Abstract
Cardiovascular disease (CVD) is a common cause of death in patients with chronic kidney disease (CKD). Aortic and mitral valve calcification (AVC and MVC, respectively) are critical indicators of CVD and all-cause mortality in CKD patients.
We conducted a single center retrospective study of Chinese inpatients with CKD to identify risk factors associated with valve calcification (VC).
Of 288 enrolled CKD patients, 22.9% had VC, all of which exhibited AVC, while 21.2% exhibited MVC. The VC group were significantly older than the non-VC group (70.42 ± 11.83 vs 56.47 ± 15.00, P < .001), and contained more patients with history of coronary artery disease (12.1% vs 4.5%, P = .025) or stroke (18.2% vs 5.4%, P < .001). Subjective global assessment scoring indicated that more VC patients were mid/severely malnourished. Levels of prealbumin, cholesterol (Ch), triglycerides, low-density lipoprotein (LDL), apolipoprotein E, ejection fraction, and fraction shortening were significantly lower, and blood C reactive protein, IL-6, left ventricular internal end diastole diameter measured in end diastole, and interventricular septum thickness (IVST) levels were significantly higher in the VC group. Bone metabolism did not differ significantly between the 2 groups. Multivariable logistic regression analysis indicated that age, blood Ch, and LDL levels were significantly associated with VC.
Advanced age, increased IVST, hypocholesterolemia, and hyper-LDL cholesterolemia were key risk factors for VC in Han patients with CKD.
Keywords: cholesterol, chronic kidney disease, heart valve calcification, inflammation, malnutrition
1. Introduction
Cardiovascular disease (CVD) is the most common cause of death in patients with chronic kidney disease (CKD). In 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) CKD–Mineral and Bone Disorder (MBD) Work Group suggested that patients with CKD and heart valve calcification (VC) should be considered at the highest risk of CVD.[1] Approximately 120 million patients have CKD in China, representing a prevalence of 10.8%,[2] which does not differ significantly from the rate of CKD reported in the United States of America (13.1%) or Norway (10.2%).[3,4] Nevertheless, a national survey conducted in 2013 found that awareness of CKD (10.04%) remains low in China.[3–5]
VC is a common complication of CKD and is a critical indicator of CVD and all-cause mortality in patients with CKD[6–8] and in patients with end-stage renal disease (ESRD).[9] The incidence of VC increases with CKD progression, from around 40% in stage 3 CKD to 80% to 99% in stage 5 CKD.[10–14] Typical manifestations include calcification of the vessel wall, myocardium, and heart valves, which cannot only impair cardiac conduction and cause arrhythmia, but also serve as an indicator of coronary artery disease progression.
Many risk factors have been associated with VC in patients with CKD, including canonical factors such as age, hypertension, diabetes, and dyslipidemia as well as noncanonical factors such as MBDs, inflammation, and malnutrition. Nevertheless, the exact contributions of these factors have not been well characterized in Asian populations. The incidence and severity of heart VC have been observed to increase with age,[15] and the onset of VC has been reported to occur several decades earlier in patients with CKD than in other populations.[16] A Chinese study indicated that the relative risk of heart VC increased by 2.22 to 2.66-fold with each decade of life, while the rate of VC remains higher in patients with CKD.[17] Vascular calcification is reported to increase left ventricular after load and is the most important contributor to the development of left ventricular hypertrophy (LVH) in ESRD patients.[18] Atherosclerosis and vascular calcification were previously reported to be independent predictors of LVH in hemodialysis patients.[19] Serum phosphorus can be cardiotoxic, leading to LVH, but this effect can be successfully reversed with adequate control of serum phosphorus.[20] Furthermore, the level of fibroblast growth factor-23, a hormone associated with vascular calcification, increases very early in CKD and is strongly associated with CVD, including LVH, and mortality.[21] Therefore, strategies to address cardiovascular risk in early CKD are imperative and vascular calcification is a potential therapeutic target.
Here, we describe a single center retrospective study of Han Chinese inpatients with CKD designed to examine the risk factors associated with VC.
