Abstract
Purpose
Pancreatic cystic lesions are common incidental findings on imaging, but up to half may be forerunners of pancreatic cancer. Therefore, accurate differential diagnosis is crucial for correct patient management. Unfortunately, currently available diagnostic methods cannot robustly identify premalignant and malignant pancreatic cystic lesions.
Methods
Cyst fluid samples obtained by routine endoscopic ultrasound-guided aspiration were used for the analyses. In a cohort of 24 patients, eight biomarker candidates for malignant potential and high-grade dysplasia/cancer were identified by an explorative proteomic approach. Subsequently, a quantitative analysis, using 30 heavy-labeled peptides from the biomarkers and parallel reaction monitoring mass spectrometry, was devised, tested in a training cohort of 80, and prospectively evaluated in a validation cohort of 68 patients. End points were surgical pathology diagnosis/clinical follow-up. Diagnostic assessments were blinded to mass spectrometry results.
Results
The optimal set of markers for detecting malignant potential was a panel of peptides from mucin-5AC and mucin-2, which could discriminate premalignant/malignant lesions from benign with an accuracy of 97% (95% CI, 89% to 99%) in the validation cohort. This result compared favorably with the accuracy of standard analyses: cyst fluid carcinoembryonic antigen (61%; 95% CI, 46% to 74%; P < .001) and cytology (84%; 95% CI, 71% to 92%; P = .02). A combination of proteins mucin-5AC and prostate stem-cell antigen could identify high-grade dysplasia/cancer with an accuracy of 96% (95% CI, 90% to 99%), and detected 95% of malignant/severely dysplastic lesions, compared with 35% and 50% for carcinoembryonic antigen and cytology (P < .001 and P = .003, respectively).
Conclusion
Targeted mass spectrometry analysis of just three cyst fluid biomarkers provides highly accurate identification and assessment of cystic precursors to pancreatic adenocarcinoma. Additional studies should determine whether the method can facilitate timely cancer diagnosis, successful intervention, and prevention.
INTRODUCTION
Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States and Western Europe.1 Unlike other cancer forms, its prognosis has changed little over time.1 The foremost reason is late diagnosis, mainly due to the lack of specific symptoms. Moreover, pancreatic cancer precursors have been considered to be invisible on imaging, growing as microscopic lesions in the ducts.1-3
However, the fact that pancreatic cancer also develops from cystic, grossly visible precursors is increasingly recognized.2-4 Cystic forerunners of pancreatic adenocarcinoma consist of two mucin-producing tumor entities: intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms.1,2 Together, these neoplasms comprise 10% to 50% of pancreatic cystic lesions (PCLs).5,6 However, PCLs also encompass intrinsically benign tumors, that is, serous cystic neoplasms and inflammatory pseudocysts.5,6
PCLs are incidentally discovered in up to 20% of abdominal magnetic resonance imaging scans in adults.7,8 Most have not caused any discomfort.5,6 The high prevalence of PCLs, their usually asymptomatic nature, and the perils of pancreatic surgery naturally exclude general prophylactic intervention, rendering accurate differential diagnosis critical.9,10
Radiology is seldom sufficient for the assessment of PCLs.5,6,11 The preferred method is endoscopic ultrasound (EUS), with aspiration of cyst fluid for cytology and quantification of carcinoembryonic antigen (CEA).5,6,12,13 However, even these analyses cannot robustly distinguish between benign, premalignant, and malignant PCLs.5,6 The utility of cytology is hampered by scant cellular yield and the focal nature of the dysplasia.12 CEA levels, although generally higher in premalignant/malignant tumors, do not correlate with the degree of dysplasia, and there is substantial overlap with benign lesions.13 Moreover, both methods frequently give inconclusive results, largely because of insufficient cyst fluid yield.14
In view of the risks of pancreatic surgery, exact diagnostic assessment is crucial for correct patient management. In a young patient, the removal of a premalignant lesion will likely forestall the development of cancer later in life.5,6,15,16 For an elderly individual with comorbidities, surgery may be indicated only for a malignant PCL, but will then usually be lifesaving.6,15,16 Furthermore, the time course for malignant transformation is variable. In IPMNs, the risk of malignancy largely depends on the epithelial subtype and is highest for pancreatobiliary-type IPMNs.17 Intestinal-type IPMNs have a somewhat better prognosis, whereas gastric-type IPMNs are generally indolent.17 Unfortunately, IPMN subtypes can rarely be distinguished preoperatively.
Proteomic analysis by mass spectrometry (MS) is largely used for experimental research, exploring the protein content of cells, tissues, or body fluids in health and disease. In contrast, targeted proteomic techniques enable high-throughput absolute quantification of biomarkers and are predicted to replace antibody-based assays.18 Pitfalls of antibody-dependent analyses, such as variable protein glycosylation as observed in dysplasia/cancer, are avoided, and many biomarkers can be analyzed simultaneously.
The primary objective of this study was to devise and validate a targeted, quantitative proteomic analysis to identify and distinguish between premalignant PCLs and cystic neoplasms with manifest high-grade dysplasia (HGD)/cancer. A secondary aim was to find and evaluate markers for different epithelial subtypes of IPMNs, which may be used to predict the risk of malignant transformation.
METHODS
Study Design and Recruitment of Patients
The study was approved by the regional ethics committee. Patients older than 18 years of age referred to Sahlgrenska Hospital, a tertiary center (catchment area, 1.6 million), for EUS-guided fine-needle aspiration (FNA) of PCLs during 10 years (January 2007 to January 2016) were consecutively included. Participants provided written informed consent. Exclusion criteria were (1) solid-pseudopapillary neoplasm and (2) neuroendocrine tumor.
