Abstract
Introduction
Antiretroviral treatment (ART) guidelines have changed over the past decade, recommending earlier initiation and more tolerable regimens. The study objective was to examine the CD4 response to ART, depending on the year of ART initiation, in HIV-positive patients in the Asia-Pacific.
Methods
We included HIV-positive adult patients who initiated ART between 2003–2013 in our regional cohort from eight urban referral centres in seven countries within Asia. We used mixed-effects linear regression models to evaluate differences in CD4 response by year of ART initiation during 36 months of follow-up, adjusted a priori for other covariates.
Results
Overall, 16962 patients were included. Patients initiating in 2006–09 and 2010–13 had an estimated mean CD4 count increase of 8cells/μL and 15cells/μL, respectively, at any given time during the 36 month follow-up, compared to those in 2003–05. The median CD4 count at ART initiation also increased from 96 cells/μL in 2003–05 to 173 cells/μL in 2010–13.
Conclusions
Our results suggest that the CD4 response to ART is modestly higher for those initiating ART in more recent years. Moreover, fewer patients are presenting with lower absolute CD4 counts over time. This is likely to reduce their risk of opportunistic infections and future non-AIDS defining cancers.
Keywords: Asia, HIV, epidemiology, CD4 trends, immunological response, ART
Introduction
CD4 counts are used as prognostic markers of HIV disease progression [1, 2]. Untreated HIV-infected persons have a gradual depletion in CD4 cell levels, leading to increased risk of AIDS-defining illnesses and death [3–6]. Combination antiretroviral therapy (ART) has been highly effective in preventing HIV disease progression and restoring CD4 cell levels as well as reducing viral replication and lowering rates of HIV-associated morbidity and mortality [7–9].
Initial World Health Organization (WHO) treatment guidelines, released in 2002, recommended ART initiation for those in advanced stages of HIV or in asymptomatic stages with CD4 count <200 cells/μL [10]. Delayed ART initiation was earlier suggested in stable patients to reduce the risk of developing and transmitting drug resistant HIV caused by suboptimal adherence [11, 12]. However, recent research has shown strong evidence to support earlier ART initiation at higher CD4 cell levels is beneficial in preventing disease progression and transmission, and also prevents the incidence of opportunistic infections (OIs) [8, 13–15]. After subsequent guideline revisions steadily increased the CD4 threshold for ART, in 2015, WHO treatment guidelines recommended ART initiation among all adults, regardless of CD4 count [16].
Others changes to the WHO treatment guidelines have also occurred over time including the use of more tolerable and convenient ART regimens, increased support and counselling services for patients, and routine monitoring of CD4 count and HIV viral load [16, 17]. These changes have been accompanied by improvements in patient outcomes, with reduced mortality rates for patients receiving care in recent years [18–20]. Although part of these improvements has been attributed to earlier ART initiation at higher CD4 counts, year of ART initiation has also shown an independent association with improved overall survival [21, 22].
A greater CD4 count response has previously been associated with younger age, female sex, lower pre-ART HIV viral load and CD4 count [23–25]. Yet, there has been little exploration as to whether CD4 count response has improved in recent years of ART initiation [23, 26]. The changes to treatment guidelines and patient management over time could result in an improved CD4 count response for patients initiating ART in recent years. Specifically, the move towards newer ARV drugs, associated with fewer side effects, for patients receiving care in Asia could lead to greater patient adherence [21]. In addition, certain ARV drugs classes, such as protease inhibitor-based regimens, may also evoke an increased CD4 count response [26–28]. Our study objective was to examine the time trends in and factors associated with CD4 response to first-line ART, by calendar year of ART initiation, in HIV-positive patients receiving care in an Asian regional observational cohort study.
Methods
Data collection and Participants
The TREAT Asia HIV Observational Database Low Intensity Transfer (TAHOD-LITE) cohort is a sub-study of the TREAT Asia HIV Observation Database (TAHOD) and currently consists of eight sites from the Asia-Pacific region including Cambodia, Hong Kong, India, Indonesia, Singapore, South Korea and Vietnam. TAHOD collects detailed patient data on a subset of patients seen at 20 treatment sites in the Asia-Pacific region [29]. Conversely, TAHOD-LITE collects routine clinical data on all patients seen at the 8 participating treatment sites. Thus, TAHOD-LITE is representative of the entire clinical population within our participating sites. Data are collected routinely when patients attend care at the treatment sites and include patient demographics, hepatitis serology, HIV-related laboratory test results and ART history. A more detailed description of TAHOD-LITE has been described elsewhere [21]. After being anonymized, data are transferred electronically to the Kirby Institute, University of New South Wales and are subjected to quality control procedures. Data include patient follow-up until May 2014. TAHOD-LITE was granted ethical approvals from Institutional Review Boards (IRB) at each participating clinical site, the University of New South Wales and the coordinating center at TREAT Asia/amfAR. Written consent was not obtained unless required by the site-specific IRBs.
The data selected for this analysis included all patients who were aged over 18 years when they initiated an ART regimen, consisting of three or more drugs, between 01 January 2003 and 31 December 2013, and had at least one subsequent visit after the date of ART initiation. There were also site-based exclusions where patients were excluded if they had initiated ART prior to: 2006 for Singapore; 2010 for Vietnam; and 2004 for Cambodia. Prior to these years, sites were unable to provide data on all patients that had been seen at the clinic.
Statistical analyses
The primary study objective was to evaluate CD4 count changes and factors associated with CD4 count change over 36 months of ART. A pseudo intention-to-treat approach was taken whereby any changes to treatment after ART initiation, including treatment interruptions were ignored. All patients were censored at the last clinic visit or date of death or 36 months from ART initiation, whichever occurred earlier. Pre-ART laboratory measurements, including CD4 count and HIV viral load, were defined as those within 6 months prior, and closest to or on the date of ART initiation.