2. Methods
2.1. Subjects
The study consecutively enrolled and retrospectively analyzed 288 Han Chinese inpatients with CKD admitted to the Department of Nephrology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine for the first time between January 1 and June 1, 2015. CKD was staged according to the estimated glomerular filtration rate (eGFR), as reported by the Kidney Disease Outcomes Quality Initiative.[22] The eGFR was calculated using the CKD-Epidemiology Collaboration Equation (CKD-EPI).[23]
The inclusion criteria were as follows: patients ≥18 years of age diagnosed with CKD according to the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of CKD (defined as abnormalities of kidney structure or function present for >3 months impacting the health).[24,25] CKD was staged according to eGFR (mL/min/1.73 m2) as follows, GFR ≥ 90, G1; GFR = 60–89, G2; GFR = 45–59, G3a; GFR = 30–44, G3b; GFR = 15–29, G4; and GFR ≤ 15, G5. Patients with acute kidney injury, active inflammatory diseases, parathyroidectomy, or evident malignancies; concomitant diseases that affect calcium (Ca) status and soft tissue calcifications such as sarcoidosis, multiple myeloma, HIV, and amyloidosis; conditions prohibiting arterial calcification measurements; or pregnancy or lactation were excluded. Written informed consent was obtained from all patients included in the study and the ethics committee of Shanghai General Hospital approved the study.
2.2. Echocardiography
Color ultrasound (Philips-ie33, Philips North America Corporation, Andover, MA) was used to examine VC in patients with CKD. If bright, dense echoes exceeding 1 mm were observed on one or more leaflets of the mitral and/or aortic valves, the patient was diagnosed with VC.[26] Ultrasound images were evaluated by an experienced ultrasound physician. Based on echocardiography, the patients were divided into 2 groups: VC and non-VC. All echocardiography examinations were performed according to the recommendations of the American Society of Echocardiography (ASE).[27,28] Left ventricular mass was calculated according to the Devereux formula 26: LVM (g) = 0.8 × 1.04 ([LVDd + IVST + LVPWTd]3 − LVDd3) + 0.6 (left ventricular internal end diastole diameter [LVDd], interventricular septum thickness [IVST], left ventricular internal posterior wall thickness measured in end diastole [LVPWTd]). Left ventricular mass index was calculated by dividing LVM by the body surface area.[29] LVH was diagnosed when LVMI was ≥125 g/m2 in males or ≥110 g/m2 in females, according to the recommendations of the ASE.3 clinical indices.
2.3. Data collection
History of primary disease, hypertension, diabetes, coronary artery disease, and stroke was scored using the subjective global assessment by 2 experienced physicians.[30] Subjective evaluations were validated by consistency analysis. Blood pressure and body mass index (kg/m2) were recorded at admission. Hemoglobin, albumin (Alb), pre-Alb (pAlb), blood urea, serum creatinine, uric acid, Ca, phosphorus (P), intact parathyroid hormone, 25-hydroxy vitamin D, N-terminal/mid-region osteocalcin, β carboxy-terminal cross-linking telopeptide of type I collagen (measured at 6 and 24 hours), total cholesterol (Ch), triglycerides (TGs), high-density lipoprotein, low-density lipoprotein (LDL), lipoprotein a, apolipoprotein (APO)-AI, APO-B, APO-E, and urine protein and Alb levels were measured routinely.
2.4. Treatments
Blood pressure, anemia, dyslipidemia, and renal osteopathy were treated by the nephrologists according to the KDIGO guidelines (http://kdigo.org/home/). Use of antihypertensive drugs including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, Ca antagonists and β receptor blockers, statin, erythropoietin, active vitamin D3, and Ca carbonate was recorded.
2.5. Statistical analysis
All continuous data were evaluated for normality using the Kolmogorov–Smirnov test. Normally distributed continuous variables were presented as mean ± standard deviation and analyzed using the Student t test. Nonnormally distributed variables were presented as median (interquartile range) and analyzed using the Mann–Whitney U test. Categorical variables were presented as number and frequencies, and analyzed using the chi-square test or Fisher exact test. Multivariable logistic regression analysis was used to identify factors associated with heart VC. Candidate variables were included in multivariable logistic regression analysis if P < .4 in univariable analyses. Odds ratios and 95% confidence intervals were calculated. Forward conditioning was used for variable selection. Hence, 0.05 was set for variable inclusion and 0.10 was set for variable exclusion. SPSS 16.0 (IBM, NY) was used for statistical analysis. P < .05 was considered to indicate significant differences.
