Abstract
Modifiable lifestyle factors play an important role regarding the development and outcomes in solid tumors. Whereas smoking has been attributed to bladder cancer and cessation leads to better outcome, we show that exercise may provide similar benefits regarding bladder cancer mortality
Background
The aim of this study was to investigate modifiable lifestyle factors of smoking, exercise, and obesity with bladder cancer mortality.
Patients and Methods
We used mortality-linked data from the National Health Information Survey from 1998 through 2006. The primary outcome was bladder cancer-specific mortality. The primary exposures were self-reported smoking status (never- vs. former vs. current smoker), self-reported exercise (dichotomized as “did no exercise” vs. “light, moderate, or vigorous exercise in ≥ 10-minute bouts”), and body mass index. We utilized multivariable adjusted Cox proportional hazards regression models, with delayed entry to account for age at survey interview.
Results
Complete data were available on 222,163 participants, of whom 96,715 (44%) were men and 146,014 (66%) were non-Hispanic whites, and among whom we identified 83 bladder cancer-specific deaths. In multivariate analyses, individuals who reported any exercise were 47% less likely (adjusted hazard ratio [HRadj], 0.53; 95% confidence interval [CI], 0.29–0.96; P = .038) to die of bladder cancer than “no exercise”. Compared with never-smokers, current (HRadj, 4.24; 95% CI, 1.89–9.65; P = .001) and former (HRadj, 2.95; 95% CI, 1.50–5.79; P = .002) smokers were 4 and 3 times more likely, respectively, to die of bladder cancer. There were no significant associations of body mass index with bladder cancer mortality.
Conclusion
Exercise decreases and current smoking increases the risk of bladder cancer-specific mortality. These data suggest that exercise and smoking cessation interventions may reduce bladder cancer death.
Keywords: Bladder cancer, Exercise, Modifiable risk factor, Mortality, Smoking
Introduction
Bladder cancer is the seventh most common cancer in the world and accounts for approximately 50,000 deaths a year in the United States (US) and European Union.1 The US population prevalence is estimated to be at least 600,000 individuals and continues to grow annually.2,3 Bladder cancer is the fourth most frequently diagnosed cancer among men and—owing to the high costs of diagnosis, treatment, and posttreatment surveillance—the single most expensive cancer to treat.4 These observations underscore the considerable challenges that bladder cancer poses to the public health and highlight an important need to develop innovative, novel therapies.
A potential method of decreasing the morbidity and mortality of bladder cancer is through behavior change interventions focused on modifiable risk factors. Modulation of lifestyle factors—for example, exercise, weight loss, and diet change—may exert beneficial, disease-specific health effects.5–8 Smoking is strongly associated with increased bladder cancer incidence, and smoking cessation is a potential target for improving outcomes in patients with bladder cancer.9–11 However, other modifiable risk factors, such as obesity12–15 and exercise,16–19 also may play important roles in bladder cancer outcomes. Therefore, we investigated associations of obesity, physical activity, and smoking with bladder cancer mortality in the National Health Information Survey (NHIS).