There were three patient cohorts. In the first, explorative, cohort biomarker candidates were identified. This retrospective cohort consisted of well-characterized PCLs, representing three of each of the most common tumor types/subtypes and six pseudocysts. Biomarkers were tested in a training cohort (included January 2007 to April 2014) and prospectively evaluated in a validation cohort (May 2014 to January 2016). Patients completed a questionnaire regarding their symptoms. The study was conducted according to the Standards for the Reporting of Diagnostic Accuracy Studies guidelines (www.stard-statement.org).
EUS, Cytology, and Cyst Fluid CEA Quantification
EUS-FNA was performed as detailed in the Data Supplement. Cytology with periodic acid–Schiff staining for mucus and cyst fluid CEA quantification by immune-chemoluminescence were routinely performed and prioritized over proteomic analysis. Established CEA cutoffs of > 192 ng/mL and > 1,000 ng/mL were used for diagnosing premalignancy and malignancy/HGD, respectively.13,19
Development of a Method for Targeted Biomarker Quantification Using Parallel Reaction Monitoring
A detailed description of the method is provided in the Data Supplement. Initially, the cyst fluid proteomes of the patients from the explorative cohort were analyzed by nano-liquid chromatography-tandem MS. Biomarker candidates for malignant potential, HGD/cancer, and IPMN histologic subtypes were determined based on differences in protein identifications and relative protein abundances between the relevant lesion categories. From the selected biomarkers, peptides suitable for quantification were identified, using criteria listed in the Data Supplement.
On the basis of the results of the explorative analysis, a targeted, quantitative MS analysis was devised, using parallel reaction monitoring.20 In this method, the mass spectrometer is programmed to select only the mass/charge windows corresponding to the peptides of interest.18,20 All their fragment ions are then scanned.20 Before analysis, known amounts of stable-isotope–labeled versions of the selected biomarker peptides were spiked into each cyst fluid sample (0.5 µL). This approach allows for accurate biomarker quantification through the comparison of the signals of the native and labeled peptides.18,20 Sample preparation and analysis took 2 to 3 days; many samples were then analyzed in parallel.
End Points
For the question of malignant potential, either histology or clinical assessment was accepted as a diagnostic standard to allow for the analysis of a representative spectrum of PCLs, including benign lesions. Clinical evaluation was typically based on structured follow-up, consistent with international (Sendai) or European guidelines, but individually modified according to patient characteristics and preferences.10,21 Ambiguous cases were discussed by a multidisciplinary board. Lesions with indeterminate final diagnosis were excluded.
Because HGD, like cancer, is an absolute indication for surgery in PCLs (provided the intent is curative), biomarkers were selected to target both conditions.9,10 For the detection/exclusion of HGD/cancer, four end points were accepted: (1) histology, (2) confirmed metastasis of pancreatic cancer, (3) follow-up > 3 years without morphologic changes, and (4) unambiguous diagnosis of an intrinsically benign lesion (serous cystic neoplasm/pseudocyst). IPMN epithelial subtypes were identified by histology. Diagnostic assessments were blinded to MS results. Conversely, the MS-based biomarker quantification was blinded to clinical data.
Statistics
Detailed information on the statistical analysis is provided in the Data Supplement. Categorical data with binomial distribution were compared using Fisher´s exact test; quantitative data were compared through Mann-Whitney U-test or Kruskal-Wallis test. The threshold for statistical significance was set at .05 and adjusted by the Bonferroni method, when appropriate. P values were two-sided.
For the training cohort, receiver operating characteristic curves were generated to compare biomarker performances and to establish cutoff levels, using the Youden index. Final biomarker panels for the different study questions were selected to maximize the area under the curve. However, biologic rationale and the simplicity of the model were also considered.
Given the lack of previous data on the novel analysis, sample size estimations for the validation cohort were based on results from the training cohort. The desired width of the 95% CI for the targeted MS analysis, as appraised from training cohort data and the performance of traditional analyses in previous reports, formed the basis for the calculations (Data Supplement). These were performed a priori, using the normal approximation method.22
RESULTS
The Study Cohorts
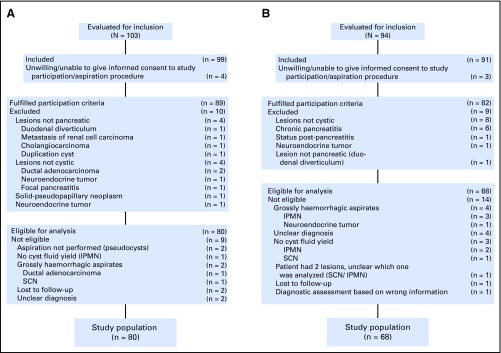
Demographic and clinical information on the study cohorts is listed in Table 1. Biomarkers were identified in an explorative cohort of 24 patients with well-characterized lesions. Subsequently, a targeted method was devised, tested in a cohort of 80 and prospectively evaluated in a validation cohort of 68 patients. Flow charts for patient inclusion/exclusion are provided in Figure 1. For six patients (4%), the final diagnosis remained unclear; these patients were excluded.
Table 1.
