Abstract
Bone marrow edema (BME) represents a reversible but highly painful finding in magnetic resonance imaging (MRI) of patients with knee osteoarthritis. The aim of this retrospective study was to evaluate the efficacy of extracorporeal shock wave treatment (ESWT) on painful BME in osteoarthritis of the knee.
This study focuses on people who had early-to-mid stage osteoarthritis with knee pain and MRI findings of BME. Patients who underwent ESWT treatment or prescribed alendronate treatment in our department were analyzed. Knee pain and function were measured using the visual analog scale (VAS) for pain and the Western Ontario and McMaster University Osteoarthritis Index (WOMAC), respectively. The degree of BME was measured with MRI scans.
A total of 126 patients who received ESWT treatment (Group A, n = 82) or alendronate treatment (Group B, n = 44) were included. All patients were followed up clinically and radiographically for a minimum of 12 months. The mean follow-up was 23.5 months (range, 12–38 months). The VAS and WOMAC score decreased more significantly after treatment in Group A than that in Group B (P <.01) within 3 months. In 6-month MRI follow-ups, there was higher incidence of distinct reduction and complete regression of BME of the affected knee in Group A than that in Group B (P <.01).
ESWT is an effective, reliable, and noninvasive treatment in patients with painful BME in osteoarthritis of the knee followed by a rapid normalization of the MRI appearance. It has the potential to shorten the natural course of this disease.
Keywords: alendronate, bone marrow edema, MRI, osteoarthritis, pain, shock wave
1. Introduction
Bone marrow edema (BME) represents a reversible but highly painful finding in MR-imaging of patients with knee joint pain.[1] Various diagnoses, especially such as degenerative arthritis, are known to contribute to BME.[1,2] The exact pathogenetic processes of painful BME in osteoarthritic knees and the role are not currently known.[3–5] BME in bone underneath cartilage significantly increases the risk for structural progression in knee osteoarthritis, and it is explained to be strongly related to malalignment toward the side affected by the lesion.[2,5] The increased mechanical load in knee osteoarthritic cases can cause microfractures to occur in the subchondral metaphyseal area, leading to the involved compartment collapses.[6] Schweitzer and White[4] also stated that altered weight bearing may rightly be ranked as one of the main causes of increased marrow edema lesions on MR images. BME is recognized to be related to biomechanical changes of knee osteoarthritis.[1,3,5,6]
BME is usually self-limiting in the nature course and the symptoms resolve spontaneously over a period of 6 months, or occasionally 12 months,[7] which is invariably associated with severe and long-lasting disability.[8,9] Various treatments have been recommended in order to shorten the natural course of the disease. Little is known about the optimal treatment of patients with this condition. The Osteoclast inhibitors such as bisphosphonate[9,10] and parenteral prostaglandin inhibitors such as iloprost[11] have been reported as being beneficial in the treatment of BME of different etiologies. Moreover, the prostaglandin inhibitors seem to act faster and more efficacious in treatment of BME than other drugs,[11] which were considered to be an ideal drug for treating this disease. Sometimes, these patients undergo nonsurgical or surgical treatments, and the pain symptom is not relieved or it may recur. Recent research supports the use of extracorporeal shock wave therapy (ESWT) in the treatment of the first stages of avascular osteonecrosis of the proximal femur and in other conditions where BME is present.[9,12–14] ESWT appears to be valid, reliable, and noninvasive to rapidly resolve intractable BME syndrome of the hip, and it has a low complication rate and relatively low cost compared with other conservative and surgical treatment approaches.[15] It has been shown to activate many cellular processes critical to neovascularization and tissue regeneration.[16,17]
However, there have been no reports on the clinical results of BME in osteoarthritis of the knee treated with ESWT. In this study, we retrospectively evaluated the efficacy of ESWT on painful BME in osteoarthritis of the knee, to explore whether shock wave treatment can shorten the natural course of this disease. We hypothesized that topical ESWT would result in rapid pain relief and functional improvement of the affected knee with BME without substantial complications.
2. Methods
The comparative historical cohort study was approved by the Institutional Review Board on Human Studies of the Ethical Committee of China–Japan Friendship Hospital, and the study procedures adhered to the 1975 Declaration of Helsinki. Informed consent was obtained from all the patients.