Patients and Methods
NHIS
The NHIS is the primary source of information on the United States population conducted by the National Center for Health Statistics as part of the Centers for Disease Control and Prevention. The annual survey provides information regarding illness and disability since the National Health Survey Act of 1956. The NHIS is an annual representative cross-sectional household interview survey of the civilian noninstitutionalized population performed in the US (http://www.cdc.gov/nchs/nhis/about_nhis.htm). The surveys are collected through personal household interviews conveyed by the US Census Bureau. On average, 42,000 households including 100,000 people are interviewed per year. We used baseline data from 1998 through 2004 linked to mortality data reporting deaths or survival through 2006. Individuals who could not be matched to mortality data were not included in the analysis. The data was collected through stratified sampling schemes that oversampled for African American and Hispanic people. Survey sample weights (provided by NHIS) were used to provide population-level estimates. The NHIS-linked Mortality Files include the NHIS years 1986 to 2004 with mortality follow-up data from the date of survey participation through December 31, 2006. Mortality status is ascertained primarily through probabilistic record matching with the National Death Index (NDI). The National Center for Health Statistics employed a matching methodology for the NHIS Linked Mortality Files that is similar, but not identical, to the standard methodology offered by the National Death Index.20
Outcomes and Exposures
The primary outcome was bladder cancer-specific mortality. The primary exposures were self-reported smoking status, self-reported physical activity, and obesity as measured by body mass index (BMI). BMI was categorized into underweight (BMI greater than 15 and less than 18.5), normal weight (18.5 to < 25, used as the reference category), overweight (25 to < 30), and obese (30+). Individuals who were severely underweight (BMI < 15) were excluded from the analysis. We excluded the very low BMI because we wanted to identify patients in which weight (BMI) could be a modifiable risk factor that exercise could impact. We also assumed that underweight people may have had other issues impacting the mortality of bladder cancer. We investigated physical actively in context of the Physical Activity Guidelines for Americans developed in 2008 as an objective for Healthy People 2020, which is a US Department of Health and Human Services program to support prevention of disease and a healthier nation. The physical activity requirements consist of 150 minutes/week of moderate-intensity aerobic exercise, 75 minutes/week of vigorous-intensity aerobic exercise, or an equivalent combination of the 2 goals. We examined the amount of recommended exercise compared with the future risk of bladder cancer mortality. However, in multivariable analysis, owing to low overall bladder cancer incidence in the general population, physical activity was dichotomized into any exercise versus no exercise. Smoking status was defined as current (had smoked 100+ cigarettes and now smoke every day or some days) or former smokers (now smoke not at all) or never-smokers (reference category, had not smoked at least 100 cigarettes in their lifetime).
Statistical Analysis
We computed frequencies of each demographic category (ie, age, ethnicity, gender), other (ie, nonkidney) cancer diagnosis, and lifestyle variable (physical activity, smoking, obesity). We utilized the χ2 test to compare recommended exercise with no exercise. We then used multivariable adjusted Cox proportional hazards regression models, with delayed entry to account for age at survey interview. Models predicted death because of bladder cancer and were adjusted for age at survey, race/ethnicity (non-Hispanic white vs. other), gender, history of bladder cancer, and history of any cancer diagnosis at time of survey. Analyses were weighted to provide population-level estimates. All analyses were conducted using SAS version 9.2.
Results
Complete data were available on 222,163 participants, of whom 96,715 (44%) were men and 146,014 (66%) non-Hispanic whites, and among whom we identified 83 bladder cancer-specific deaths (Table 1). Bladder cancer mortality was noted lowest in those patients who obtained the recommended amount of exercise from the Healthy People 2020 Physical Activity Guidelines as compared with no exercise (0.05%; n = 56 of 116,390 vs. 0.01%; n = 4 of 30,945; P = .006). Figure 1 displays a bar graph for mortality rates of subjects in relation to whether they met the Healthy People 2020 Physical Activity Guidelines. “None” corresponds to no physical activity reported. Comparisons made included none versus below standard (P = .11), none versus meets standard (P = .006), and none versus exceeds standard (P = .013). The variables for multivariable analysis were chosen prior to analysis, and none were eliminated. The model was adjusted for age at survey and age at either death or 2006, gender, race/ethnicity, BMI (in categories), exercise (any exercise vs. none), whether they had been diagnosed with bladder cancer at survey, whether they had been diagnosed with any other cancer at survey, and whether they were current smokers at survey. Individuals who reported any exercise were 47% less likely (adjusted hazard ratio [HRadj], 0.53; 95% confidence interval [CI], 0.29–0.96; P = .038) to die of bladder cancer than those who reported “no exercise” (Figure 2). Compared with never-smokers, current (HRadj, 4.24; 95% CI, 1.89–9.65; P = .001) and former (HRadj, 2.95; 95% CI, 1.50–5.79; P = .002) smokers were 4 and 3 times more likely, respectively, to die of bladder cancer. There were no significant associations of BMI with bladder cancer mortality.