Summary of the Study Cohorts
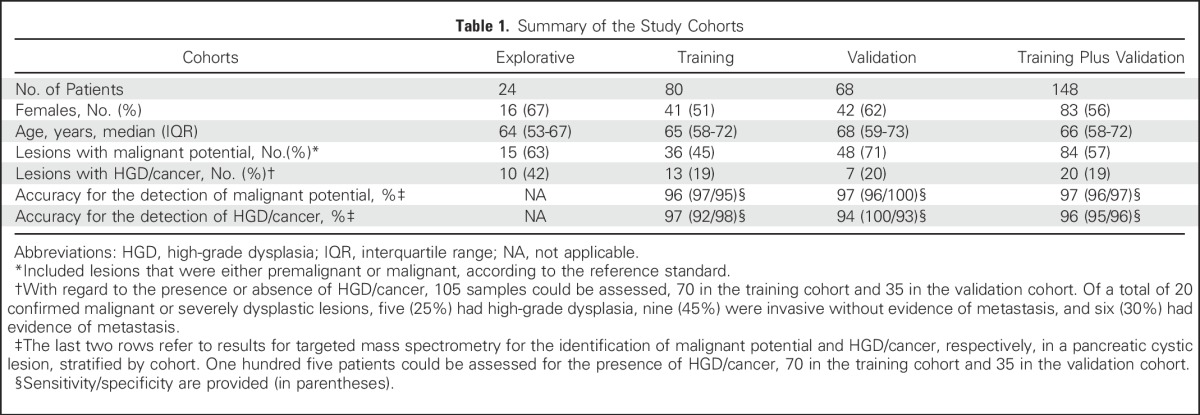
Fig 1.
Flow diagrams of patient inclusion/exclusion. (A) Training cohort and (B) validation cohort. IPMN, intraductal papillary mucinous neoplasm; SCN, serous cystic neoplasm.
The proportions of lesions with malignant potential or HGD/cancer were, respectively, 63% (15 of 24) and 42% (10 of 24) in the explorative cohort, 45% (36 of 80) and 19% (13 of 70) in the training cohort, and 71% (48 of 68) and 20% (7 of 35) in the validation cohort. One hundred five patients could be assessed for the presence of HGD/cancer (training cohort, 70; validation cohort, 35) because they fulfilled predetermined end point criteria. The distribution of diagnoses in the study population is detailed in the Data Supplement, including clinical information for the different lesion types and end points. Histologic confirmation was available for 20 (83%), 28 (35%), and 20 (29%) patients from the explorative, training, and validation cohorts, respectively. Surgery took place within 6 months from aspiration. Median follow-up with imaging was 10 months (IQR, 6 to 19 months).
Identification of Protein and Peptide Biomarkers
In the first cohort of 24 patients, an explorative proteomic analysis was performed to identify biomarker candidates for malignant potential, HGD/cancer, and/or IPMN histologic subtypes. Only secreted and plasma membrane proteins were regarded as potentially reliable and biologically relevant cyst fluid biomarkers. The final eight biomarkers are listed in the Data Supplement, along with their performance in the explorative cohort.
On the basis of criteria listed in the Data Supplement, peptides from the selected biomarkers were chosen for targeted quantification, 1 to 10 per protein (Data Supplement). Standard curves for the heavy-labeled peptides are presented in the Data Supplement. Reproducibility analysis of three replicates for 23 samples (16%) revealed minimal variation (Data Supplement).
Mucin-5AC and Mucin-2 Identify Malignant Potential in PCLs
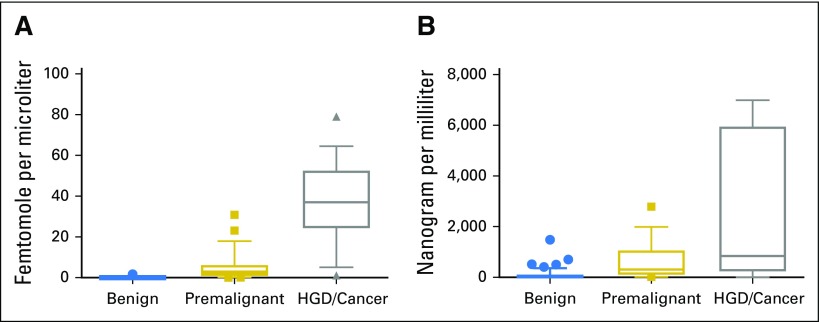
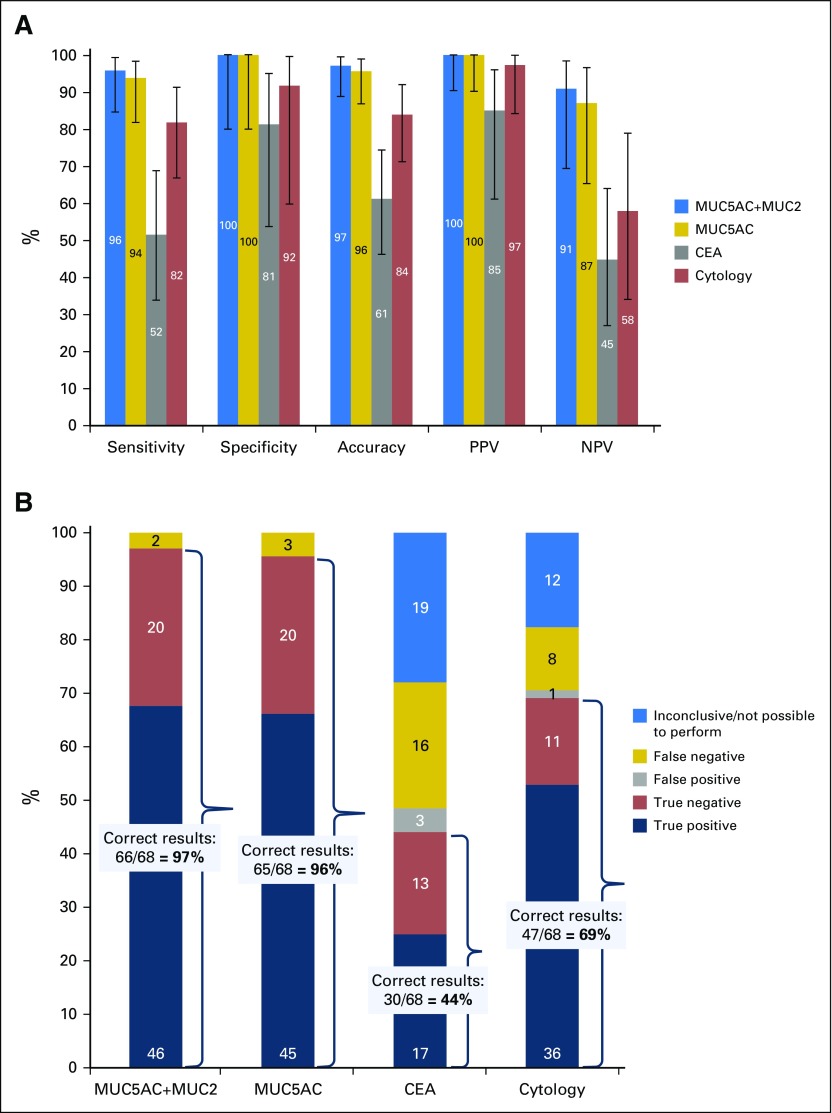
In the training cohort, the best marker for cystic cancer precursors was the mucin-5AC (MUC5AC). A box plot of cyst fluid MUC5AC levels for benign, premalignant, and malignant/HGD lesions is provided in Figure 2A and corresponding results for CEA in Figure 2B. However, intestinal-type IPMNs, like the intestinal epithelium, secrete mainly mucin-2 (MUC2), which motivated the inclusion of this protein in the analysis.5,17 For the combined analysis of MUC5AC and MUC2, a cutoff of 0.01 fmol/µL, representing the summed protein concentration levels, was established in the training cohort. The diagnostic accuracy of this panel for malignant potential was 97% (95% CI, 89% to 99%) in the validation cohort, which significantly exceeded the accuracy values for CEA (61%; P < .001) and cytology (84%; P = .02; Fig 3A23; Data Supplement). Full performance characteristics are provided for MUC5AC plus MUC2, MUC5AC only, and traditional methods in Figure 3A and the Data Supplement. Accuracy results for all biomarker candidates and receiver operating characteristic curves are provided in the Data Supplement.
Fig 2.