Data were modelled using repeated-measures, random-intercept linear regression using generalized least squares estimation to evaluate differences in the CD4 response between the year periods of ART initiation (2003–05, 2006–09, 2010–13). Covariates, selected a priori, included clinical site, age at ART initiation, sex, mode of HIV exposure, pre-ART HIV viral load (copies/mL), pre-ART CD4 count (cells/μL), first ART regimen, hepatitis B and hepatitis C co-infection, time from ART initiation and squared time from ART initiation. These covariates were selected based on previous literature and available patient data collected in TAHOD-LITE. Continuous variables, including age at ART initiation, pre-ART HIV viral load and pre-ART CD4 count, were categorized in the model. First ART regimen was categorized based on the drug classes included. The squared time from ART initiation was included to allow for the predicted CD4 count to be modelled as a quadratic curve from ART initiation. We also evaluated whether there was an interaction between the year period of ART initiation and time from ART initiation in a sensitivity analysis. This model was selected for the analysis as it includes all CD4 count measurements during the 36 months of follow-up and determines whether certain factors influence the CD4 response over the entire follow-up time rather than at one time point (eg. 12 months from ART initiation). As we modelled the CD4 count change from ART initiation, patients without a CD4 count result within 6 months prior to ART initiation (i.e. without a pre-ART CD4 count) were excluded from the model.
CD4 count response was also summarized by the median CD4 count, with interquartile range (IQR), and the proportion of patients within each CD4 count category (≤50, 51–100, 101–200, 201–350, 351–500 and ≥501 cells/μL) every 6 months up to 36 months from ART initiation, by year of ART initiation, overall and for each country. For these crude summaries, we only included CD4 count measurements that were closest to and within ±3 months of the given time point.
Data were analysed using Stata version 12 (Stata Corporation, College Station, Texas, USA) and SAS (version 9.4 for Windows).
Results
A total of 18 441 patients aged over 18 years had initiated ART between 1 January 2003 and 31 December 2013. Of these, 777 patients were excluded for not attending the clinic after ART initiation and 702 patients were excluded due to site-based exclusions (see Methods; Singapore, n=70; Vietnam, n=568; Cambodia, n=64). The remaining 16 962 were included in the analysis.
Patient Characteristics
A summary of the patient characteristics across all countries by year of ART initiation is given in Table 1. Briefly, the majority of patients were male (2003–05: 75%; 2006–09: 69%; 2010–13: 66%), reported heterosexual mode of HIV exposure (2003–05: 88%; 2006–09: 85%; 2010–13: 73%), initiated in recent years (2003–05: 17%; 2006–09: 37%; 2010–13: 46%) and had a first ART regimen consisting of nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI) (2003–05: 92%; 2006–09: 97%; 2010–13: 98%). The median age at ART initiation was relatively consistent between periods of ART initiation (2003–05: 35 years, IQR: 30–40; 2006–09: 36 years, IQR: 31–42; 2010–13: 36 years, IQR: 30–43). Over 80% of the patients had a pre-ART CD4 count measurement, regardless of year of ART initiation. A minority of patients had a pre-ART HIV viral load measurement (2003–05: 14%; 2006–09: 15%; 2010–13: 29%) and the median pre-ART HIV viral load increased from 106 000 copies/mL (IQR: 32 000–261 000 copies/mL) in 2003–05 to 110 564 (IQR: 30 563–402 000 copies/mL) in 2010–13.
Table 1.
Summary of the patient characteristics across all countries.
| 2003–05 | 2006–09 | 2010–13 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
|
| |||
| Total | 2874 | 6248 | 7840 |
| Age | |||
| ≤30 | 736 (25) | 1505 (24) | 2087 (26) |
| 31–40 | 1440 (50) | 2865 (46) | 3343 (43) |
| 41–50 | 508 (18) | 1272 (20) | 1555 (20) |
| 51+ | 190 (7) | 606 (10) | 855 (11) |
| Median [IQR] | 35 [30, 40] | 36 [31, 42] | 36 [30, 43] |
| Sex | |||
| Male | 2149 (75) | 4312 (69) | 5176 (66) |
| Female | 721 (25) | 1931 (31) | 2657 (34) |
| Transgender | 4 (<0.2) | 5 (<0.1) | 7 (<0.1) |
| Mode of HIV exposure | |||
| Heterosexual | 2529 (88) | 5272 (85) | 5748 (73) |
| Homosexual | 111 (4) | 405 (6) | 784 (10) |
| Injecting drug user | 84 (3) | 125 (2) | 571 (7) |
| Other/Unknown | 150 (5) | 446 (7) | 737 (10) |
| HCV (ever) | |||
| Negative | 754 (26) | 2609 (42) | 4093 (52) |
| Positive | 80 (3) | 171 (3) | 776 (10) |
| Not tested | 2040 (71) | 3468 (55) | 2971 (38) |
| HBV (ever) | |||
| Negative | 939 (33) | 2869 (46) | 4702 (60) |
| Positive | 108 (4) | 305 (5) | 473 (6) |
| Not tested | 1827 (63) | 3074 (49) | 2665 (34) |
| Pre-ART CD4 (cells/μL) | |||
| ≤50 | 708 (25) | 1295 (21) | 1620 (21) |
| 51–100 | 543 (19) | 915 (15) | 833 (10) |
| 101–200 | 793 (27) | 1748 (28) | 1307 (17) |
| >200 | 377 (13) | 1325 (21) | 2990 (38) |
| Not tested | 453 (16) | 967 (15) | 1094 (14) |
| Median [IQR] | 96 [45, 171] | 128 [52, 201] | 172 [53, 286] |
| Pre-ART viral load (copies/mL) | |||
| ≤105 | 191 (7) | 458 (7) | 1073 (14) |
| >105 | 204 (7) | 518 (8) | 1154 (15) |
| Not tested | 2479 (86) | 5272 (85) | 5613 (71) |
| Median [IQR] | 106 000 [32 000, 261 000] | 114 000 [29 785, 351 500] | 110 564 [30 563, 402 000] |
| First ART regimen | |||
| NRTI+NNRTI | 2719 (95) | 5930 (95) | 7381 (95) |
| NRTI+PI | 148 (5) | 290 (5) | 381 (5) |
| Other | 7 (<0.3) | 28 (<0.5) | 78 (1) |
| Previous mono/dual therapy | |||
| No | 2632 (92) | 6053 (97) | 7680 (98) |
| Yes | 242 (8) | 195 (3) | 160 (2) |
Summary of the CD4 count response
Of the 14 448 patients with pre-ART CD4 count measurements, ART was initiated in 2003–05 for 2 421 patients, in 2006–09 for 5 281 patients and in 2010–13 for 6 746 patients. The median follow-up time for patients initiating in 2003–05 was 2.6 years, in 2006–09 was 2.5 years and in 2010–13 was 1.6 years.