Table 1.
Demographic Distribution and Incidence of Bladder Cancer Deaths Among Sample Population (NHIS 1998–2004)
| Demographic | Total | Died of Bladder Cancer | ||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 222,163 | 83 | 0.0344 | |
| Age, y | ||||
| <60 | 167,262 | 78.4 | 7 | 0.0044 |
| 60–74 | 34,514 | 14.2 | 28 | 0.0944 |
| 75+ | 20,387 | 7.4 | 48 | 0.2367 |
| Race/ethnicity | ||||
| Non-Hispanic white | 146,014 | 73.2 | 76 | 0.0432 |
| Hispanic | 37,439 | 11.1 | 2 | 0.0067 |
| African American | 30,197 | 11.2 | 2 | 0.0029 |
| Asian/Pacific Island | 5492 | 3.0 | 1 | 0.0329 |
| Other | 3021 | 1.5 | 2 | 0.0473 |
| Male gender | 96,715 | 48.0 | 47 | 0.0466 |
| Body mass index | ||||
| Underweight | 4229 | 2.0 | 5 | 0.0934 |
| Normal weight | 85,023 | 40.3 | 39 | 0.0426 |
| Overweight | 74,752 | 35.4 | 24 | 0.0263 |
| Obese | 48,502 | 22.3 | 14 | 0.0306 |
| Exercise | ||||
| Any | 101,657 | 49.2 | 20 | 0.016 |
| None | 116,390 | 50.0 | 56 | 0.048 |
| Below recommended | 21,530 | 10.5 | 5 | 0.023 |
| Recommended | 30,935 | 14.5 | 4 | 0.013 |
| Two-fold higher than recommended | 49,192 | 23.3 | 11 | 0.022 |
| Cigarette smoker | ||||
| Current | 50,062 | 22.4 | 18 | 0.032 |
| Former | 48,361 | 22.1 | 46 | 0.0965 |
Abbreviation: NHIS = National Health Interview Survey.
Figure 1.
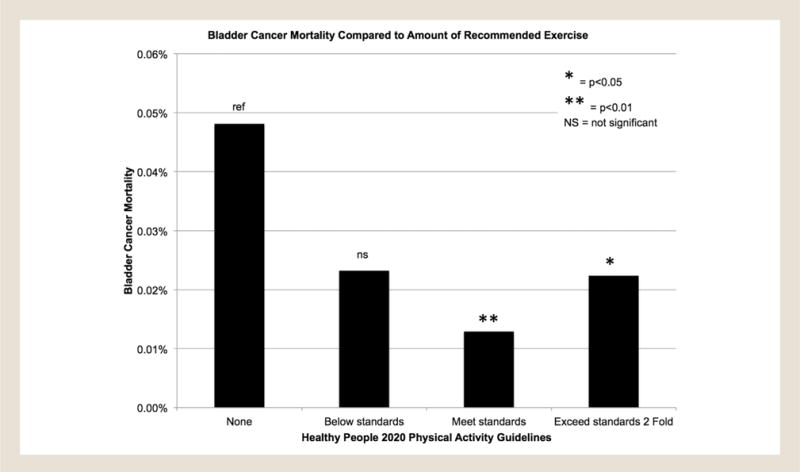
Bladder Cancer Mortality Compared With Amount of Recommended Exercise. A Bar Graph Displays the Mortality Rate of Subjects in Relation to Whether They Met the Healthy People 2020 Physical Activity Guidelines. “None” Corresponds to No Activity. Comparisons Made Included None Versus Below Standard (P = .11), None Versus Meets Standard (P = .006), and None Versus Exceeds Standard (P = .013)
Figure 2.