Mucin-5AC (MUC5AC) and carcinoembryonic antigen (CEA) concentrations in benign, premalignant, and malignant/severely dysplastic pancreatic cystic lesions. Boxes represent the 25th to 75th percentile, the line represents the median, and the whiskers represent the 10th to 90th percentile. (A) MUC5AC concentrations in (1) intrinsically benign pancreatic cystic lesions, (2) premalignant lesions (ie, IPMNs or mucinous cystic neoplasms with low-grade or intermediate-grade dysplasia), and (3) lesions with high-grade dysplasia (HGD) or invasive cancer. Three high outliers for the group with HGD/cancer do not appear in the figure (1,631, 1,312 and 341 fmol/µL). Two outliers high in MUC5AC among the premalignant lesions, which were wrongly classified as HGD/cancer by targeted mass spectrometry, are included in the figure. In both cases, histology of the surgical specimen revealed IPMNs with main duct involvement and intermediate-grade dysplasia. Taken together, this means that the risk of future malignant progression would have been high and that surgery was the correct treatment option. The P value for the comparison of the three groups is statistically significant: < .001 (Kruskal-Wallis test with Bonferroni adjustment of the significance threshold to .017). (B) Carcinoembryonic antigen (CEA) concentrations in (1) intrinsically benign pancreatic cystic lesions; (2) premalignant lesions (ie, IPMNs or mucinous cystic neoplasms with low-grade or intermediate-grade dysplasia); and (3) lesions with high-grade dysplasia (HGD)/invasive cancer. Two high outliers for the HGD/cancer group do not appear in the figure (11,246 and 33,700 ng/mL). There is a substantial overlap between patient groups.
Fig 3.
Identification of cystic lesions with malignant potential. Malignant potential is defined as either premalignancy or malignancy. Cutoff values were mucin-5AC (MUC5AC) plus mucin-2 (MUC2), 0.01 fmol/µL cyst fluid (summed protein concentration levels); MUC5AC, 0.01 fmol/µL; and carcinoembryonic antigen (CEA), 192 ng/mL. A positive result for cytology was defined as either presence of mucus in the sample or evidence of dysplasia. The results are from the validation cohort. (A) Performance characteristics for the identification of cystic lesions with malignant potential; inconclusive results not included (validation cohort). The accuracy for MUC5AC plus MUC2 was statistically significantly higher than that of CEA (P < .001) and cytology (P = .02). Comparative statistical analysis was performed by Fisher´s exact test with Bonferroni correction for multiple comparisons (threshold for significance, .025). Error bars represent 95% CIs, calculated using the Wilson procedure,23 with correction for continuity. (B) Diagnostic outcomes for the identification of malignant potential in a cystic lesion (validation cohort). Percentages are displayed on the y-axis, and the number of individuals are displayed in labels on the stacked bar chart. The preferred panel for the identification of malignant potential, MUC5AC plus MUC2, gave a statistically significantly higher proportion of correct results than CEA (P < .001) and cytology (P < .001). Comparative statistical analysis was performed by Fisher´s exact test with Bonferroni correction for multiple comparisons (threshold for significance, .025). NPV, negative predictive value; PPV, positive predictive value.