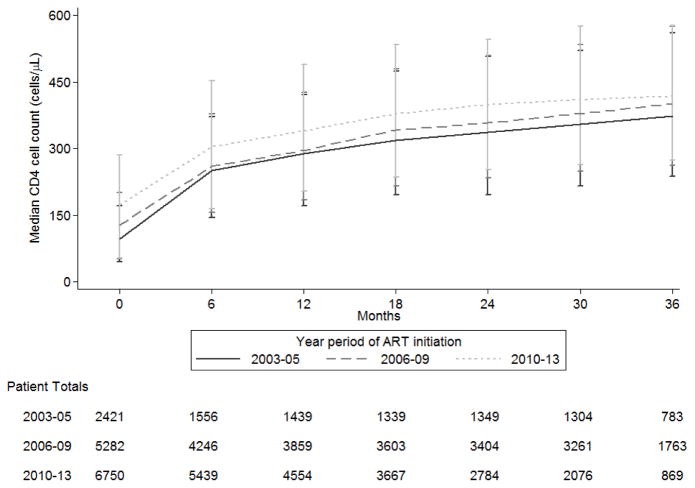
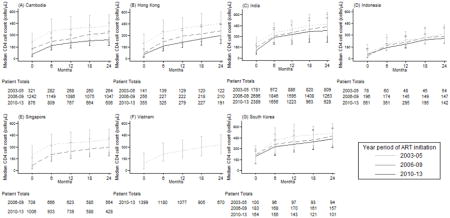
The median CD4 count increases from ART initiation were: in 2003–05, from 96 cells/μL (IQR: 45–171 cells/μL) at ART initiation to 374 cells/μL (IQR: 237–561 cells/μL) at 36 months; in 2006–09, from 128 cells/μL (IQR: 52–201 cells/μL) at ART initiation to 401 cells/μL (IQR: 263–575 cells/μL) at 36 months; and in 2010–13, from 173 cells/μL (IQR: 53–286 cells/μL) at ART initiation to 418 cells/μL (IQR: 274–577 cells/μL) at 36 months (Figure 1). Overall, there was an increasing trend where those initiating in 2010–13 had a higher median CD4 count at ART initiation follow-up compared to prior year periods. However, this was not found be significant in the Kruskal-Wallis median test (p value = 0.368). Similar trends were observed when examined by country (Appendix 1).
Figure 1.
The median CD4 count (cells/μL) and patient totals over the time (months) since ART initiation, by the year period of ART initiation.
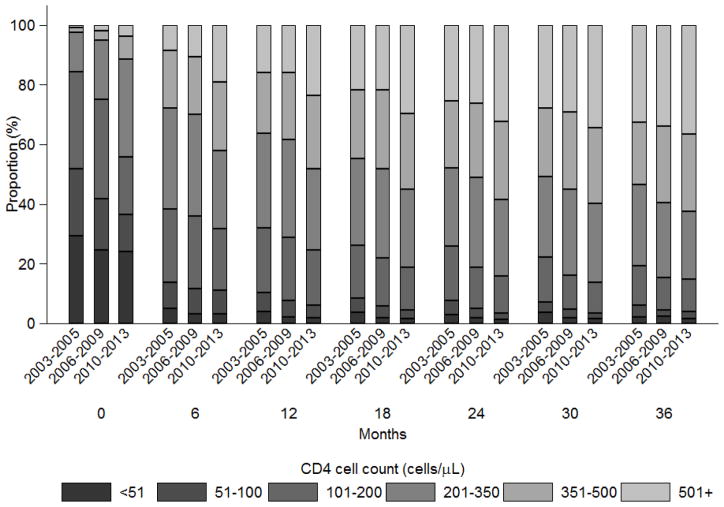
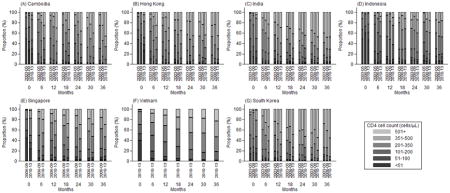
The proportion of patients within each CD4 count category (≤50, 51–100, 101–200, 201–350, 351–500 and ≥501 cells/μL) up to 36 months from ART initiation is summarized in Figure 2. Largely as a result of ART initiation at higher CD4 counts, there was an increasing trend where those initiating in 2010–13 had a greater proportion of patients at higher CD4 counts than in previous year periods. The proportion of patients with CD4 count ≥201 cells/μL increased for patients initiating: in 2003–05, from 16% at ART initiation to 81% at 36 months; in 2006–09, from 25% at ART initiation to 85% at 36 months; and in 2010–13, from 44% at ART initiation to 85% at 36 months. This trend was also apparent by country (Appendix 2).
Figure 2.
The proportion of patients in each CD4 count (cells/μL) category over time since ART initiation, by year period of ART initiation.
Modelling the CD4 count response up to 36 months
The model indicated that several factors were significantly associated with the CD4 count response over time (Table 2). In the univariate analysis, year period of ART initiation was significantly (p value <0.001) associated with the CD4 cell response. Those initiating in 2006–09 and 2010–13 had a mean CD4 count that at any given time during follow-up, was 8 cells/μL (95% CI: 3 to 13 cells/μL) and 13 cells/μL (95% CI: 8 to 19 cells/μL) higher than those initiating in 2003–05. In the multivariate model, the year period of ART initiation remained significant while adjusting for clinical site and other relevant covariates (p value <0.001). Here, the mean CD4 cell count was higher at any given time during follow-up, for those initiating in 2006–09 and 2010–13 by 8 cells/μL (95% CI: 3 to 13 cells/μL) and 15 cells/μL (95% CI: 9 to 20 cells/μL), respectively, compared to those initiating in 2003–05.
Table 2.
