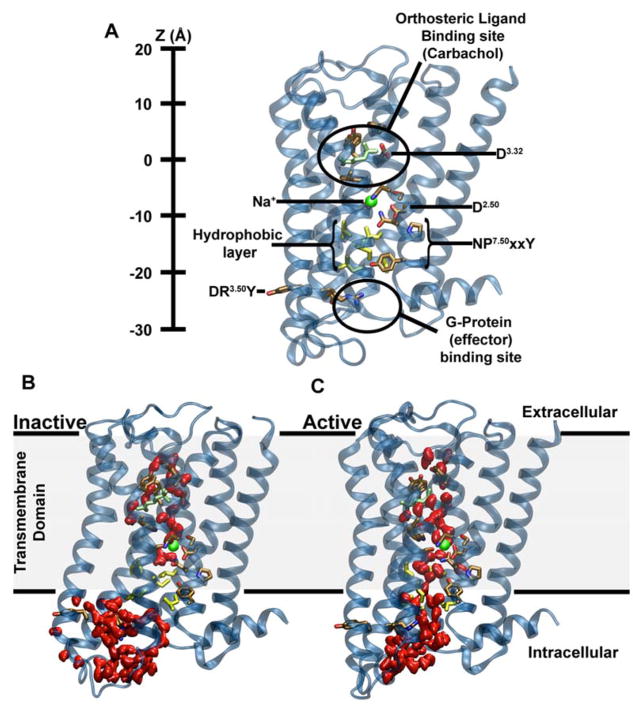
Figure 1. Major structural features and internal hydration of class A GPCRs in the inactive and active state as shown by the m2r.
(A) The main structural features of class A GPCRs, as exemplified by m2r, include 7 TM helices (blue), an extracellular ligand binding site, the intracellular effector (G-protein) binding site as well as conserved and functionally important residues termed microswitches (selected ones are highlighted). The vertical axis (Z-coordinate) and all positions stated in the text use the Cα atom of D1033.32 as a reference. (B) Conformation of inactive m2r (PDB: 3UON) during the simulations showing the presence of the hydrophobic layer separating the hydrophilic pocket and effector binding site. (C) After transition to the active state (PDB: 4MQT), and further simulation, m2r displays a continuous water channel connecting the orthosteric ligand binding site, hydrophilic pocket and effector binding site. Note that in (B) and (C) the upward conformation of the Y4407.53 is shown in order to highlight the changes in hydration levels only; please see Fig S3 for a detailed comparison of the upward and downward tyrosine conformations. Water molecules are shown in red (surface representation); the position of the allosteric Na+ ion, as obtained from our initial simulations, is shown as a green sphere, and residues forming the hydrophobic layer (yellow) as well as the bound ligand (carbachol, light green) are depicted in stick representation.