However, this analysis does not consider the high proportion of inconclusive results for CEA and cytology because of a low yield.14 Importantly, the minimal cyst fluid requirements (0.5 µL) for the targeted MS analysis enabled biomarker quantification for all lesions. Figure 3B details the diagnostic outcomes for the evaluated markers/methods for the entire validation cohort. MUC5AC plus MUC2 correctly classified lesions in 66 of 68 patients (97%), cytology in 47 of 68 (69%; P < .001 compared with MUC5AC plus MUC2), and CEA in 30 of 68 patients (44%; P < .001).
MUC5AC and Prostate Stem-Cell Antigen Identify HGD and Cancer in PCLs
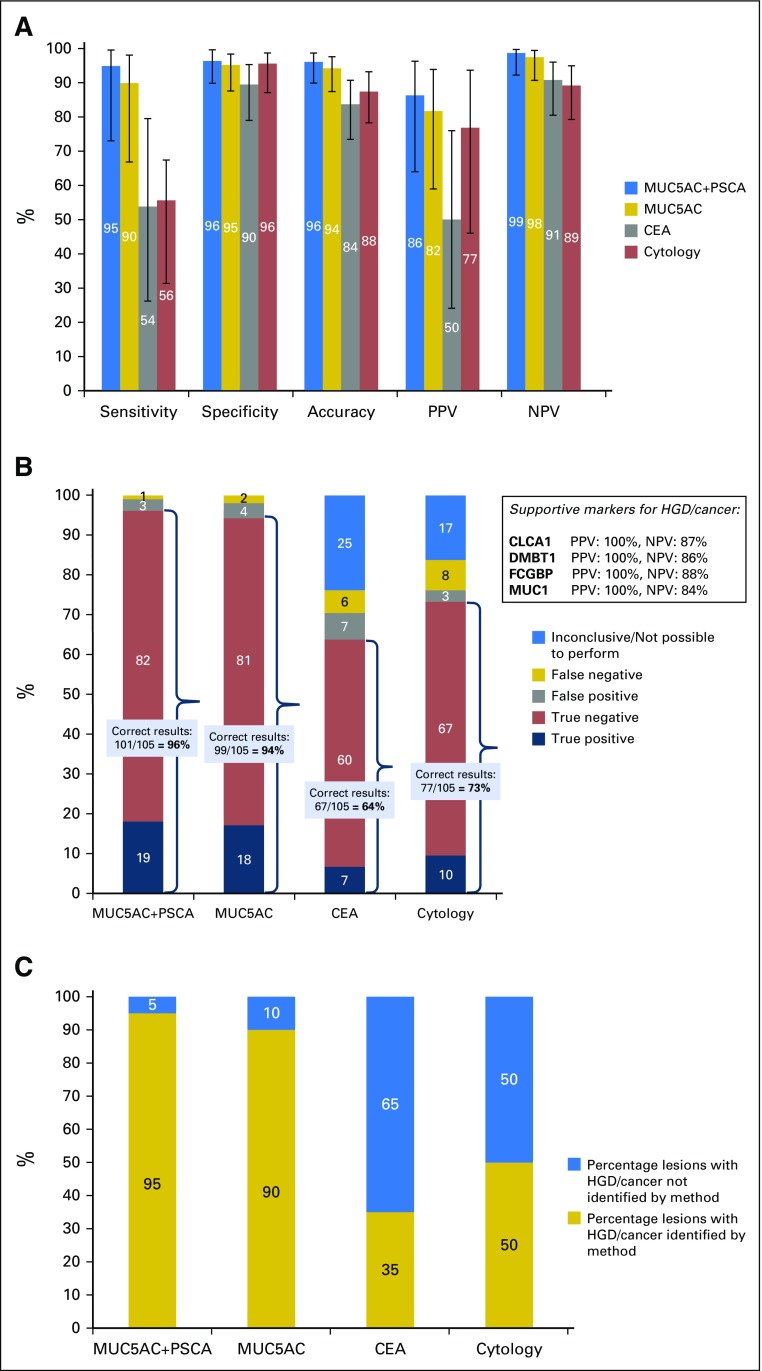
In the training cohort, the optimal biomarker panel for the detection of HGD/cancer was MUC5AC plus prostate stem-cell antigen (PSCA; cutoff, 12 fmol/µL; summed protein levels). PSCA levels were only considered when MUC5AC was present in the sample. The overall accuracy of this panel was 96% (95% CI, 90% to 99%); 94% in the validation cohort (Fig 4A; Data Supplement). In the entire study population, the sensitivity of MUC5AC plus PSCA for HGD/cancer was 95%, which significantly exceeded that of CEA (54%; P = .008) and cytology (56%, P = .007), whereas specificity was comparably high for all methods (Fig 4A23). Full results, by cohort, are presented in the Data Supplement.
Fig 4.