Estimated mean CD4 count (cells/μL) change up to 36 months from ART initiation.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Diff. | 95% CI | p value | Mean Diff. | 95% CI | p value | |
| Year of ART Initiation | <0.001 | <0.001 | ||||
| 2003–2005 | ref | ref | ||||
| 2006–2009 | 8 | (3, 13) | 0.003 | 8 | (3, 13) | 0.002 |
| 2010–2013 | 13 | (8, 19) | <0.001 | 15 | (9, 20) | <0.001 |
|
| ||||||
| Time from ART initiation (per month) | 16 | (15, 16) | <0.001 | 16 | (16, 17) | <0.001 |
|
| ||||||
| Age at ART initiation (years) | <0.001 | <0.001 | ||||
| ≤30 | ref | ref | ||||
| 31–40 | −11 | (−15, −6) | <0.001 | −9 | (−13, −5) | <0.001 |
| 41–50 | −21 | (−26, −16) | <0.001 | −20 | (−25, −15) | <0.001 |
| 51+ | −19 | (−25, −13) | <0.001 | −17 | (−24, −11) | <0.001 |
|
| ||||||
| Sex | ||||||
| Male | ref | ref | ||||
| Female | 18 | (14, 22) | <0.001 | 17 | (13, 21) | <0.001 |
|
| ||||||
| Mode of HIV Exposure | <0.001 | <0.001 | ||||
| Heterosexual contact | ref | ref | ||||
| Homosexual contact | 16 | (9, 23) | <0.001 | 18 | (11, 25) | <0.001 |
| Injecting drug use | −21 | (−30, −12) | <0.001 | −7 | (−18, 3) | 0.186 |
| Other/unknown | 6 | (0, 13) | 0.061 | 4 | (−2, 11) | 0.167 |
|
| ||||||
| Pre-ART HIV viral load (copies/mL) | ||||||
| ≤100000 | ref | ref | ||||
| >100000 | 41 | (34, 47) | <0.001 | 38 | (32, 45) | <0.001 |
| Not tested | 7 | (1, 13) | 0.024 | 5 | (−1, 11) | 0.117 |
|
| ||||||
| Pre-ART CD4 (cells/μL) | <0.001 | <0.001 | ||||
| ≤50 | ref | ref | ||||
| 51–100 | 10 | (4, 15) | 0.001 | 9 | (3, 14) | 0.002 |
| 101–200 | 5 | (1, 10) | 0.040 | 3 | (−2, 8) | 0.230 |
| 201+ | −8 | (−13, −4) | <0.001 | −15 | (−20, −10) | <0.001 |
|
| ||||||
| First ART regimen | 0.031 | 0.109 | ||||
| NRTI1+NNRTI2 | ref | ref | ||||
| NRTI1+PI3 | −4 | (−12, 4) | 0.331 | −5 | (−13, 3) | 0.246 |
| Other | 24 | (4, 44) | 0.020 | 16 | (−4, 36) | 0.108 |
|
| ||||||
| Hepatitis B co-infection | ||||||
| Negative | ref | ref | ||||
| Positive | −12 | (−20, −5) | 0.001 | −11 | (−18, −4) | 0.003 |
| Not tested | −5 | (−10, −1) | 0.033 | −5 | (−12, 2) | 0.134 |
|
| ||||||
| Hepatitis C co-infection | ||||||
| Negative | ref | ref | ||||
| Positive | −20 | (−28, −12) | <0.001 | −12 | (−21, −3) | 0.008 |
| Not tested | −4 | (−9, 1) | 0.145 | 3 | (−5, 10) | 0.478 |
Note: Global p-values for year of ART initiation, age and pre-ART CD4 count are test for trend. Other global p-values are test for heterogeneity.
NRTI = nucleoside reverse transcriptase inhibitor.
NNRTI = nonnucleoside reverse transcriptase inhibitor.
PI = protease inhibitor.
We conducted several sensitivity analyses to evaluate the robustness of our results. First, we evaluated whether there was an interaction between the year period of ART initiation and time from ART initiation. This interaction term was significant for those initiating in 2006–09 compared to 2003–05 (p value=0.002). The estimated mean CD4 count difference was not significantly higher for those initiating 2006–09 compared to 2003–05 from ART initiation, except at 3 months (Appendix 3). There was little evidence to suggest an interaction between time from ART initiation and those initiating in 2010–13, compared to 2003–05 (p value=0.527). The estimated mean CD4 count difference was significantly higher for those initiating in 2010–13, compared to 2003–05, up to 15 months and 27 to 36 months from ART initiation (Appendix 3). Second, we evaluated whether the inclusion of pre-ART CD4 count in the model biased our estimated CD4 count change. Using a mixed model approach with random intercept and random slope for time from ART initiation, we found minimal differences in the parameter estimates when pre-ART CD4 count was excluded (Appendix 4). We also found excluding other covariates with large proportions of missing data, such as pre-ART HIV viral load, HBV and HCV status, did not significantly affect the estimated mean CD4 count change for the covariates (Appendix 5). Third, we evaluated whether a mixed linear model with random intercept and random slope for time from ART initiation produced significantly different parameter estimates. The parameter estimates from this model was not substantially different from the primary analysis. The mean CD4 count difference was significantly higher, at any given time during follow-up, for those initiating in 2006–09 (p value=0.001) and 2010–13 (p value <0.001) compared to those initiating in 2003–05 (Appendix 5).
Other factors in the multivariate model significantly associated with a higher CD4 count response, at any given time during follow-up, included younger age, female gender, homosexual contact (compared to heterosexual contact), higher pre-ART HIV viral load, lower pre-ART CD4 count, and HBV or HCV negative (compared to positive).
Discussion
In this analysis consisting of 16 962 HIV-positive patients receiving care in the Asia-Pacific region, our findings have shown that long-term CD4 response to ART is greater in those initiating in 2010–13 and 2006–09 compared to those initiating in 2003–05, regardless of CD4 count at ART initiation. There was an increasing trend with those initiating in more recent years having a greater CD4 response compared to previous years. Over the follow-up period, the median CD4 count was also consistently higher, and the proportion of patients with higher CD4 counts increased in more recent years.