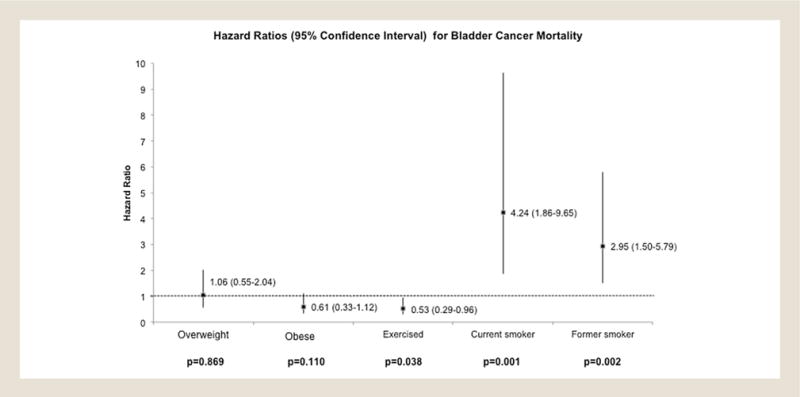
Multivariable Analysis of Bladder Cancer Mortality and Modifiable Risk Factors. Box and Whisker Plot Displaying the Hazard Ratios and 95% Confidence Intervals of Body Mass Index, Exercise, and Smoking in Association With Bladder Cancer Mortality
Discussion
In this study, we observed a 47% decreased risk of bladder cancer death in individuals who reported any form of exercise compared with those who did not exercise. Current and former smokers were 4 and 3 times, respectively, more likely to die of bladder cancer than never-smokers. Therefore, a prospective clinical trial encouraging smoking cessation and physical exercise could provide evidence that behavioral modification may impact bladder cancer outcomes.
Bladder cancer was one of the first cancers to be associated with environmental exposures.21 Currently, tobacco smoking is the most firmly established modifiable risk factor for bladder cancer.9,22 Our research adds to the evidence associating the importance of abstaining from smoking to potentially reduce mortality of bladder cancer. The data are obtained from a survey given to people in the community; therefore, we are unable to draw relationships to dose-response and causal relationships between smoking and bladder cancer mortality. We also noted an additional survival benefit by obtaining any amount of physical activity.
Exercise has been shown to reduce cancer incidence and mortality in more than 25 different cancer types, including bladder cancer.23,24 Additionally, our group has published a systematic review on the impact of obesity and exercise specifically on bladder cancer outcomes, noting a beneficial effect of exercise.8 We also identified only 1 article that focused on bladder cancer mortality, setting up the premise for investigating the NHIS dataset specifically for bladder cancer mortality. Unfortunately, physical activity has been defined in various ways, including but not limited to amount of exercise, exercise vigor, work-related, household chore-related, or calculated by metabolic equivalents (MET). We chose to use the US Department of Health and Human Services Healthy People 2020 guidelines to define if recommended exercise is able to improve bladder cancer mortality. The results may then be used to validate health policy.
Previous investigations of population data regarding physical activity have usually included results for obesity, and both have had mixed associations.25–27 Additionally, there is difficulty regarding interpretation between studies as the outcome variable definitions can be different. For obesity, one can use weight, BMI, or body proportion measurements. Herein, we used BMI to determine “obesity” and did not find an association with bladder cancer mortality that differs from previous reports.13 Owing to the questionnaire being given at one point in time, we are unable to follow an individual BMI over time. Additionally, we could not control for confounding by disease stage and performance status.
Limitations of the study include the inherent limitations of survey data and the variation in question format over different year versions of the survey. Personal in-home surveyors conducted the survey at the time of the US Census, which provides complete data and accurate collection and limits selection bias. Our study also lacks the specific grade and stage of bladder cancer; however, we focused our primary outcome on mortality data. Bladder cancer prevalence, stage, and other outcomes are beyond the scope of this article. We are unable to investigate specific comorbidities that may have a confounding effect on obesity and exercise. Only cause of death data was available for linkage, which is subject to documentation error or multiple causes. The cause of death specifically from bladder cancer may have less contributing comorbidities and likely strengthens the associations we identified. Moreover, owing to small numbers, there may be interactions between smoking, obesity, and exercise that we are unable to identify or analyze separately.