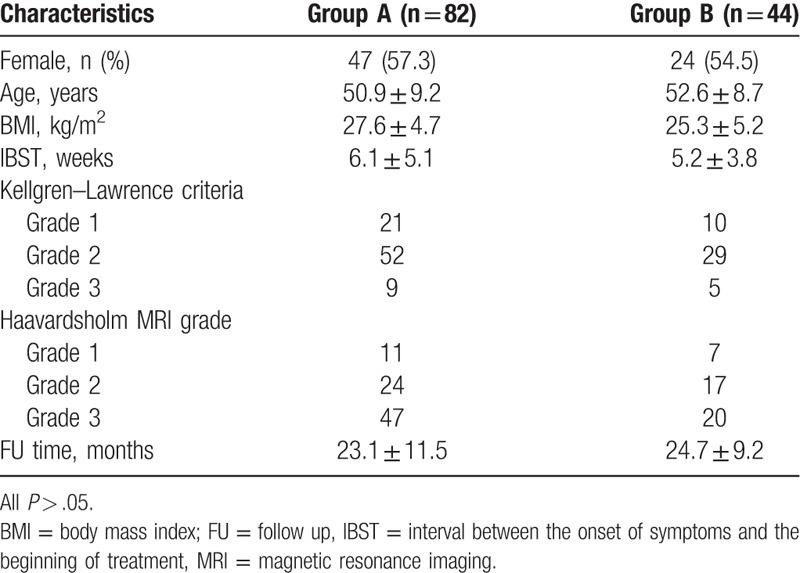
The study comprised early-to-mid stage osteoarthritic patients who had presented between January 2012 and July 2015 with knee pain and who had magnetic resonance imaging (MRI) findings of BME. All the patients included in this study had early-to-mid stage osteoarthritis with knee pain, no history or recall of trauma, and no valgus or varus deformity. Patients who had received any previous treatment or other diagnoses were also excluded, along with those who had contraindications for ESWT.[12] The study included 126 osteoarthritic patients who had presented with knee pain and whose MRIs showed BME at our center (Fig. 1). There were 71 females and 55 males, and their mean age was 51.9 years (range, 39–73 years) in this study. An evaluation of body mass index (BMI) showed as 26.8 ± 4.1 kg/m2. Patients who underwent ESWT treatment (Fig. 2) (Group A, n = 82) or prescribed alendronate treatment (Group B, n = 44) in our department were analyzed (70 mg po qw; Merck & Co, Inc, Peking) (Table 1). They complained of continuous pain while walking, which eased with rest. The average time between the onset of symptoms and the beginning of treatment was 5.6 weeks (range 1–12 weeks). The pain was in the right knee in 79 patients and in the left knee in 47 patients. The mean follow-up period was 23.5 months (range, 12–38 months).
Figure 1.
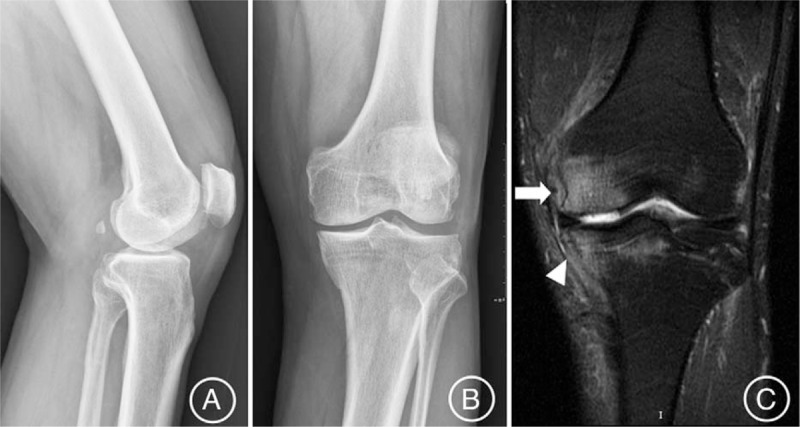
The MRI T2-weighted images (C) showing a large bone marrow edema within the medial femoral condyle (white arrow) and medial tibial plateau (white arrowhead) of the left osteoarthritic knee (K-L Grade 2), (A) posteroanterior view and (B) lateral view of x-ray, in a 64-year-old male patient. MRI = magnetic resonance imaging.
Figure 2.