Similar temporal trends in CD4 count at ART initiation has been shown in other studies. A large multiregional comparison of HIV-positive adults initiating ART between 2002 and 2009 found a steady increase in the median CD4 count at ART initiation in most countries. This trend was apparent regardless of the income status of the country, although the greatest increases were seen in low-income and middle-income countries rather than in high-income countries and, was also higher in females than males [30]. In contrast, a meta-analysis of 44 studies did not find a significant increasing trend in the mean CD4 count at presentation for newly presenting HIV-positive adults. The annual estimated change in CD4 count at presentation was 1.6 cells/μL (95% CI: −4.4 to 5.4 cells/μL) which was not significant (p>0.05), adjusting for study inclusion criteria, data type and study location [31].
Overall, there were few studies that explored whether the year of ART initiation influenced the CD4 count response in HIV-positive patients. One study based in a London hospital showed an association between calendar year of ART initiation and CD4 response from ART initiation [23]. During the first 3 months of ART initiation, this association was not significant. However, beyond 3 months, patients initiating from 1997 to 2003 had a yearly CD4 count increase that was 84 cells/μL (95% CI: −48 to −120 cells/μL) higher than those initiating in 1996 and before.
It is also difficult to ascertain which factors of patient care have contributed to the improved CD4 count response in recent years. A move towards greater adherence in patients, either due to physician advice, support services, or more tolerable and convenient regimens, could have played an important role [17, 32]. Previous studies have highlighted that patients who are more adherent have greater and more sustained gains in CD4 count than non-adherent patients [33–35]. Other predictors significantly associated with improved CD4 count recovery are also consistent with former studies, including older age, female sex, pre-ART HIV viral load, HBV and HCV co-infection [36–38].
The clinical implications of our findings are fairly limited as those initiating in 2010–13 were only 15 cells/μL higher compared to those in 2003–05. But, our analysis has highlighted that the proportion of patients at high range CD4 counts has drastically increased over time, in particular, at ART initiation but also through to 36 months follow-up. Therefore, over time, fewer patients are being exposed to lower CD4 counts where they are at higher risk of OIs [39] and non-AIDS defining cancers (NADCs) [40, 41]. Patients are also experiencing shorter durations at lower CD4 counts, which leads to a better overall prognosis [42].
An advantage of our study was the large patient sample size yet, our patient data were limited to a few variables. As such, we were unable to explore other important trends relating to lower CD4 counts, including the occurrence of NADCs or OIs. In addition, HIV viral load was not routinely collected at the clinical sites and had large proportions of missing data. Hence, we could not expand the scope of our analysis to also examine the HIV viral load response by year of ART initiation. Our model estimates for the pre-ART HIV viral load may also be bias and caution is advised when interpreting these findings.
We used observational data on CD4 counts collected during routine clinic visits for HIV-positive adults presenting between 2003 and 2013. Patients lost to follow-up (LTFU) can introduce potential bias that can impede on the analysis because it is unclear how many remain in care elsewhere or have died. The LTFU rate previously reported in TAHOD-LITE was relatively low and consistent between the years of ART initiation (2003–05: 2.1 per 100 person-years; 2006–09: 2.9 per 100 person-years; 2010–13: 2.8 per 100 person-years) [21]. We also had 8 clinical sites represent 7 countries across the region, and hence, our results are reflective of trends occurring within the clinical sites rather than their respective countries. The presence of country-level differences in when patients present for care, the available treatment options and the patient care provided, as well as other unmeasured confounding factors could have also contributed to heterogeneity. However, our analysis by clinical site has shown similar trends to the overall analysis where there is an increasing trend in CD4 response over time by the year of ART initiation. Furthermore, the model used in our analysis is adjusted for clinic site to account for these differences between sites.
In summary, we found that the CD4 response to ART is greater in those initiating in 2010–13 and 2006–09 compared to those in 2003–05, with greater proportions of patients starting treatment at higher CD4 counts in recent years. Patients initiating in more recent years spend less time exposed in lower CD4 count ranges, reducing their risk for serious OIs and future NADCs that are associated with lower CD4 count. As guidelines recommending immediate ART are more widely implemented, it will be important to monitor their impact on immediate and long-term clinical outcomes.
Acknowledgments
TAHOD-LITE (TREAT Asia HIV Observational Database Low-Intensity TransfEr) is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia (The University of New South Wales). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Appendix 1. The median CD4 count (cells/μL) and patient totals over the time since ART initiation, by the year period of ART initiation and country
Appendix 2. The proportion of patients in each CD4 count (cells/μL) category over time since ART initiation, by country and year period of ART initiation
Appendix 3. Estimated mean CD4 count (cells/μL) change when considering an interaction between year period of ART initiation and follow-up time from ART initiation
| Month of follow-up | Year period of ART initiation | Mean Diff. | 95% CI | p value |
|---|---|---|---|---|
|
| ||||
| 3 | 2003–05 | ref | ||
| 2006–09 | 10 | (4, 15) | 0.001 | |
| 2010–13 | 18 | (12, 24) | <0.001 | |
|
| ||||
| 6 | 2003–05 | ref | ||
| 2006–09 | 5 | (−1, 11) | 0.093 | |
| 2010–13 | 15 | (9, 22) | <0.001 | |
|
| ||||
| 9 | 2003–05 | ref | ||
| 2006–09 | 2 | (−4, 9) | 0.497 | |
| 2010–13 | 13 | (6, 19) | <0.001 | |
|
| ||||
| 12 | 2003–05 | ref | ||
| 2006–09 | 1 | (−6, 7) | 0.856 | |
| 2010–13 | 10 | (4, 16) | 0.002 | |
|
| ||||
| 15 | 2003–05 | ref | ||
| 2006–09 | 0 | (−6, 6) | 0.979 | |
| 2010–13 | 8 | (1, 14) | 0.017 | |
|
| ||||
| 18 | 2003–05 | ref | ||
| 2006–09 | 0 | (−6, 6) | 0.888 | |
| 2010–13 | 6 | (0, 12) | 0.067 | |
|
| ||||
| 21 | 2003–05 | ref | ||
| 2006–09 | 1 | (−5, 8) | 0.666 | |
| 2010–13 | 5 | (−2, 12) | 0.132 | |
|
| ||||
| 24 | 2003–05 | ref | ||
| 2006–09 | 3 | (−4, 9) | 0.425 | |
| 2010–13 | 5 | (−2, 12) | 0.126 | |
|
| ||||
| 27 | 2003–05 | ref | ||
| 2006–09 | 4 | (−3, 10) | 0.233 | |
| 2010–13 | 7 | (0, 14) | 0.046 | |
|
| ||||
| 30 | 2003–05 | ref | ||
| 2006–09 | 5 | (−1, 11) | 0.128 | |
| 2010–13 | 10 | (3, 18) | 0.004 | |
|
| ||||
| 33 | 2003–05 | ref | ||
| 2006–09 | 6 | (−2, 13) | 0.150 | |
| 2010–13 | 16 | (7, 24) | <0.001 | |
|
| ||||
| 36 | 2003–05 | ref | ||
| 2006–09 | 5 | (−6, 17) | 0.354 | |
| 2010–13 | 23 | (10, 36) | <0.001 | |
Note: Multivariate model adjusts for age at ART initiation, gender, HIV mode of exposure, pre-ART HIV viral load, pre-ART CD4 count, first ART regimen, hepatitis B co-infection, hepatitis C co-infection and clinical site.