Identification of cystic lesions with high-grade dysplasia (HGD) or invasive cancer. Cutoff values were mucin-5AC (MUC5AC) plus prostate stem-cell antigen (PSCA), 12 fmol/µL cyst fluid (summed protein concentration levels); MUC5AC, 7.6 fmol/µL; and carcinoembryonic antigen (CEA), 1,000 ng/mL. PSCA levels were only considered if MUC5AC was present in the sample. One hundred five patients could be assessed for the presence of HGD/cancer, 70 from the training cohort and 35 from the validation cohort. The results are from the entire study population (training and validation cohorts). (A) Performance characteristics for the identification of HGD/malignant lesions; inconclusive results not included (entire study population). The sensitivity of MUC5AC plus PSCA was higher than that of CEA (P = .008) and cytology (P = .007; Fisher´s exact test). Error bars represent 95% CIs, calculated using the Wilson procedure,23 with correction for continuity. (B) Diagnostic outcomes for the identification of malignant/severely dysplastic cystic lesions (entire study population). Percentages are displayed on the y-axis and the number of individuals in labels on the stacked bar chart. The preferred panel for the identification of HGD/cancer, MUC5AC plus PSCA, gave a statistically significantly higher proportion of correct results than CEA (P < .001) and cytology (P < .001). Comparative statistical analysis was performed by Fisher´s exact test with Bonferroni correction for multiple comparisons (threshold for significance, 0.025). Supportive biomarkers for the identification of HGD/cancer, along with their positive and negative predictive values, are provided in a text box. (C) Proportion of cystic lesions with HGD/cancer identified by the different diagnostic methods. MUC5AC plus PSCA identified a statistically significantly higher proportion of HGD/malignant lesions than CEA (P < .001) and cytology (P = .003; Fisher´s exact test with Bonferroni correction). The results are from the entire study population (105 patients). CLCA1, calcium-activated chloride channel regulator 1; DMBT1, deleted in malignant brain tumors 1; FCGBP, IgGFc-binding protein; MUC1, mucin-1; NPV, negative predictive value; PPV, positive predictive value.
Again, the utility of cytology and CEA was compromised by a high proportion of inconclusive results. As shown in Figure 4B, MUC5AC plus PSCA provided correct assessments in 101 of 105 patients (96%), compared with 67 of 105 (64%) for CEA and 77 of 105 (73%) for cytology (P < .001 for both). In particular, MUC5AC plus PSCA identified 19 of 20 (95%) lesions with HGD/cancer, compared with 7 of 20 (35%) for CEA and 10 of 20 (50%) for cytology (P < .001 and P = .003; Fig 4C).
Clinical guidelines for the identification of malignant lesions rely primarily on imaging features, cyst diameter, and symptoms, and have been criticized for poor specificity.9,10,21,24 The sensitivity and specificity results of EUS morphology, cyst size (cutoff ≥ 3 cm) and the presence of pancreas-related symptoms for HGD/cancer in this study were, respectively, 50 and 94%, 55 and 39%, and 85 and 31%. In combination (with one positive result considered indicative of HGD/cancer), they reached a sensitivity of 90%, but with a specificity of merely 18% (Data Supplement).
MUC2 and PSCA May Be Used for Risk Stratification of Premalignant PCLs
Histologic specimens were reviewed by an expert pathologist (C.S.V.). For IPMNs, the epithelial subtype was determined to be gastric (best prognosis), intestinal (intermediate prognosis), or pancreatobiliary (worst prognosis).17 A fourth variant, oncocytic IPMN, is rare17 and was represented by only one patient (Data Supplement). As shown in the Data Supplement, PSCA levels were significantly elevated in pancreatobiliary-type IPMN and MUC2 in intestinal-type IPMN, compared with other subtypes (P = .007 and P = .002, respectively). Thus, these biomarkers may also be useful for risk-stratification of premalignant PCLs, the vast majority of which are IPMNs.
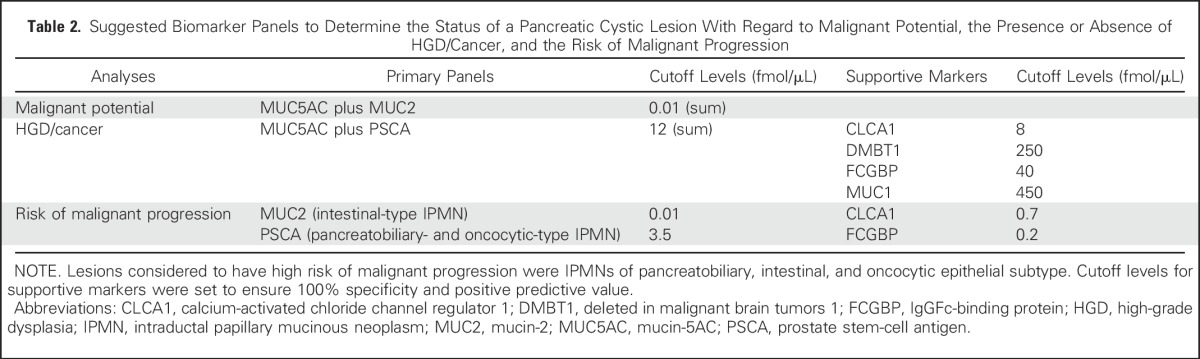
Table 2 lists the primary biomarker panels to identify malignant potential and HGD/cancer and to risk-stratify premalignant lesions. A number of supportive markers are also listed at cutoffs that provide 100% specificity. Supportive markers may be considered when the results of the primary panels are near cutoff levels or when the diagnosis remains ambiguous. Their performance characteristics are provided in the Data Supplement.
Table 2.
Suggested Biomarker Panels to Determine the Status of a Pancreatic Cystic Lesion With Regard to Malignant Potential, the Presence or Absence of HGD/Cancer, and the Risk of Malignant Progression
DISCUSSION
In this study, targeted proteomic analysis of three cyst fluid biomarkers, MUC5AC, MUC2, and PSCA, identified cystic pancreatic cancers and precursor lesions with high accuracy, significantly exceeding that of current diagnostic methods.
PCLs are frequently detected as incidentalomas on imaging.7,8 However, unlike renal and hepatic cysts, PCLs are generally a manifestation of an underlying inflammatory or neoplastic disease, and up to 50% have the potential to transform into pancreatic cancer.5,6 Still, because malignant transformation may not occur during the patient´s lifetime, careful evaluation is needed.15,16 If pancreatic cancer does develop, the prognosis is dismal.1 However, the risks and costs of unnecessary pancreatic surgery are substantial.25 Thus, the stakes for the accurate assessment of PCLs are high.