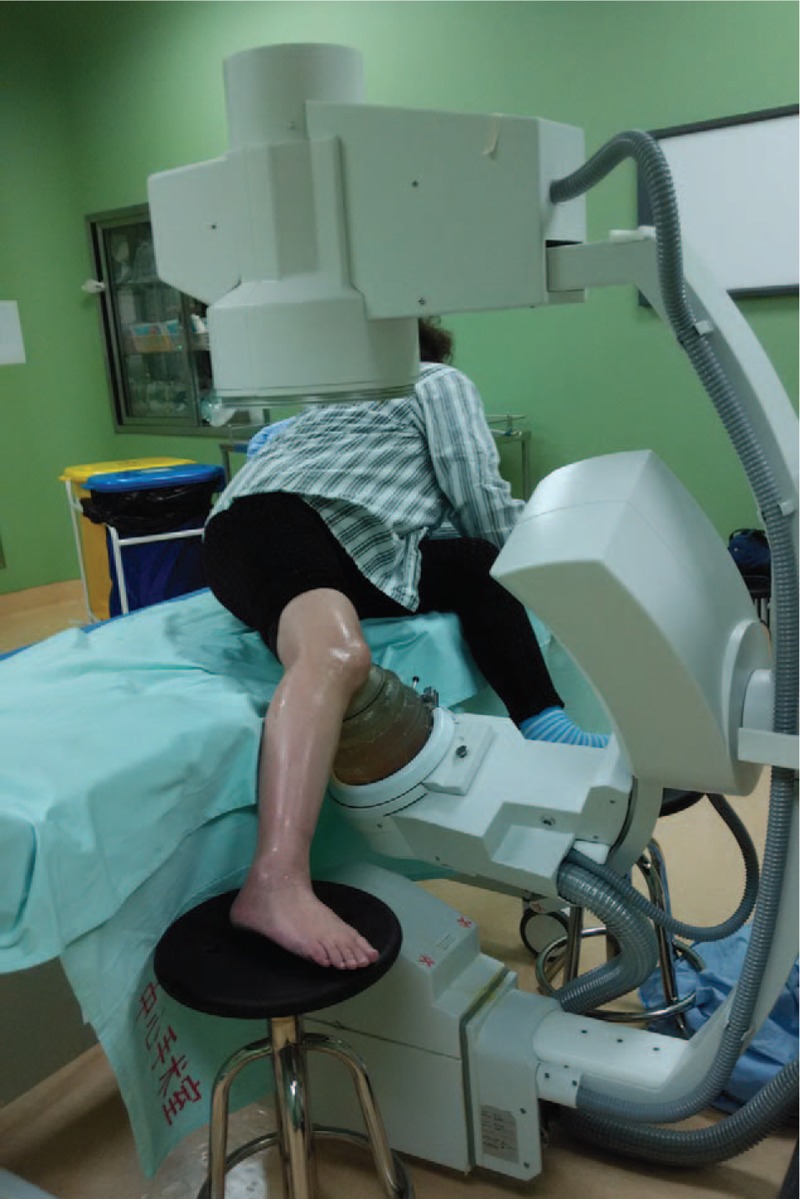
Intratherapeutic extracorporeal shock wave photograph of patients involved in the study.
Table 1.
Patients characteristics.
The degree of osteoarthritis was categorized radiologically according to the Kellgren–Lawrence (K-L) grading system[18] (Fig. 1A and B). According to the K-L criteria, 31 cases were Grade 1, 81 cases were Grade 2, and 14 cases were Grade 3. An experienced radiologist evaluated the extent of edema on 1 slide with the most obvious edema of the resulting MRI films with the same fluid-sensitive sequence using the PACS software (Kodak version 11.0, MA) to verify whether the edema lesion showed unchanged, reduced, or regressed completely. The BME was categorized according to the width of the lesions extending into the joint surface subchondral area on MRI T2 sequences as follows: Grade 0, no edema; Grade 1, minimal (<5 mm diameter); Grade 2, mild (5–20 mm diameter); Grade 3, severe (>20 mm diameter)[19] (Fig. 1C). The degree of BME on MRI was evaluated as follows: 18 cases of Grade 1, 41 cases of Grade 2, and 67 cases of Grade 3.
2.1. Shock wave treatment
The shock wave treatment was applied under intensified surface anesthesia (lidocaine hydrochloride gel) combined with once-through intravenous flurbiprofen axetil (Beijing Tide Pharmaceutical Co, Ltd, Beijing) using an Electromagnetic Shock Wave Emitter (Dornier Compact DELTA II, Germany) (Fig. 2), with a penetration depth of between 0 and 150 mm and a focus diameter of 4 mm. Shock waves were focused around (on the margins of) the knee under radiographic guidance. The treatment area was prepared with a coupling gel to minimize the loss of shock wave energy at the interface between the head of the device and the skin. The patients were subjected to high-energy ESWT,[13,15,29] and the parameters are prepared and used as follows: number of levels, 3–4; at a high energy flux density of >0.44 mJ/mm2 (level 3); 3000 to 4000 impulses at a frequency of 2 to 3 Hz. Each patient underwent 2 therapy sessions (the time interval between successive procedures was 1 week). The number of the frequency selected depends on the patient's condition.
