOI] [PubMed] [Google Scholar]
- [36].Powell N, Jung SE, Krishnan B. Clostridium difficile infection and inflammatory bowel disease: a marker for disease extent? Gut 2008;57:1183–4. [PubMed] [Google Scholar]
- [37].Schneeweiss S, Korzenik J, Solomon DH, et al. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther 2009;30:253–64. [DOI] [PubMed] [Google Scholar]
- [38].Tang YM, Stone CD. Clostridium difficile infection in inflammatory bowel disease: challenges in diagnosis and treatment. Clin J Gastroenterol 2017;10:112–23. [DOI] [PubMed] [Google Scholar]
- [39].Law CC, Tariq R, Khanna S, et al. Systematic review with meta-analysis: the impact of Clostridium difficile infection on the short- and long-term risks of colectomy in inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:1011–20. [DOI] [PubMed] [Google Scholar]
- [40].Peng JC, Shen J, Zhu Q, et al. The impact of Clostridium difficile on surgical rate among ulcerative colitis patients: a systemic review and meta-analysis. Saudi J Gastroenterol 2015;21:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis 2005;5:549–57. [DOI] [PubMed] [Google Scholar]
- [42].Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect 2008;70:298–304. [DOI] [PubMed] [Google Scholar]
- [43].Gerding DN, Muto CA, Owens RC., Jr Treatment of Clostridium difficile infection. Clin Infect Dis 2008;46(suppl 1):S32–42. [DOI] [PubMed] [Google Scholar]
- [44].Rao K, Higgins PD. Epidemiology, diagnosis, and management of Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:1744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bricker E, Garg R, Nelson R, et al. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev 2005;25:CD004610. [DOI] [PubMed] [Google Scholar]
- [47].Napolitano LM, Edmiston CE., Jr Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. Surgery 2017;162:325–48. [DOI] [PubMed] [Google Scholar]
- [48].Harbord M, Eliakim R, Bettenworth D, et al. Corrigendum: Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017;11:1512. [DOI] [PubMed] [Google Scholar]
- [49].Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol 2009;7:981–7. [DOI] [PubMed] [Google Scholar]
- [50].Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol 2004;16:775–8. [DOI] [PubMed] [Google Scholar]
- [51].Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C. difficile infection. JAMA 2009;301:954–62. [DOI] [PubMed] [Google Scholar]
- [52].Kariv R, Navaneethan U, Venkatesh PG, et al. Impact of Clostridium difficile infection in patients with ulcerative colitis. J Crohns Colitis 2011;5:34–40. [DOI] [PubMed] [Google Scholar]
- [53].Hardt C, Berns T, Treder W, et al. Univariate and multivariate analysis of risk factors for severe Clostridium difficile-associated diarrhoea: importance of co-morbidity and serum C-reactive protein. World J Gastroenterol 2008;14:4338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Razik R, Rumman A, Bahreini Z, et al. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol 2016;111:1141–6. [DOI] [PubMed] [Google Scholar]
- [55].Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Abdelfatah M, Nayfe R, Nijim A, et al. Factors predicting recurrence of Clostridium difficile infection (CDI) in hospitalized patients: retrospective study of more than 2000 patients. J Investig Med 2015;63:747–51. [DOI] [PubMed] [Google Scholar]
- [57].Joshi NM, Marks IH, Crowson R, et al. Incidence and outcome of Clostridium difficile infection in hospitalized patients with inflammatory bowel disease in the UK. J Crohns Colitis 2017;11:70–6. [DOI] [PubMed] [Google Scholar]
- [58].Rocha MF, Maia ME, Bezerra LR, et al. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes. Infect Immun 1997;65:2740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012;35:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ananthakrishnan AN, McGinley EL, Saeian K, et al. Temporal trends in disease outcomes related to Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:976–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