Appendix 4. Comparison of estimated mean CD4 count (cells/μL) change up to 36 months from ART initiation using a mixed linear model, with random intercept and random slope for time from ART initiation
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Diff. | 95% CI | p value | Mean Diff. | 95% CI | p value | |
| Year of ART Initiation | <0.001 | <0.001 | ||||
| 2003–2005 | ref | ref | ||||
| 2006–2009 | 6 | (2, 10) | 0.001 | 7 | (3, 11) | 0.001 |
| 2010–2013 | 9 | (5, 13) | <0.001 | 11 | (7, 15) | <0.001 |
|
| ||||||
| Time from ART initiation (per month) | 16 | (15, 16) | <0.001 | 16 | (15, 16) | <0.001 |
|
| ||||||
| Age at ART initiation (years) | <0.001 | |||||
| ≤30 | ref | ref | ||||
| 31–40 | −5 | (−8, −2) | 0.003 | −5 | (− −9, −2) | 0.001 |
| 41–50 | −11 | (−15, −7) | <0.001 | −12 | (−16, −8) | <0.001 |
| 51+ | −7 | (−12, −2) | 0.004 | −8 | (−13, −3) | 0.001 |
|
| ||||||
| Sex | ||||||
| Male | ref | ref | ||||
| Female | 1 | (−2, 4) | 0.445 | 1 | (−2, 5) | 0.332 |
|
| ||||||
| Mode of HIV Exposure | <0.001 | <0.001 | ||||
| Heterosexual contact | ref | ref | ||||
| Homosexual contact | 11 | (5, 16) | <0.001 | 11 | (6, 16) | <0.001 |
| Injecting drug use | −17 | (−24, −10) | <0.001 | −17 | (−24, −10) | <0.001 |
| Other/unknown | 4 | (−1, 9) | 0.111 | 4 | (−1, 9) | 0.111 |
|
| ||||||
| Pre-ART CD4 cell count (cells/μL) | ||||||
| ≤50 | ref | |||||
| 51–100 | 11 | (7, 16) | 0.000 | |||
| 101–200 | 9 | (5, 13) | 0.000 | |||
| 201+ | −3 | (−6, 1) | 0.169 | |||
|
| ||||||
| First ART regimen | 0.009 | 0.013 | ||||
| NRTI1+NNRTI2 | ref | ref | ||||
| NRTI1+PI3 | −4 | (−10, 3) | 0.263 | −3 | (−9, 3) | 0.374 |
| Other/unknown | 20 | (6, 35) | 0.007 | 20 | (5, 35) | 0.007 |
Note: Global p-values for year of ART initiation, pre-ART CD4 count and age are test for trend. Other global p-values are test for heterogeneity.
NRTI = nucleoside reverse transcriptase inhibitor.
NNRTI = nonnucleoside reverse transcriptase inhibitor.
PI = protease inhibitor.
Appendix 5. Comparison of estimated mean CD4 count (cells/μL) change up to 36 months from ART initiation using a mixed linear model, with random intercept and random slope for time from ART initiation
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Diff. | 95% CI | p value | Mean Diff. | 95% CI | p value | |
| Year of ART Initiation | <0.001 | <0.001 | ||||
| 2003–2005 | ref | ref | ||||
| 2006–2009 | 6 | (2, 10) | 0.001 | 6 | (2, 10) | 0.002 |
| 2010–2013 | 9 | (5, 13) | <0.001 | 9 | (5, 13) | <0.001 |
|
| ||||||
| Time from ART initiation (per month) | 16 | (15, 16) | <0.001 | 16 | (15, 16) | <0.001 |
|
| ||||||
| Age at ART initiation (years) | <0.001 | <0.001 | ||||
| ≤30 | ref | ref | ||||
| 31–40 | −5 | (−8, −2) | 0.003 | −6 | (−9, −3) | <0.001 |
| 41–50 | −11 | (−15, −7) | <0.001 | −13 | (−17, −9) | <0.001 |
| 51+ | −7 | (−12, −2) | 0.004 | −9 | (−14, −4) | <0.001 |
|
| ||||||
| Sex | ||||||
| Male | ref | ref | ||||
| Female | 1 | (−2, 4) | 0.445 | 2 | (−1, 5) | 0.319 |
|
| ||||||
| Mode of HIV Exposure | <0.001 | <0.001 | ||||
| Heterosexual contact | ref | ref | ||||
| Homosexual contact | 11 | (5, 16) | <0.001 | 11 | (6, 16) | <0.001 |
| Injecting drug use | −17 | (−24, −10) | <0.001 | −11 | (−19, −2) | 0.011 |
| Other/unknown | 4 | (−1, 9) | 0.111 | 3 | (−1, 8) | 0.168 |
|
| ||||||
| First ART regimen | 0.009 | 0.030 | ||||
| NRTI1+NNRTI2 | ref | ref | ||||
| NRTI1+PI3 | −4 | (−10, 3) | 0.263 | −4 | (−10, 3) | 0.252 |
| Other | 20 | (6, 35) | 0.007 | 17 | (2, 31) | 0.026 |
|
| ||||||
| Pre-ART HIV viral load (copies/mL) | ||||||
| ≤100000 | ref | |||||
| >100000 | 30 | (25, 35) | <0.001 | |||
| Not tested | 6 | (1, 10) | 0.014 | |||
|
| ||||||
| Pre-ART CD4 (cells/μL) | 0.724 | |||||
| ≤50 | ref | |||||
| 51–100 | 12 | (8, 16) | <0.001 | |||
| 101–200 | 10 | (6, 14) | <0.001 | |||
| 201+ | 0 | (−4, 3) | 0.895 | |||
|
| ||||||
| Hepatitis B co-infection | 0.086 | |||||
| Negative | ref | |||||
| Positive | −6 | (−11, −1) | 0.042 | |||
| Not tested | −3 | (−8, 2) | 0.291 | |||
|
| ||||||
| Hepatitis C co-infection | 0.016 | |||||
| Negative | ref | |||||
| Positive | −10 | (−16, −3) | 0.005 | |||
| Not tested | 1 | (−4, 7) | 0.601 | |||
Note: Global p-values for year of ART initiation, age at ART initiation, pre-ART HIV viral load and pre-ART CD4 count are test for trend. Other global p-values are test for heterogeneity.