3. Results
3.1. Participant characteristics
Of the 288 Han inpatients with CKD (190 male and 98 female) admitted to our department, 66 (22.9%) had VC, of which all exhibited aortic valve calcification and 14 (21.2%) exhibited mitral valve calcification. The gender distribution did not differ significantly between the VC and non-VC groups, but patients in the VC group were significantly older than in the non-VC group (70.42 ± 11.83 vs 56.47 ± 15.00, P < .001; Table 1). The types of drugs used in the VC and non-VC group did not differ significantly (Table 1).
Table 1.
Demographic and clinical characteristics of participants.
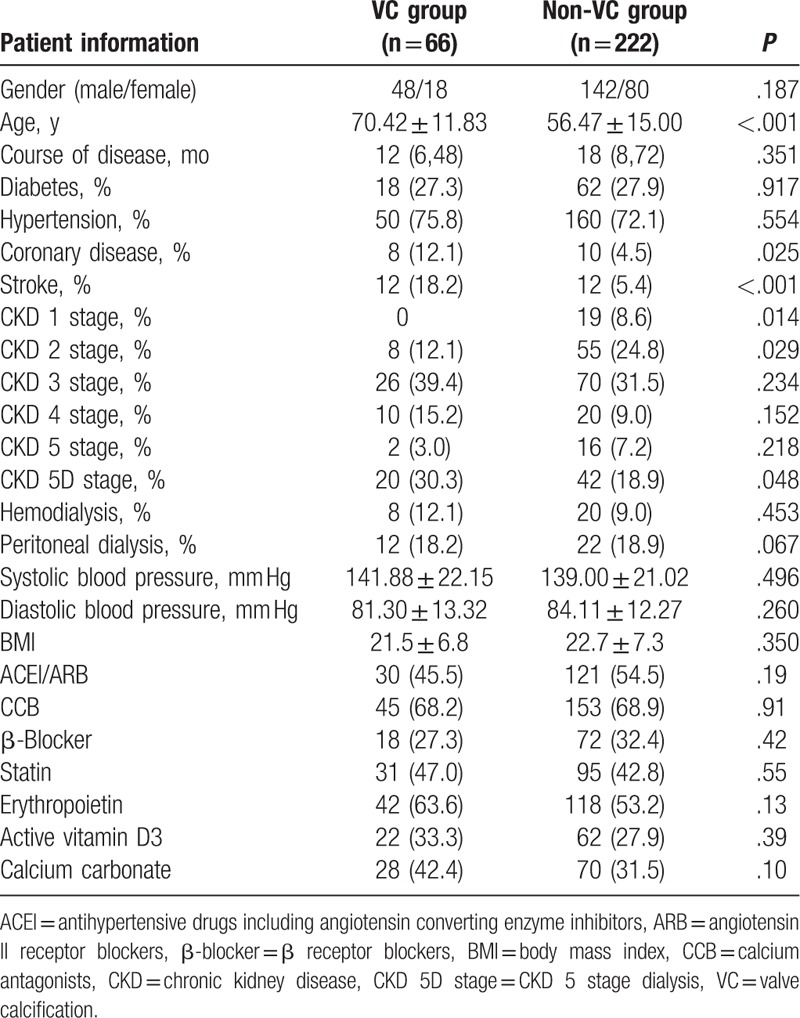
Chronic glomerulonephritis, diabetes, and hypertension were the most frequently reported primary diseases. The types and frequency of primary diseases, hypertension, diabetes, and blood pressure did not differ significantly between the VC and non-VC groups, but the rates of coronary artery disease and stroke were higher in the VC group than in the non-VC group (12.1% vs 4.5%, P = .025, and 18.2% vs 5.4%, P < .001, respectively). The percentage of patients with stage 1 and 2 CKD in the VC group was significantly lower than in the non-VC group, and the percentage of patients in stage 5 CKD was significantly higher in the VC group that in the non-VC group. The rate of hemo- and peritoneal dialysis was also higher in the VC group than in the non-VC group, but not statistically significantly higher.