2.2. Postintervention management and follow-up
All patients were mobilized with partial weight bearing and walking aids for 6 weeks and analgesics on demand with restrictions for impact sports such as sprinting or jumping. Patients in both groups received intravenous alprostadil (10 μg, qd for 2 weeks; Beijing Tide Pharmaceutical Co, Ltd; Beijing). The postintervention results were evaluated by the visual analog scale (VAS), WOMAC, and MRI scans as well as plain radiographs at 2 weeks (without imaging examination), 1 months (without imaging examination), 3 months, 6 months, 1 year post-treatment, and after. Routine evaluation of the mechanical axis was made by full-weight-bearing anterior–posterior-lateral knee radiographs and leg length radiographs.
2.3. Statistical analysis
All data analyses were performed using SPSS version 16.0.0 software (SPSS; Chicago, IL). The means and standard deviations (SD) were calculated for all patients, and 95% confidence intervals (CIs) were determined. The paired t test (Gaussian population) or Wilcoxon test (non-Gaussian population) was used to determine the changes in the VAS and WOMAC knee scores. A probability (P) value <.05 was considered to be of statistical significance.
3. Results
3.1. Clinical outcome
The compared results of the development of the VAS for pain and the Western Ontario and McMaster University Osteoarthritis Index (WOMAC) between both groups are shown in Figures 3 and 4. In this study, the overall VAS and WOMAC score decreased significantly in both groups at the final follow-up time (P <.01). All patients described the daily life function as significantly improved. This might be mainly caused by pain relief. Compared with Group B, all patients in Group A showed a greater and earlier improvement in VAS pain score and WOMAC score at the last follow-up after therapeutic intervention (P <.01). Moreover, VAS pain score of all patients in Group A continued to improve more obviously over the follow-up period than that in Group B (Fig. 3). Significant improvement in the VAS score was observed in Group A, from 8.5 ± 1.3 to 3.4 ± 2.1 points at 2 weeks, to 2.0 ± 1.4 points at 1 month, to 1.1 ± 0.9 points at 3 months, to 0.9 ± 0.6 points at 6 months, and to 0.5 ± 0.5 points at 12 months after therapeutic intervention. Gradual improvement in the VAS was shown in Group B, from 8.2 ± 1.5 points to 5.8 ± 1.9 points at 2 weeks, to 4.6 ± 1.8 points at 1 month, to 3.1 ± 2.0 points at 3 months, to 2.2 ± 1.1 points at 6 months, and to 1.4 ± 0.9 points at 12 months after therapeutic intervention. The mean improvement of WOMAC score after therapeutic intervention between both groups during the follow-up time was statistically significant (P <.01) (Fig. 4). Significant improvement in the WOMAC score was observed in Group A, from 67.1 ± 9.7 to 27.9 ± 10.1 points at 2 weeks, to 19.5 ± 11 points at 1 month, to 9.8 ± 9.8 points at 3 months, to 8.6 ± 9.5 points at 6 months, and to 7.4 ± 5.8 points at 12 months after therapeutic intervention. Gradual improvement in the WOMAC was shown in Group B, from 65.9 ± 10.4 to 48.2 ± 11.2 points at 2 weeks, to 41.1 ± 9.8 points at 1 month, to 26.3 ± 10.2 points at 3 months, to 21.9 ± 9.1 points at 6 months, and to 19.4 ± 8.3 points at 12 months after therapeutic intervention.
Figure 3.
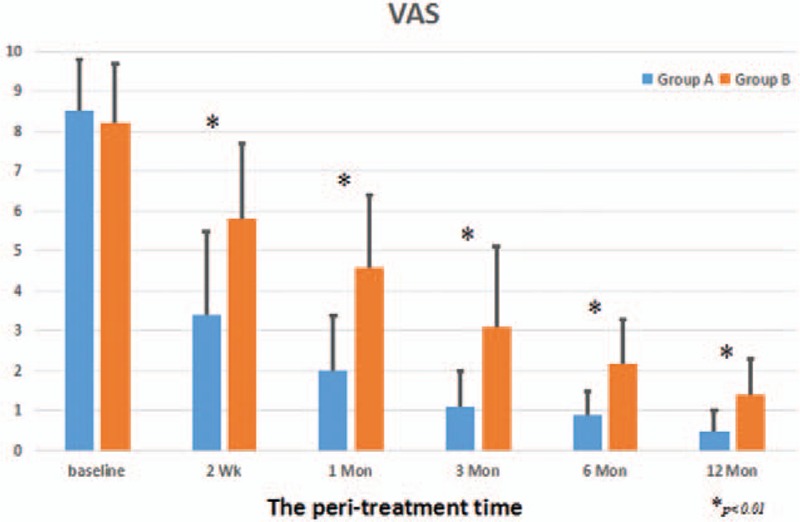
The development of the VAS during therapeutical intervention on BME in the knee osteoarthritis between 2 groups. BME = bone marrow edema, VAS = visual analog scale.