NRTI = nucleoside reverse transcriptase inhibitor.
NNRTI = nonnucleoside reverse transcriptase inhibitor.
PI = protease inhibitor.
TAHOD-LITE study members
PS Ly and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
MP Lee, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
TP Merati, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
OT Ng, PL Lim, LS Lee and R Martinez-Vega, Tan Tock Seng Hospital, Singapore;
JY Choi, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
TT Pham, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam;
KV Nguyen, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
AH Sohn, JL Ross and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
NL De La Mata, A Jiamsakul, DC Boettiger and MG Law, The Kirby Institute, UNSW Australia, Sydney, Australia.
Footnotes
Competing Interests
The authors do not have any competing interests to declare.
Authors’ contributions
NLD and ML contributed to the concept development. KN, PSL, OTN, KVN, TPM, TTP, MPL and JYC contributed data for the analysis. NLD performed the statistical analysis and wrote the first draft of the manuscript. All authors commented on the draft manuscript and approved of the final manuscript.
References
- 1.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of Internal Medicine. 1997 Jun 15;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Korenromp EL, Williams BG, Schmid GP, Dye C. Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection--a quantitative review. PloS One. 2009;4(6):e5950. doi: 10.1371/journal.pone.0005950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapadula G, Chatenoud L, Gori A, Castelli F, Di Giambenedetto S, Fabbiani M, et al. Risk of Severe Non AIDS Events Is Increased among Patients Unable to Increase their CD4+ T-Cell Counts >200+/mul Despite Effective HAART. PloS One. 2015;10(5):e0124741. doi: 10.1371/journal.pone.0124741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clinical Infectious Diseases. 2014 May;58(9):1312–21. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray S, Gedeon J, Hadi A, Kotb A, Rahman T, Sarwar E, et al. Predictive value of CD4 cell count nadir on long-term mortality in HIV-positive patients in Uganda. HIV AIDS (Auckl) 2012;4:135–40. doi: 10.2147/HIV.S35374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Research. 2015 May;117:69–74. doi: 10.1016/j.antiviral.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Reniers G, Slaymaker E, Nakiyingi-Miiro J, Nyamukapa C, Crampin AC, Herbst K, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA) AIDS. 2014 Nov;28( Suppl 4):S533–42. doi: 10.1097/QAD.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermund SH. Treatment as prevention for HIV in China. Lancet. 2013 Oct 5;382(9899):1159–61. doi: 10.1016/S0140-6736(12)62005-4. [DOI] [PubMed] [Google Scholar]
- 9.van Sighem A, Danner S, Ghani AC, Gras L, Anderson RM, de Wolf F, et al. Mortality in patients with successful initial response to highly active antiretroviral therapy is still higher than in non-HIV-infected individuals. Journal of Acquired Immune Deficiency Syndromes. 2005 Oct 1;40(2):212–8. doi: 10.1097/01.qai.0000165911.97085.d0. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organisation. Scaling up Antiretroviral Therapy in resource-limited settings. Geneva, Switzerland: 2002. [Google Scholar]
- 11.Booth CL, Garcia-Diaz AM, Youle MS, Johnson MA, Phillips A, Geretti AM. Prevalence and predictors of antiretroviral drug resistance in newly diagnosed HIV-1 infection. Journal of Antimicrobial Chemotherapy. 2007 Mar;59(3):517–24. doi: 10.1093/jac/dkl501. [DOI] [PubMed] [Google Scholar]
- 12.Bangsberg DR, Moss A. When should we delay highly active antiretroviral therapy? Journal of General Internal Medicine. 1999 Jul;14(7):446–8. doi: 10.1046/j.1525-1497.1999.05109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans SM, van Leth F, Manabe YC, Hoepelman AI, Lange JM, Kambugu A. Earlier initiation of antiretroviral therapy, increased tuberculosis case finding and reduced mortality in a setting of improved HIV care: a retrospective cohort study. HIV Medicine. 2012 Jul;13(6):337–44. doi: 10.1111/j.1468-1293.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- 14.Romley JA, Juday T, Solomon MD, Seekins D, Brookmeyer R, Goldman DP. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Affairs. 2014 Mar;33(3):370–7. doi: 10.1377/hlthaff.2013.0623. [DOI] [PubMed] [Google Scholar]
- 15.Sterling TR, Chaisson RE, Keruly J, Moore RD. Improved outcomes with earlier initiation of highly active antiretroviral therapy among human immunodeficiency virus-infected patients who achieve durable virologic suppression: longer follow-up of an observational cohort study. Journal of Infectious Diseases. 2003 Dec 1;188(11):1659–65. doi: 10.1086/379741. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Available online at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. [verified January 2016] [PubMed]
- 17.World Health Organisation. Consolidated guidelines on the use of Antiretroviral Drugs for treating and preventing HIV infection: Recommendations for a Public Health approach. Geneva, Switzerland: 2013. Available online at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [verified October 2015] [PubMed] [Google Scholar]