Thirty patients in the VC group had chronic glomerulonephritis, 12 had diabetes, 10 had hypertension, 4 had obstructive nephropathy, 3 had systemic vasculitis, 2 had focal segmental glomerulosclerosis, 2 had gout, 2 had tumor-associated nephropathy, and 1 had renal artery stenosis. In the non-VC group, 114 patients had chronic glomerulonephritis, 32 had diabetes, 18 had hypertension, 14 had polycystic kidney disease, 12 had IgA nephropathy, 10 had systemic vasculitis, 6 had membranous nephropathy, 4 had obstructive nephropathy, 2 had focal segmental glomerulosclerosis, 2 had gout, 2 had hepatitis B virus-associated nephritis, 2 had systemic lupus erythematosus, 2 had purpura nephritis, 1 had multiple myeloma, and renal artery stenosis.
3.2. Biochemical parameters
Patients in the VC group had significantly lower pAlb levels than those of the non-VC group, and levels of Ch, TG, LDL, and APOE were significantly lower in the VC group than in the non-VC group. According to subjective global assessment scoring, the percentage of patients with mid/severe malnutrition in the VC group was significantly higher than in the non-VC group. Serum levels of inflammatory markers serum CRP and IL-6 were significantly higher in the VC group than in the non-VC group. Moreover, hemoglobin levels were lower in the VC group but not statistically significantly lower, and bone metabolism did not differ significantly between the 2 groups (Table 2).
Table 2.
Participant biochemical parameters.
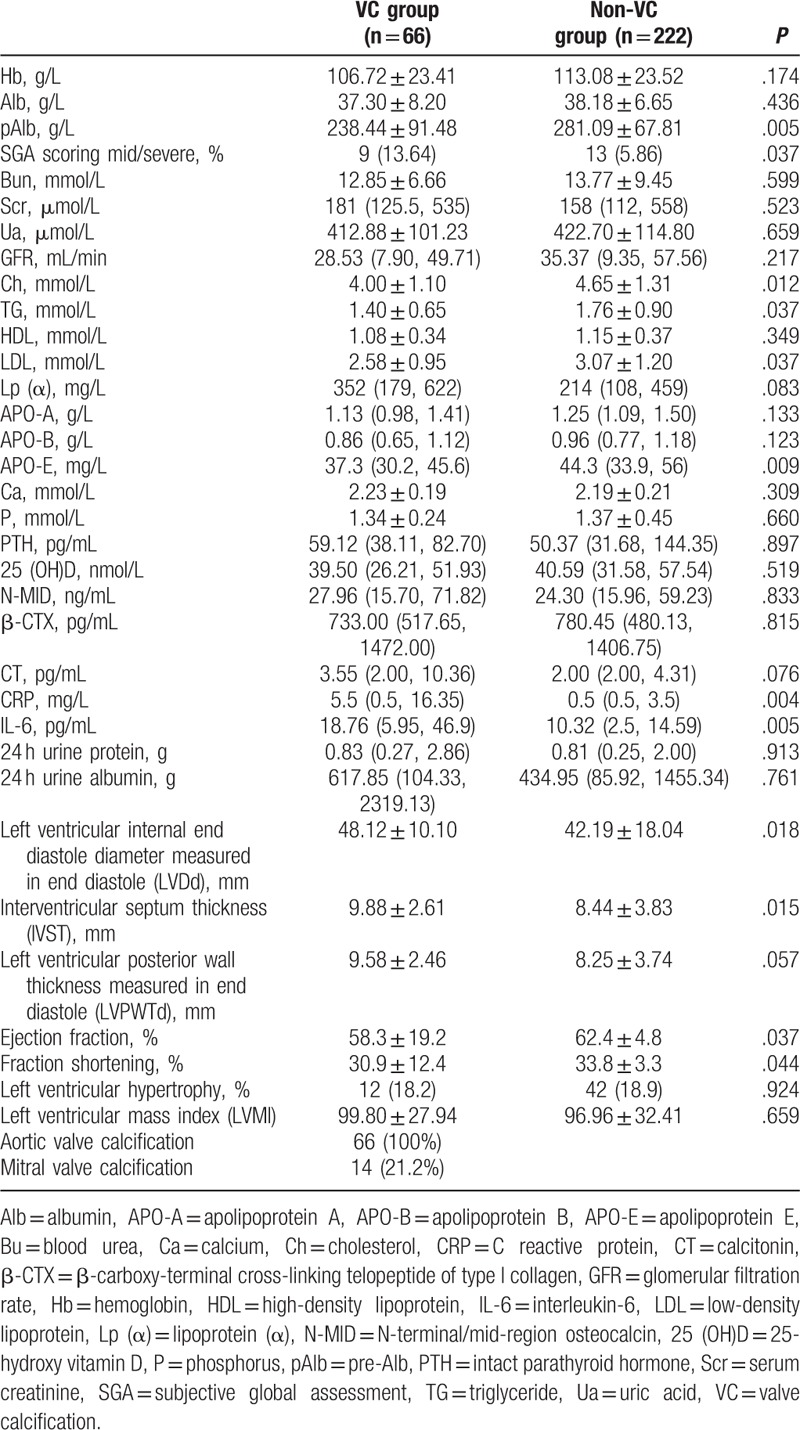
LVDd, LVPWTd, and IVST were significantly greater in the VC group than in the non-VC group (P < .05). Ejection fraction was significantly lower in the VC group (P < .05), but the rates of LVH and left ventricular mass index did not differ significantly between these groups (Table 2).