In conclusion, the amount of physical activity may be associated with a lower risk of bladder cancer than a sedentary lifestyle. In addition to avoiding or cessation of smoking along with exercise, interventions may potentially prolong the life of patients diagnosed with bladder cancer.
Clinical Practice Points.
Several studies have shown obesity to be an adverse feature for bladder cancer outcomes; however, only one study has used bladder cancer mortality as an outcome and is over 20 years old.
We have identified that physical activity is an independent modifiable risk factor that may reduce bladder cancer mortality.
Providers caring for bladder cancer patients not only should encourage smoking cessation, but also engage in a discussion regarding physical activity.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 5.Stein CJ, Colditz GA. Modifiable risk factors for cancer. Br J Cancer. 2004;90:299–303. doi: 10.1038/sj.bjc.6601509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons JK, Pierce JP, Mohler J, et al. A randomized trial of diet in men with early stage prostate cancer on active surveillance: rationale and design of the Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) Contemp Clin Trials. 2014;38:198–203. doi: 10.1016/j.cct.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons JK, Pierce JP, Natarajan L, et al. A randomized pilot trial of dietary modification for the chemoprevention of noninvasive bladder cancer: the dietary intervention in bladder cancer study. Cancer Prev Res (Phila) 2013;6:971–8. doi: 10.1158/1940-6207.CAPR-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noguchi JL, Liss MA, Parsons JK. Obesity, physical activity and bladder cancer. Curr Urol Rep. 2015;16:74. doi: 10.1007/s11934-015-0546-2. [DOI] [PubMed] [Google Scholar]
- 9.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–45. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crivelli JJ, Xylinas E, Kluth LA, Rieken M, Rink M, Shariat SF. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol. 2014;65:742–54. doi: 10.1016/j.eururo.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90:228–39. doi: 10.1046/j.1464-410x.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 12.Wyszynski A, Tanyos SA, Rees JR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120:408–14. doi: 10.1002/cncr.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chromecki TF, Cha EK, Fajkovic H, et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. 2013;111:249–55. doi: 10.1111/j.1464-410X.2012.11322.x. [DOI] [PubMed] [Google Scholar]
- 14.Kluth LA, Xylinas E, Crivelli JJ, et al. Obesity is associated with worse outcomes in patients with T1 high grade urothelial carcinoma of the bladder. J Urol. 2013;190:480–6. doi: 10.1016/j.juro.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 15.Qin Q, Xu X, Wang X, Zheng XY. Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:3117–21. doi: 10.7314/apjcp.2013.14.5.3117. [DOI] [PubMed] [Google Scholar]
- 16.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 17.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 18.Laake I, Thune I, Selmer R, Tretli S, Slattery ML, Veierod MB. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev. 2010;19:1511–22. doi: 10.1158/1055-9965.EPI-09-0813. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–95. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. The National Health Interview Survey (1986–2004) Linked Mortality Files, Mortality Follow-up Through 2006: Matching Methodology. Hyattsville, MD: Office of Analysis and Epidemiology; 2009. [Google Scholar]
- 21.Case RA, Hosker ME, Mc DD, Pearson JT. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. I. The role of aniline, benzidine, alphanaphthylamine, and beta-naphthylamine. Br J Ind Med. 1993;50:389–411. [PMC free article] [PubMed] [Google Scholar]
- 22.Rink M, Zabor EC, Furberg H, et al. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol. 2013;64:456–64. doi: 10.1016/j.eururo.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Int Med. 2016;176:816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Int Med. 2015;175:959–67. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paffenbarger RS, Jr, Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: a preliminary report. Am J Clin Nutr. 1987;45(1 Suppl):312–7. doi: 10.1093/ajcn/45.1.312. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi A, Folsom AR, Anderson KE, Iowa Women’s Health Study Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women’s Health Study. Cancer. 2002;95:2316–23. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 27.Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140–6. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]