Figure 4.
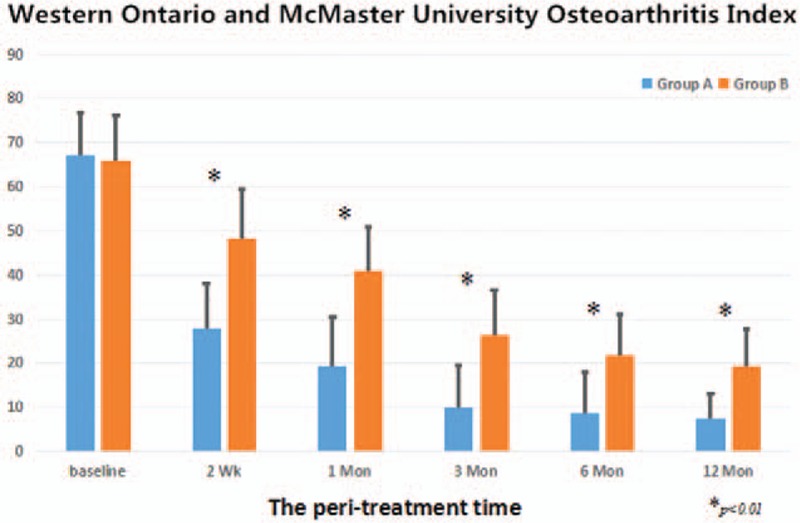
The development of the WOMAC score during therapeutical intervention on BME in the knee osteoarthritis between 2 groups. BME = bone marrow edema, WOMAC = Western Ontario and McMaster University Osteoarthritis Index.
3.2. Radiological outcome
The MRI findings demonstrated the progressive regression of the BME in both groups. MRI scans of both groups showed that the patients in Group A had a higher incidence of distinct reduction and complete regression of BME at 3 months (90.2% vs 61.4%; P <.001). The MRI at 6-month follow-up in Group A showed that there were a distinct reduction of BME in 61 patients and complete regression in 17 patients, so total improvement rate was 95.1% (78/82). In Group B, there was a reduction in BME in 21 patients and complete regression in 14 patients, moreover, total improvement rate was 79.5% (35/44). There was an apparent significant difference between 2 groups in the MRI improvement rate (P = .006). However, the MRI at 12-month follow-up showed that there were complete regression in all patients in Group A (100%) and most of patients in Group B (97.7%). However, 1 case in Group B continued to normalize over the subsequent follow-up period (18 months). Therefore, these MRI results at the final follow-up were not statistically significant. In the final follow-up, there was no joint space narrowing found, no necrosis, or stress fracture of the knee seen in this study.
3.3. Side-effects
Only minor complications occurred after ESWT, such as transient soft tissue swelling or minor bruising. No clinically detectable neuromuscular, systemic, or device-related adverse effects were observed after ESWT. No adverse events were detected with alendronate. Over the study period, no case of osteonecrosis of the jaw or a kidney dysfunction was noted in the alendronate oral medication. No other adverse effects were noted. But there was a special case of intra-articular shifting painful BME of knee osteoarthritis with treated ESWT plus alprostadil. We described the case hereinbelow.
3.4. One case with intra-articular shifting BME
There was 1 case with intra-articular shifting BME, hence which was excluded in this study. Painful BME in the left knee osteoarthritis was diagnosed in a 50-year-old male driver. ESWT rapidly produced positive effects with regard to both pain and BME. Symptoms of pain were significantly relieved. The painful VAS score dropped from 9 points preoperatively to 2 points at 2 weeks post-treatment, and disappeared at 1 month post-treatment. However, the sudden intense pain occurs in his left knee at 9 weeks post-treatment. MRI showed BME recurred and was involved in lateral condyle of femur. He received the same treatment option. Patients treated with ESWT recover quickly and experience a rapid relief from pain. Accompanied by improvements in the WOMAC Osteoarthritis Index and VAS, MRI showed a significant regression in edema between the pretreatment and 3 months post second treatment. At the 12-month follow-up MRI BME was not seen to have recurred in the patient.