3.3. Multivariable logistic regression analysis
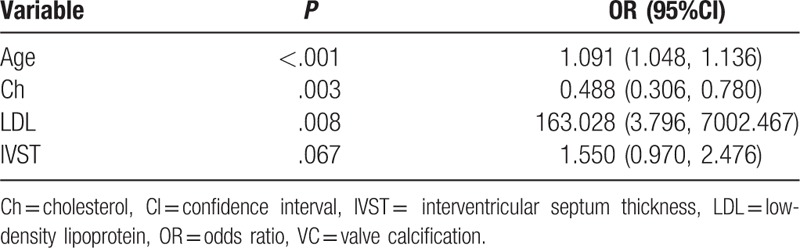
Factors which univariable analysis indicated to be significantly associated with VC were assessed via multivariable logistic regression analysis. As indicated in Table 3, higher age (OR 1.091, 95%CI 1.048, 1.136), higher LDL levels (OR 163.028, 95%CI 3.796, 7002.467), thicker IVST (OR 1.550, 95%CI 0.970, 2.476), and lower total Ch levels (OR 0.488, 95% CI 0.306, 0.780) were associated with vascular calcification (P < .05).
Table 3.
Risk factors significantly correlated with VC.
4. Discussion
To analyze risk factors for VC in patients with CKD, we retrospectively analyzed 288 CKD patients. Over one-fifth of patients had VC, of which all exhibited aortic valve calcification and roughly one-fifth of which exhibited mitral valve calcification. The patients in the VC group were significantly older than those in the non-VC group and had higher rates of coronary artery disease and stroke. Significantly more VC patients were malnourished, and levels of pAlb, Ch, TGs, LDL, and APO E were significantly lower in VC patients than in non-VC patients. EF and fraction shortening were also lower in VC patients. Markers of inflammation, CRP, and IL-6 levels were elevated in VC patients, and left ventricular internal end diastole diameter measured in end diastole and IVST were significantly higher in the VC group.
The risk of death from CVD and non-CVD is reported to be higher in patients with ESRD.[31] Oxidative stress, anemia, inflammation, protein-energy wasting (PEW), Ca, P, and lipoprotein disorders may also contribute to this increased risk.[31] Nevertheless, some risk factors for CVD defined in the general population appear to have the opposite effect in this patient population.[32–35] For example low Ch levels have been reported to be associated with poor prognosis of patients with ESRD; and Ch levels appear protective.[32–35] This “reverse epidemiology” phenomenon could be explained by the fact that inflammation, PEW, and other complications associated with severe ESRD can reduce Ch levels.[36] Both short- and long-term[37] factors can influence mortality. In the general population, hypercholesterolemia is a long-term risk factor for progression of atherosclerosis and death, acting over several decades whereas inflammation and PEW are associated with mortality within several months to years. As the mortality of patients with ESRD was far higher than that of the general population, most patients do not live long enough to reveal the impact of long-term risk factors such as high Ch level.[38] In addition, some studies have suggested that higher lipid concentrations directly improve survival of patients with chronic diseases including ESRD.[39] Thorough investigation of APOE polymorphisms may reveal the mechanisms responsible for poor prognosis in ESRD patients with low Ch levels.[40]
The reverse epidemiology phenomenon has been reported not only in patients with CKD,[41] but also in chronic heart[42] and respiratory[43] failure, and rheumatoid arthritis.[44] Chronic microinflammation was also commonly seen in these patients, which typically have a long disease course. Although some studies have only observed the Ch reverse epidemiology phenomenon in inflammatory disorders complicated with malnutrition,[34] other studies did not show the same results.[35,45]
Serum CRP and IL-6 levels reflect chronic systemic microinflammation and have been associated with the risk of VC in CKD. This pathway may be affected by multiple factors including oxidative stress, hyperglycemia, and hyperlipidemia.[46] Inflammation may accelerate VC in patients with ESRD by interfering with LDL receptor-mediated signaling pathways, as observed in atherosclerosis.[47] Krasniak et al[48] examined the average carotid intima-media thickness, coronary artery calcification score, and VC associated factors in 73 patients under maintenance HD and found that both CRP and IL-6 were positively correlated, indicating that inflammation may contribute to VC. Nuclear factor-κB is a key factor in many inflammatory responses,[49] and nuclear factor-κB activation promotes secretion of many cytokines (including tumor necrosis factor-α, IL-6, and CRP) that are positive regulators of VC that may promote calcification of vascular membranes and soft tissues.