Unfortunately, state-of-the-art diagnostic methods, that is, EUS-FNA with cytology and cyst fluid CEA quantification, provide insufficient support for these difficult decisions.12,13 Not only is their diagnostic accuracy unsatisfactory, inconclusive results are also common.14 In this study, CEA could not be analyzed in nearly 40% (32 of 84; 38%) of premalignant and malignant tumors.
In response to this clinical problem, several studies have attempted to identify new biomarkers for premalignant/malignant PCLs, for example, KRAS and GNAS mutations, telomerase activity, microRNAs, and proteome alterations.26-33 So far, small sample sizes, lack of validation, doubtful clinical feasibility, and/or dearth of superiority over established methods have precluded clinical introduction of these assays. The scant cellular yield from cystic lesions presents a particular obstacle for genomic analyses.
In this prospective phase IIc study of nearly 150 patients, just three protein biomarkers proved sufficient to answer the most important diagnostic questions regarding PCLs: they correctly classified > 95% of lesions as benign, premalignant, or malignant/severely dysplastic. Two of these markers are secreted mucins, MUC5AC and MUC2. Mucins are densely O-glycosylated glycoproteins, which can be secreted or membrane-bound.34 Aberrant expression of both forms has been observed for several tumors, including pancreatic cancer.34,35 The mechanisms of de novo expression of secreted mucins in (pre)neoplastic lesions are unknown, but may involve epigenetic signaling.35 Mucin secretion by tumor cells may provide protection against antitumor immunity.34,36 The third marker, PSCA, is a membrane-bound protein of unknown function, postulated to participate in intracellular signaling.37 PSCA is overexpressed in pancreatic adenocarcinoma and, in conjunction with MUC5AC, accurately identified HGD/cancer in this study.38 Moreover, combined analysis of MUC2 and PSCA could detect high-risk IPMNs and therewith extended the diagnostic value of the assessment. All biomarkers in this study are either membrane-bound (with potential to be shed into the cyst fluid) or secreted proteins and thus directly reflect aberrations of the dysplastic epithelium.
Targeted MS, as performed in this study, allows for absolute quantification of low-abundant biomarkers.18 Minimal amounts of cyst fluid (0.5 µL) were sufficient for analysis, approximately 1,000 times less than the volume required for conventional CEA quantification. Thus, unlike traditional methods, targeted MS gave conclusive results for all lesions from which cyst fluid was obtained. The analysis was reproducible, the preparation simple, and the focus on selected peptides allows for high-throughput analysis.18 Consequently, the method fulfils basic requirements for clinical feasibility. Potential challenges for its large-scale implementation include analysis costs and education of personnel. Still, similar mass spectrometers are widely available in universities and hospital laboratories. Reagents and standards could be produced as kits, keeping costs low, and the targeted approach along with dedicated analytic software would eliminate the need for complex data processing. Moreover, we believe that targeted MS, given its advantages over antibody-based assays—including the ability to simultaneously analyze many biomarkers—is likely to rapidly evolve into a widespread clinical tool. Thus, the design of our method could potentially serve as a blueprint for the development of similar analytical platforms for other conditions.
Our investigation has some limitations, including its basic design as a single-center, phase II diagnostic study. Additional validation through randomized controlled multicenter trials is warranted.39 Certain precancerous/cancerous lesions may be missed by the method, for example, solid pseudopapillary neoplasms and cystic endocrine neoplasms, which were excluded from this study. These rare entities can be readily identified through their unique cytologic/immunocytochemical characteristics.40,41 Patients with IPMNs may also be at increased risk for concomitant pancreatic adenocarcinoma developing from separate microscopic/macroscopic precursors elsewhere in the organ.5,9,42,43 In the absence of cystic components that can be targeted by FNA, these neoplasms would be beyond the scope of our analysis. Finally, to avoid selection bias, the study was not limited to patients who underwent surgery. Diagnostic assessments are typically based on structured follow-up and evaluation by a multidisciplinary board.
The increasing detection of PCLs offers an unprecedented possibility for timely intervention and prevention of pancreatic cancer. However, this opportunity has been deterred by a lack of robust diagnostic methods. Here, we present a high-throughput analysis that can identify and distinguish between cystic precursor lesions and manifest pancreatic cancer/HGD with high accuracy. Thus, it is expected to improve the identification of early-stage tumors, which is crucial to reduce pancreatic cancer mortality.
ACKNOWLEDGMENT
The authors thank Mehmet Akif Demir, MD, Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden, for cytology assessments and Lisbeth Eklund, Department of Gastroenterology, Sahlgrenska University Hospital, Gothenburg, Sweden, for assistance with sample collection.
Footnotes
Supported by the Swedish Cancer Foundation, BioCARE research foundation, Swedish Research Council (Grant No. 7461), Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgrenska University Hospital, Wilhelm and Martina Lundgren’s Foundation, Health and Medical Care Committee of the Regional Executive Board, Region Västra Götaland (Grants No. VGFOUREG-564381 and VGFOUREG-144591), and Swedish Society of Medicine (Grants No. SLS-404261 and SLS-325061). The sponsors had no involvement in the design/conduct of the study, nor in the analysis and interpretation of data or the preparation, review, and approval of the manuscript.