4. Discussion
Several reasons are known for pain in osteoarthritic knees. All patients with BME can also suffer severe pain. Several studies have stated a relationship between BME and pain and that a decrease in BME lesions reduces the pain.[2,5,15,20] In our study, the regression in the BME and the reduction in pain following ESWT plus alprostadil can also support the idea of a relationship between BME and pain. However, the exact frequency of BME in patients with osteoarthritis of the knee is not known. The presence of BME was noted on MRI in 50% to 73% of painful knees and also in a few of pain-free knees.[5,21] BME is strongly related to malalignment toward the affected side, which increases the risk for structural progression in knee osteoarthritis.[2,5] The altered weight bearing may be 1 reason why marrow edema lesions on MR images increased in the subchondral metaphyseal area.[4] And biomechanical changes of knee osteoarthritis can cause microfractures to occur, leading to BME in the involved compartment.[6,22] Besides, high BMI and knee deformities are the high-risk factors affecting the occurrence of BME lesions on MRI in knee osteoarthritis,[2] which, in turn, would show a tendency to speed up the progression of osteoarthritis. Histologically, it showed that abnormal bone trabeculae, small areas of osteonecrosis, and wide areas of remodeling can be observed in the area of BME.[23] The venous stasis and increased bone pressure may occur in the bone marrow lesions near the painful joint where abnormally high uptake of the radiotracer appears.[24] However, there is still debate regarding the pathogenesis and implications of BME in knee osteoarthritis.
There is no consensus regarding treatment for BME seen on MRI in osteoarthritic knees.[2,3,5,8,25] Conservative treatment is recommended to be able to regress BME, including reduction of weight-bearing load, analgesic and anti-inflammatory medication, and physiotherapy.[3,8,26,27] The potent osteoclast inhibitor alendronate has proved to be effective and tolerant in metabolic bone disease such as osteoporosis conditions and BME.[10,28,29] However, the pathophysiological mechanism of action of alendronate in BME is still unclear. It seems that bisphosphonate treatment can regulate the increased bone turnover in BME to shorten the natural course of the disease.[10] However, conservative treatment approaches take too long time or are unable to relieve symptoms in some cases.[1,8] Surgeries such as core decompression or open-wedge tibial osteotomy, which can shorten the clinical course, are costly and associated with risks.[2,8,26,30] Moreover, some considered that surgery was too invasive for a self-limiting disease with a variable clinical course.[12,30] Clinical trials have confirmed the effectiveness of ESWT in treating the early stages of avascular necrosis, reducing bone edema, and pain.[13,31] There have been currently a few reports addressing the use of ESWT in idiopathic BME of the hip.[9,12,15] Our study showed that ESWT would result in more highly effective pain relief and functional improvement of the affected knee without substantial complications in painful BME in knee osteoarthritic patients than alendronate oral medication. And the mean VAS showed a more dramatic improvement from pretreatment values at all follow-up time, especially at 1 month. The clinical improvement in WOMAC Osteoarthritis Index observed following ESWT was more obvious in most patients at 1 month post-treatment than the control Group (P <.01). At the final follow-up point, all patients had already regained a significant level of autonomy in their daily lives with a marked reduction in pain, which correlated with the progressive normalization of MRI features. It showed that ESWT method was a valid tool to shorten the natural course of this disease.
However, the exact mechanism by which ESWT operates remains relatively unknown. Tischer et al[17] stated that the amount of new bone formation seemed to be related to the energy dose for shock wave applications. The positive effects of ESWT on bone metabolism showed the close anatomical and functional links between vascular elements, marrow stromal, and active bone cells, and ESWT can facilitate bone reparative processes. Meanwhile it also can activate many critical cellular processes of neovascularization and tissue regeneration,[9,13,16] which was confirmed to be associated with increased expression of angiogenic growth factors, including vascular endothelial growth factor, proliferating cell nuclear antigen, endothelial nitric oxide synthase, and BMP-2.[16,32–36] It is reasonable to speculate that neovascularization plays a role in improvement in the local blood supply and may promote bone regeneration in cases of BME.[37,38] The neoangiogenetic effect of ESWT appears to reduce the time to symptom remission. All BME patients following ESWT in our study presented a dramatic pain reduction by eliminating the BME and ESWT shortens the natural course of the self-limiting lesion.
There are several limitations associated with this study. So far, the mechanisms and indications of ESWT have not been very clear. The indications are mainly based on the supported literatures and our previous clinical observation. This study is limited by virtue of the retrospective analysis. There was no randomized and blinded control group with conservative treatment in this study. Intravenous prostacyclin can achieve a reduction in BME, with a considerable improvement of painful symptoms, by improving tissue blood supply in a variety of situations through multiple mechanisms, such as vasodilatation and inhibition of platelet aggregation.[8,30] It is that pain relief and rapid regression of BME due to the action of prostacyclin in reducing capillary permeability and dilating vessels.[27] Based on this, all patients in both groups were treated with combined alprostadil in our study. The functional improvement in the knee was assessed subjectively using the VAS and functional scores, but no objective measures were utilized. The follow-up time was relatively short, but similar to prior studies on this subject. This study was only a pilot clinical study. However, the results of this study were inspiring. Moreover, we will consider multicenter randomized controlled trials to validate this conclusion in future studies.