Low or normal serum Ch level and inflammation are associated with CVD and all-cause mortality in HD patients. Not only are levels of the acute inflammatory factor CRP increased, but also levels of the antiinflammatory cytokine IL-10 are decreased. Moreover, the increased incidence of hospitalization seemed to more strongly indicate upregulation of inflammatory markers.[50] The release of IL-6 and its soluble receptors by peripheral blood mononuclear cells was elevated in HD patients with low Ch levels.[51] Increased serum IL-6 and depressed sgp130 (inhibitor of IL-6 soluble receptor) are independent indicators of CVD and all-cause hospitalization. In addition, mortality was strongly influenced by malnutrition (cachexia) and Ch levels, which are good indicators of nutrition status.[52]
Furthermore, MBDs were specific risk factors for VC in patients with CKD. VC in patients with CKD is reported to be similar to osteogenesis, but it has also been reported that VC was not correlated with Ca, P, or PTH levels.[53] We observed no correlation between VC and blood Ca, P, and intact parathyroid hormone, or with indicators of bone metabolism including 25-hydroxy vitamin D, N-terminal/mid-region osteocalcin, β-CTX, and CT. Whether these factors are associated with advanced age, malnutrition, and drug intervention in the VC group requires further investigation.
This research is limited by the scope of this single center study of inpatients. A study with a larger sample size and a wider range of patients (including outpatients with less severe disease) may allow stratified multivariable logistic regression analysis of factors including age, and whether or not patients achieved ESRD or dialysis. Crucially, although we observed higher rates of coronary artery disease, hypertension, stroke, and late stage CKD in the VC group, whether VC increased IVST, hypocholesterolemia, and hyper-LDL cholesterolemia, or vice versa cannot be determined by this retrospective study. A longitudinal study may be able to further probe the causative relationship between these factors.
5. Conclusion
Taken together, our results suggest that advanced age, increased IVST, hypocholesterolemia, and hyper-LDL cholesterolemia were key risk factors for VC in patients with CKD, and were closely associated with inflammation and malnutrition. Heart VC screening should be regularly performed in elderly patients with CKD, especially those aged over 60.
Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 81200542), the Natural Science Foundation of Science and Technology Commission of Shanghai Municipal (No. 16ZR1427600), and “production, learning, and research” practice plan of Shanghai university teachers in 2017 of Shanghai Municipal Education Commission for the support.
Footnotes
Abbreviations: Alb = albumin, APO = apolipoprotein, Ca = calcium, Ch = cholesterol, CKD = chronic kidney disease, CVD = cardiovascular disease, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, IVST = interventricular septum thickness, KDIGO = Kidney Disease: Improving Global Outcomes, LDL = low-density lipoprotein, LVDd = left ventricular internal end diastole diameter, LVH = left ventricular hypertrophy, LVPWTd = left ventricular internal posterior wall thickness measured in end diastole, MBD = mineral and bone disorder, pAlb = pre-Alb, PEW = protein-energy wasting, TG = triglyceride, VC = valve calcification.
SR and XQ contributed equally to this work.