- 18.Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010 Sep 10;24(14):2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Medicine. 2005 Mar;6(2):99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 20.Kee MK, Lee JH, Kim EJ, Lee J, Nam JG, Yoo BH, et al. Improvement in survival among HIV-infected individuals in the Republic of Korea: Need for an early HIV diagnosis. BMC Infectious Diseases. 2009 Aug;12:9. doi: 10.1186/1471-2334-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De La Mata NL, Kumarasamy N, Khol V, Ng OT, Van Kinh N, Merati TP, et al. Improved survival in HIV treatment programmes in Asia. Antiviral Therapy. 2016 Mar 10; doi: 10.3851/IMP3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farahani M, Price N, El-Halabi S, Mlaudzi N, Keapoletswe K, Lebelonyane R, et al. Trends and determinants of survival for over 200 000 patients on antiretroviral treatment in the Botswana National Program: 2002–2013. AIDS. 2016 Jan 28;30(3):477–85. doi: 10.1097/QAD.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, Madge S, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. Journal of Infectious Diseases. 2004 Nov 15;190(10):1860–8. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 24.Asfaw A, Ali D, Eticha T, Alemayehu A, Alemayehu M, Kindeya F. CD4 cell count trends after commencement of antiretroviral therapy among HIV-infected patients in Tigray, Northern Ethiopia: a retrospective cross-sectional study. PloS One. 2015;10(3):e0122583. doi: 10.1371/journal.pone.0122583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright ST, Petoumenos K, Boyd M, Carr A, Downing S, O’Connor CC, et al. Ageing and long-term CD4 cell count trends in HIV-positive patients with 5 years or more combination antiretroviral therapy experience. HIV Medicine. 2013 Apr;14(4):208–16. doi: 10.1111/j.1468-1293.2012.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasuriar R, Gouillou M, Spelman T, Read T, Hoy J, Law M, et al. Clinical predictors of immune reconstitution following combination antiretroviral therapy in patients from the Australian HIV Observational Database. PloS One. 2011;6(6):e20713. doi: 10.1371/journal.pone.0020713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leth F, Wit FW, Reiss P, Schattenkerk JK, van der Ende ME, Schneider MM, et al. Differential CD4 T-cell response in HIV-1-infected patients using protease inhibitor-based or nevirapine-based highly active antiretroviral therapy. HIV Medicine. 2004 Mar;5(2):74–81. doi: 10.1111/j.1468-1293.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 28.Mocroft A, Phillips AN, Ledergerber B, Katlama C, Chiesi A, Goebel FD, et al. Relationship between antiretrovirals used as part of a cART regimen and CD4 cell count increases in patients with suppressed viremia. AIDS. 2006 May 12;20(8):1141–50. doi: 10.1097/01.aids.0000226954.95094.39. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. Journal of Acquired Immune Deficiency Syndromes. 2005 Feb 1;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, Koller M, Dabis F, et al. Immunodeficiency at the Start of Combination Antiretroviral Therapy in Low-, Middle-, and High-Income Countries. Journal of Acquired Immune Deficiency Syndromes. 2014 Jan 1;65(1):E8–E16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A Systematic Review and Meta-regression of Temporal Trends in Adult CD4(+) Cell Count at Presentation to HIV Care, 1992–2011. Clinical Infectious Diseases. 2013 Oct 10;57(7):1027–37. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 32.Duda SN, Farr AM, Lindegren ML, Blevins M, Wester CW, Wools-Kaloustian K, et al. Characteristics and comprehensiveness of adult HIV care and treatment programmes in Asia-Pacific, sub-Saharan Africa and the Americas: results of a site assessment conducted by the International epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. Journal of the International AIDS Society. 2014;17:19045. doi: 10.7448/IAS.17.1.19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2004 Mar 1;35(3):261–8. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 34.Lima VD, Harrigan R, Murray M, Moore DM, Wood E, Hogg RS, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS. 2008 Nov 12;22(17):2371–80. doi: 10.1097/QAD.0b013e328315cdd3. [DOI] [PubMed] [Google Scholar]
- 35.Safren SA, Kumarasamy N, James R, Raminani S, Solomon S, Mayer KH. ART adherence, demographic variables and CD4 outcome among HIV-positive patients on antiretroviral therapy in Chennai, India. AIDS Care. 2005 Oct;17(7):853–62. doi: 10.1080/09540120500038439. [DOI] [PubMed] [Google Scholar]
- 36.van Griensven J, Phirum L, Choun K, Thai S, De Weggheleire A, Lynen L. Hepatitis B and C Co-Infection among HIV-Infected Adults while on Antiretroviral Treatment: Long-Term Survival, CD4 Cell Count Recovery and Antiretroviral Toxicity in Cambodia. PloS One. 2014 Feb 12;9(2) doi: 10.1371/journal.pone.0088552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, Fox MP. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. Journal of Women’s Health. 2013 Feb;22(2):113–20. doi: 10.1089/jwh.2012.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes RA, Sterne JAC, Walsh J, Bansi L, Gilson R, Orkin C, et al. Long-term trends in CD4 cell counts and impact of viral failure in individuals starting antiretroviral therapy: UK Collaborative HIV Cohort (CHIC) study. HIV Medicine. 2011 Nov;12(10):583–93. doi: 10.1111/j.1468-1293.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 39.Damtie D, Yismaw G, Woldeyohannes D, Anagaw B. Common opportunistic infections and their CD4 cell correlates among HIV-infected patients attending at antiretroviral therapy clinic of Gondar University Hospital, Northwest Ethiopia. BMC Research Notes. 2013;6:534. doi: 10.1186/1756-0500-6-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franzetti M, Adorni F, Parravicini C, Vergani B, Antinori S, Milazzo L, et al. Trends and predictors of non-AIDS-defining cancers in men and women with HIV infection: a single-institution retrospective study before and after the introduction of HAART. Journal of Acquired Immune Deficiency Syndromes. 2013 Apr 1;62(4):414–20. doi: 10.1097/QAI.0b013e318282a189. [DOI] [PubMed] [Google Scholar]
- 41.Calabresi A, Ferraresi A, Festa A, Scarcella C, Donato F, Vassallo F, et al. Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of Northern Italy, 1999–2009. HIV Medicine. 2013 Sep;14(8):481–90. doi: 10.1111/hiv.12034. [DOI] [PubMed] [Google Scholar]
- 42.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm(3) on long-term combination antiretroviral therapy reach same mortality rates as the general population. Journal of Acquired Immune Deficiency Syndromes. 2007 Sep 1;46(1):72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]