5. Conclusion
In conclusion, extracorporeal shock wave treatment was an effective, reliable, and noninvasive treatment in patients who have painful BME in osteoarthritis of the knee, followed by a rapid normalization of the MRI appearance. They seemed to act faster and more efficacious with a lower complication rate. It has the potential to resolve patient suffering quickly. It was explored to shorten the natural course of this disease. To ensure our results, prospective randomized trials with long-terms results should be realized. Further exploration of its mechanisms and prospects would be worthwhile.
Footnotes
Abbreviations: BME = bone marrow edema, ESWT = extracorporeal shock wave treatment, VAS = visual analog scale, WOMAC = Western Ontario and McMaster University Osteoarthritis Index.
SK, FG, JH, and TM are joint first authors.
This study was supported by the Beijing Natural Science Foundation (7174346) and National Natural Science Foundation of China (81372013, 81672236).
The authors have no conflicts of interest to disclose.
References
- [1].Berger CE, Kröner AH, Kristen KH, et al. Transient bone marrow edema syndrome of the knee: clinical and magnetic resonance imaging results at 5 years after core decompression. Arthroscopy 2006;22:866–71. [DOI] [PubMed] [Google Scholar]
- [2].Kesemenli CC, Memisoglu K, Muezzinoglu US, et al. Treatment for painful bone marrow edema by open wedge tibial osteotomy. Eur J Orthop Surg Traumatol 2013;23:825–9. [DOI] [PubMed] [Google Scholar]
- [3].Hofmann S, Kramer J, Breitenseher M, et al. Bone marrow edema in the knee. Differential diagnosis and therapeutic possibilities. Orthopade 2006;35:463–75. [DOI] [PubMed] [Google Scholar]
- [4].Schweitzer ME, White LM. Does altered biomechanics cause marrow edema. Radiology 1996;198:851–3. [DOI] [PubMed] [Google Scholar]
- [5].Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 2003;139:330–6. [DOI] [PubMed] [Google Scholar]
- [6].Kesemenli CC, Memisoglu K, Muezzinoglu US. Bone marrow edema seen in MRI of osteoarthritic knees is a microfracture. Med Hypotheses 2009;72:754–5. [DOI] [PubMed] [Google Scholar]
- [7].Aigner N, Meizer R, Petje G, et al. Natural course of intra-articular shifting bone marrow edema syndrome of the knee. BMC Musculoskelet Disord 2008;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel S. Primary bone marrow oedema syndromes. Rheumatology (Oxford) 2014;53:785–92. [DOI] [PubMed] [Google Scholar]
- [9].Baiano C, Romeo A, Zocco A, et al. Bone marrow edema syndrome of the hip: effectiveness of extracorporeal shock waves therapy associated with clodronate: a case report. Bone 2010;47:S91–2. [Google Scholar]
- [10].Bartl C, Imhoff A, Bartl R. Treatment of bone marrow edema syndrome with intravenous Ibandronate. Arch Orthop Trauma Surg 2012;132:1781–8. [DOI] [PubMed] [Google Scholar]
- [11].Meizer R, Radda C, Stolz G, et al. MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint localizations treated with the prostacyclin analogue iloprost. Wien Klin Wochenschr 2005;117:278–86. [DOI] [PubMed] [Google Scholar]
- [12].Agostino C, Romeo P, Lavanga V, et al. Effectiveness of extracorporeal shock wave therapy in bone marrow edema syndrome of the hip. Rheumatol Int 2014;34:1513–8. [DOI] [PubMed] [Google Scholar]
- [13].Wang CJ, Wang FS, Yang KD, et al. Treatment of osteonecrosis of the hip: comparison of extracorporeal shockwave with shockwave and alendronate. Arch Orthop Trauma Surg 2008;128:901–8. [DOI] [PubMed] [Google Scholar]
- [14].Gao F, Sun W, Li Z, et al. Extracorporeal shock wave therapy in the treatment of primary bone marrow edema syndrome of the knee: a prospective randomised controlled study. BMC Musculoskelet Disord 2015;16:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gao F, Sun W, Li Z, et al. Intractable bone marrow edema syndrome of the hip. Orthopedics 2015;38:e263–70. [DOI] [PubMed] [Google Scholar]