Ethic approval: Written informed consent was obtained from all patients included in the study and the ethics committee of Shanghai General Hospital approved the study.
Funding/support: This study was supported by grants from the National Natural Science Foundation of China (No. 81200542), the Natural Science Foundation of Science and Technology Commission of Shanghai Municipal (No. 16ZR1427600), and “production, learning, and research” practice plan of Shanghai university teachers in 2017 of Shanghai Municipal Education Commission.
The authors have no conflicts of interest to disclose.
References
- [1].Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;S1–30. [DOI] [PubMed] [Google Scholar]
- [2].Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. [DOI] [PubMed] [Google Scholar]
- [3].Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006;17:2275–84. [DOI] [PubMed] [Google Scholar]
- [4].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [5].Wang F, Zhang L, Wang H, et al. Awareness of CKD in China: a national cross-sectional survey. Am J Kidney Dis 2014;63:1068–70. [DOI] [PubMed] [Google Scholar]
- [6].Wang AY, Wang M, Woo J, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol 2003;14:159–68. [DOI] [PubMed] [Google Scholar]
- [7].Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol 2005;16:507–13. [DOI] [PubMed] [Google Scholar]
- [8].Ix JH, Shlipak MG, Katz R, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2007;50:412–20. [DOI] [PubMed] [Google Scholar]
- [9].Karohl C, D’Marco Gascon L, Raggi P. Noninvasive imaging for assessment of calcification in chronic kidney disease. Nat Rev Nephrol 2011;7:567–77. [DOI] [PubMed] [Google Scholar]
- [10].Chertow GM, Burke SK, Raggi P, et al. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62:245–52. [DOI] [PubMed] [Google Scholar]
- [11].Sigrist M, Bungay P, Taal MW, et al. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 2006;21:707–14. [DOI] [PubMed] [Google Scholar]
- [12].Garland JS, Holden RM, Groome PA, et al. Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis 2008;52:849–58. [DOI] [PubMed] [Google Scholar]
- [13].Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009;20:381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 2004;44:1024–30. [DOI] [PubMed] [Google Scholar]
- [15].Furukawa K, Motomura S. [Bone and calcium update; diagnosis and therapy of bone metabolism disease update. Molecular mechanism in cardiac valve calcification]. Clin Calcium 2011;21:61–6. [PubMed] [Google Scholar]
- [16].Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478–83. [DOI] [PubMed] [Google Scholar]
- [17].Li K, Yang C, Lu A, et al. Age-related changes in calcification of heart valves. Chin J Geriatr 2013;32:934–6. [Google Scholar]
- [18].Nitta K, Akiba T, Uchida K, et al. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 2004;27:47–52. [DOI] [PubMed] [Google Scholar]
- [19].Yildiz A, Memisoglu E, Oflaz H, et al. Atherosclerosis and vascular calcification are independent predictors of left ventricular hypertrophy in chronic haemodialysis patients. Nephrol Dial Transplant 2005;20:760–7. [DOI] [PubMed] [Google Scholar]
- [20].Achinger SG, Ayus JC. Left ventricular hypertrophy: is hyperphosphatemia among dialysis patients a risk factor? J Am Soc Nephrol 2006;17(12 Suppl 3):S255–61. [DOI] [PubMed] [Google Scholar]
- [21].Toussaint ND, Pedagogos E, Tan SJ, et al. Phosphate in early chronic kidney disease: associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 2012;17:433–44. [DOI] [PubMed] [Google Scholar]
- [22].National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- [23].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. [DOI] [PubMed] [Google Scholar]
- [25].Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 2013;84:622–3. [DOI] [PubMed] [Google Scholar]
- [26].Wong M, Tei C, Shah PM. Sensitivity and specificity of two-dimensional echocardiography in the detection of valvular calcification. Chest 1983;84:423–7. [DOI] [PubMed] [Google Scholar]
- [27].Schiller NB. Two-dimensional echocardiographic determination of left ventricular volume, systolic function, and mass. Summary and discussion of the 1989 recommendations of the American Society of Echocardiography. Circulation 1991;84(3 Suppl):I280–7. [PubMed] [Google Scholar]