Presented at Digestive Disease Week, Chicago, IL, May 6-9, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Karolina S. Jabbar, Riadh Sadik, Gunnar C. Hansson
Financial support: Riadh Sadik, Gunnar C. Hansson
Administrative support: Gunnar C. Hansson
Provision of study materials or patients: Riadh Sadik
Collection and assembly of data: Karolina S. Jabbar, Liisa Arike, Riadh Sadik
Data analysis and interpretation: Karolina S. Jabbar, Liisa Arike, Caroline S. Verbeke
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Karolina S. Jabbar
No relationship to disclose
Liisa Arike
No relationship to disclose
Caroline S. Verbeke
No relationship to disclose
Riadh Sadik
Honoraria: Ferring, Abbvie, Olympus, Boston Scientific, Cook Medical
Travel, Accommodations, Expenses: Cook Medical
Gunnar C. Hansson
No relationship to disclose
REFERENCES
- 1.Kamisawa T, Wood LD, Itoi T, et al. : Pancreatic cancer. Lancet 388:73-85, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Matthaei H, Schulick RD, Hruban RH, et al. : Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol 8:141-150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto MI, Hruban RH, Fishman EK, et al. : Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142:796-804, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strobel O, Rosow DE, Rakhlin EY, et al. : Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 138:1166-1177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell JJ, Fernández-del Castillo C: Pancreatic cystic neoplasms: Management and unanswered questions. Gastroenterology 144:1303-1315, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Stark A, Donahue TR, Reber HA, et al. : Pancreatic cyst disease: A review. JAMA 315:1882-1893, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Zhang XM, Mitchell DG, Dohke M, et al. : Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology 223:547-553, 2002 [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira PB, Puchnick A, Szejnfeld J, et al. : Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One 10:e0121317, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Fernández-del Castillo C, Adsay V, et al. : International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12:183-197, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Del Chiaro M, Verbeke C, Salvia R, et al. : European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 45:703-711, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Visser BC, Yeh BM, Qayyum A, et al. : Characterization of cystic pancreatic masses: Relative accuracy of CT and MRI. AJR Am J Roentgenol 189:648-656, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Thosani N, Thosani S, Qiao W, et al. : Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: A systematic review and meta-analysis. Dig Dis Sci 55:2756-2766, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. : Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 126:1330-1336, 2004 [DOI] [PubMed] [Google Scholar]
- 14.de Jong K, Poley JW, van Hooft JE, et al. : Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy 43:585-590, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Tanno S, Nakano Y, Nishikawa T, et al. : Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: Long-term follow-up results. Gut 57:339-343, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Salvia R, Crippa S, Falconi M, et al. : Branch-duct intraductal papillary mucinous neoplasms of the pancreas: To operate or not to operate? Gut 56:1086-1090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa T, Hatori T, Fujita I, et al. : Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut 60:509-516, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Gillette MA, Carr SA: Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat Methods 10:28-34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugge WR: Should all pancreatic cystic lesions be resected? Cyst-fluid analysis in the differential diagnosis of pancreatic cystic lesions: A meta-analysis. Gastrointest Endosc 62:390-391, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gallien S, Duriez E, Crone C, et al. : Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol Cell Proteomics 11:1709-1723, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Chari S, Adsay V, et al. : International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6:17-32, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Hulley SB, Cummings SR, Browner WS, et al. Designing clinical research: An epidemiologic approach (ed 4). Philadelphia, PA, Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 23. Wilson EB: Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 22:209-212, 1927. [Google Scholar]
- 24.Goh BK, Lin Z, Tan DM, et al. : Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery 158:1192-1202, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Ho CK, Kleeff J, Friess H, et al. : Complications of pancreatic surgery. HPB 7:99-108, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalid A, Zahid M, Finkelstein SD, et al. : Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc 69:1095-1102, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Kanda M, Knight S, Topazian M, et al. : Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 62:1024-1033, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrell JJ, Toste P, Wu N, et al. : Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol 108:1352-1359, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hata T, Dal Molin M, Suenaga M, et al. : Cyst fluid telomerase activity predicts the histologic grade of cystic neoplasms of the pancreas. Clin Cancer Res 22:5141-5151, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corcos O, Couvelard A, Dargère D, et al. : Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 41:169-174, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Cuoghi A, Farina A, Z’graggen K, et al. : Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts. J Proteome Res 10:2664-2670, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Ke E, Patel BB, Liu T, et al. : Proteomic analyses of pancreatic cyst fluids. Pancreas 38:e33-e42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabbar KS, Verbeke C, Hyltander AG, et al. : Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Natl Cancer Inst 106:djt439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kufe DW: Mucins in cancer: Function, prognosis and therapy. Nat Rev Cancer 9:874-885, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent A, Perrais M, Desseyn JL, et al. : Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene 26:6566-6576, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Yamazoe S, Tanaka H, Sawada T, et al. : RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res 29:53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saeki N, Gu J, Yoshida T, et al. : Prostate stem cell antigen: A Jekyll and Hyde molecule? Clin Cancer Res 16:3533-3538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argani P, Rosty C, Reiter RE, et al. : Discovery of new markers of cancer through serial analysis of gene expression: Prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res 61:4320-4324, 2001 [PubMed] [Google Scholar]
- 39.Gluud C, Gluud LL: Evidence based diagnostics. BMJ 330:724-726, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon WJ, Daglilar ES, Pitman MB, et al. : Cystic pancreatic neuroendocrine tumors: Endoscopic ultrasound and fine-needle aspiration characteristics. Endoscopy 45:189-194, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Pettinato G, Di Vizio D, Manivel JC, et al. : Solid-pseudopapillary tumor of the pancreas: A neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol 27:325-334, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi K, Kanemitsu S, Hatori T, et al. : Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40:571-580, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Pea A, Yu J, Rezaee N, et al. : Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg 266:133-141, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]