- [16].Xu JK, Chen HJ, Li XD, et al. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase. J Biol Chem 2012;287:26200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tischer T, Milz S, Weiler C, et al. Dose-dependent new bone formation by extracorporeal shock wave application on the intact femur of rabbits. Eur Surg Res 2008;41:44–53. [DOI] [PubMed] [Google Scholar]
- [18].Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Haavardsholm EA, Bøyesen P, Østergaard M, et al. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis 2008;67:794–800. [DOI] [PubMed] [Google Scholar]
- [20].Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 2001;134:541–9. [DOI] [PubMed] [Google Scholar]
- [21].Sowers MF, Hayes C, Jamadar D, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and x-ray-defined knee osteoarthritis. Osteoarthritis Cartilage 2003;11:387–93. [DOI] [PubMed] [Google Scholar]
- [22].Plenk H, Jr, Hofmann S, Eschberger J, et al. Histomorphology and bone morphometry of the bone edema syndrome of the hip. Clin Orthop Relat Res 1997;334:73–84. [PubMed] [Google Scholar]
- [23].Zanetti M, Bruder E, Romero J, et al. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 2000;215:835–40. [DOI] [PubMed] [Google Scholar]
- [24].Arnoldi CC, Djurhuus JC, Heerfordt J, et al. Intraosseous phlebography, intraosseous pressure measurements and 99mTC polyphasphate scintigraphy in patients with various painful conditions in the hip and knee. Acta Orthop Scan 1980;51:19–28. [DOI] [PubMed] [Google Scholar]
- [25].Kornaat PR, Kloppenburg M, Sharma R, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol 2007;17:3073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hofmann S, Engel A, Neuhold A, et al. Bone-marrow oedema syndrome and transient osteoporosis of the hip. An MRI-controlled study of treatment by core decompression. J Bone Joint Surg Br 1993;75:210–6. [DOI] [PubMed] [Google Scholar]
- [27].Korompilias AV, Karantanas AH, Lykissas MG, et al. Bone marrow edema syndrome. Skeletal Radiol 2009;38:425–36. [DOI] [PubMed] [Google Scholar]
- [28].Simon MJ, Barvencik MJ, Luttke M, et al. Intravenous bisphosphonates and vitamin D in the treatment of bone marrow oedema in professional athletes. Injury 2014;45:981–7. [DOI] [PubMed] [Google Scholar]
- [29].Ringe JD, Dorst A, Faber H. Effective and rapid treatment of painful localized transient osteoporosis (bone marrow edema) with intravenous ibandronate. Osteoporos Int 2005;16:2063–8. [DOI] [PubMed] [Google Scholar]
- [30].Baier C, Schaumburger J, Götz J, et al. Bisphosphonates or prostacyclin in the treatment of bone-marrow oedema syndrome of the knee and foot. Rheumatol Int 2013;33:1397–402. [DOI] [PubMed] [Google Scholar]
- [31].Wang CJ, Wang FS, Ko JY, et al. Extracorporeal shockwave therapy shows regeneration in hip necrosis. Rheumatology (Oxford) 2008;47:542–6. [DOI] [PubMed] [Google Scholar]
- [32].Frairia R, Berta L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles Ligaments Tendons J 2011;1:138–47. [PMC free article] [PubMed] [Google Scholar]
- [33].Wang CJ, Huang KE, Sun YC, et al. VEGF modulates angiogenesis and osteogenesis in shockwave-promoted fracture healing in rabbits. J Surg Res 2011;171:114–9. [DOI] [PubMed] [Google Scholar]
- [34].Haake M, Thon A, Bette M. Unchanged c-Fos expression after extracorporeal shock wave therapy: an experimental investigation in rats. Arch Orthop Trauma Surg 2002;122:518–21. [DOI] [PubMed] [Google Scholar]
- [35].Ma HZ, Zeng BF, Li XL, et al. Temporal and spatial expression of BMP-2 in sub-chondral bone of necrotic femoral heads in rabbits by use of extracorporeal shock waves. Acta Orthop 2008;79:98–105. [DOI] [PubMed] [Google Scholar]
- [36].Ma HZ, Zeng BF, Li XL. Upregulation of VEGF in subchondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcif Tissue Int 2007;81:124–31. [DOI] [PubMed] [Google Scholar]
- [37].Wang CJ, Yang YJ, Huang CC. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol Int 2011;31:871–7. [DOI] [PubMed] [Google Scholar]
- [38].Hausdorf J, Lutz A, Mayer-Wagner S, et al. Shock wave therapy for femoral head necrosis—pressure measurements inside the femoral head. J Biomech 2010;43:2065–9. [DOI] [PubMed] [Google Scholar]