- [28].Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- [29].Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55:613–8. [DOI] [PubMed] [Google Scholar]
- [30].Steiber AL, Kalantar-Zadeh K, Secker D, et al. Subjective Global Assessment in chronic kidney disease: a review. J Ren Nutr 2004;14:191–200. [PubMed] [Google Scholar]
- [31].de Jager DJ, Grootendorst DC, Jager KJ, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302:1782–9. [DOI] [PubMed] [Google Scholar]
- [32].Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 1990;15:458–82. [DOI] [PubMed] [Google Scholar]
- [33].Iseki K, Yamazato M, Tozawa M, et al. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int 2002;61:1887–93. [DOI] [PubMed] [Google Scholar]
- [34].Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004;291:451–9. [DOI] [PubMed] [Google Scholar]
- [35].Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol 2007;18:293–303. [DOI] [PubMed] [Google Scholar]
- [36].Fleischmann EH, Bower JD, Salahudeen AK. Risk factor paradox in hemodialysis: better nutrition as a partial explanation. ASAIO J 2001;47:74–81. [DOI] [PubMed] [Google Scholar]
- [37].Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003;63:793–808. [DOI] [PubMed] [Google Scholar]
- [38].Chmielewski M, Verduijn M, Drechsler C, et al. Low cholesterol in dialysis patients–causal factor for mortality or an effect of confounding? Nephrol Dial Transplant 2011;26:3325–31. [DOI] [PubMed] [Google Scholar]
- [39].Chmielewski M, Carrero JJ, Nordfors L, et al. Lipid disorders in chronic kidney disease: reverse epidemiology and therapeutic approach. J Nephrol 2008;21:635–44. [PubMed] [Google Scholar]
- [40].Zoccali C, Testa A, Spoto B, et al. Mendelian randomization: a new approach to studying epidemiology in ESRD. Am J Kidney Dis 2006;47:332–41. [DOI] [PubMed] [Google Scholar]
- [41].Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol 2007;18:304–11. [DOI] [PubMed] [Google Scholar]
- [42].Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005;165:55–61. [DOI] [PubMed] [Google Scholar]
- [43].Cano NJ, Pichard C, Roth H, et al. C-reactive protein and body mass index predict outcome in end-stage respiratory failure. Chest 2004;126:540–6. [DOI] [PubMed] [Google Scholar]
- [44].Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 2005;165:1624–9. [DOI] [PubMed] [Google Scholar]
- [45].Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 2007;49:581–91. [DOI] [PubMed] [Google Scholar]
- [46].London GM, Marchais SJ, Guerin AP, et al. Inflammation, arteriosclerosis, and cardiovascular therapy in hemodialysis patients. Kidney Int Suppl 2003;S88–93. [DOI] [PubMed] [Google Scholar]
- [47].Liu J, Ma KL, Gao M, et al. Inflammation disrupts the LDL receptor pathway and accelerates the progression of vascular calcification in ESRD patients. PLoS One 2012;7:e47217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Krasniak A, Drozdz M, Pasowicz M, et al. Factors involved in vascular calcification and atherosclerosis in maintenance haemodialysis patients. Nephrol Dial Transplant 2007;22:515–21. [DOI] [PubMed] [Google Scholar]
- [49].Zhao G, Xu MJ, Zhao MM, et al. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int 2012;82:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tsirpanlis G, Boufidou F, Zoga M, et al. Low cholesterol along with inflammation predicts morbidity and mortality in hemodialysis patients. Hemodial Int 2009;13:197–204. [DOI] [PubMed] [Google Scholar]
- [51].Tsirpanlis G, Chatzipanagiotou S, Boufidou F, et al. Release of interleukin-6 and its soluble receptors by activated peripheral blood monocytes is elevated in hypocholesterolemic hemodialysis patients. Am J Nephrol 2005;25:484–90. [DOI] [PubMed] [Google Scholar]
- [52].Stenvinkel P, Heimburger O, Lindholm B. Wasting, but not malnutrition, predicts cardiovascular mortality in end-stage renal disease. Nephrol Dial Transplant 2004;19:2181–3. [DOI] [PubMed] [Google Scholar]
- [53].Fernandez-Reyes MJ, Auxiliadora Bajo M, Robles P, et al. Mitral annular calcification in CAPD patients with a low degree of hyperparathyroidism. An analysis of other possible risk factors. Nephrol Dial Transplant 1995;10:2090–5. [PubMed] [Google Scholar]


