Abstract
Purpose
We evaluated the relationship between prostate-specific antigen (PSA) and overall survival in the context of a prospectively randomized clinical trial comparing androgen-deprivation therapy (ADT) plus docetaxel with ADT alone for initial metastatic hormone-sensitive prostate cancer.
Methods
We performed a landmark survival analysis at 7 months using the E3805 Chemohormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) database (ClinicalTrials.gov identifier: NCT00309985). Inclusion required at least 7 months of follow-up and PSA levels at 7 months from ADT initiation. We used the prognostic classifiers identified in a previously reported trial (Southwest Oncology Group 9346) of PSA ≤ 0.2, > 0.2 to 4, and > 4 ng/mL.
Results
Seven hundred nineteen of 790 patients were eligible for this subanalysis; 358 were treated with ADT plus docetaxel, and 361 were treated with ADT alone. Median follow-up time was 23.1 months. On multivariable analysis, achieving a 7-month PSA ≤ 0.2 ng/mL was more likely with docetaxel, low-volume disease, prior local therapy, and lower baseline PSAs (all P ≤ .01). Across all patients, median overall survival was significantly longer if 7-month PSA reached ≤ 0.2 ng/mL compared with > 4 ng/mL (median survival, 60.4 v 22.2 months, respectively; P < .001). On multivariable analysis, 7-month PSA ≤ 0.2 and low volume disease were prognostic of longer overall survival (all P < 0.01). The addition of docetaxel increased the likelihood of achieving a PSA ≤ 0.2 ng/mL at 7 months (45.3% v 28.8% of patients on ADT alone). Patients on ADT alone who achieved a 7-month PSA ≤ 0.2 ng/mL had the best survival and were more likely to have low-volume disease (56.7%).
Conclusion
PSA ≤ 0.2 ng/mL at 7 months is prognostic for longer overall survival with ADT for metastatic hormone-sensitive prostate cancer irrespective of docetaxel administration. Adding docetaxel increased the likelihood of a lower PSA and improved survival.
INTRODUCTION
Some patients with metastatic prostate cancer have a prolonged survival of approximately 5 or more years from the diagnosis of metastasis.1-4 This extended survival is in part a result of the advent of multiple effective second-line therapies for castration-resistant disease that, when used in sequence, contribute to prolonging life. These include taxane-based chemotherapies, next-generation antiandrogens such as enzalutamide, biosynthesis inhibitors such as abiraterone acetate, bone metastasis–homing radiation with radium-223, and an autologous dendritic cell vaccine sipuleucel-T.5-12 Given that these drugs yield only incremental survival benefits, attempts to improve the outcomes of this largely fatal disease have evolved to move these agents forward to the hormone-sensitive setting. In this setting, the disease burden is lower, and there may be fewer resistance mechanisms at play to evade elimination. Indeed, as of 2017, we now have multiple lines of therapy with level 1 evidence showing that both docetaxel and abiraterone acetate increase survival and disease control when added to luteinizing hormone–releasing hormone agonists at the diagnosis of metastatic hormone-sensitive disease.1-4
Three pivotal studies have investigated the use of docetaxel for metastatic hormone-sensitive prostate cancer (mHSPC). Although the first study, Groupe d'Étude des Tumeurs Uro-Génitales-Association Francaise d'Urologie (GETUG-AFU 15), did not show a benefit to adding docetaxel at the time of mHSPC, the subsequent two larger studies, Chemohormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) and Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE), have demonstrated a significant overall survival advantage to early docetaxel administration.3,4,13
CHAARTED was a phase III, open-label study that randomly assigned 790 patients to receive either androgen-deprivation therapy (ADT) plus six cycles of docetaxel or ADT alone. It revealed a greater than 12-month overall survival benefit to adding docetaxel to ADT compared with ADT alone. Integrated into the stratification and analysis of the study was characterization of outcomes based on volume of disease. High-volume disease was defined by the presence of four or more bone lesions with at least one beyond the vertebral bodies or pelvis or any site of visceral metastasis. A planned subgroup analysis of CHAARTED based on volume of disease showed that the survival advantage of early chemotherapy administration was restricted to patients with high-volume cancer (hazard ratio, 0.6).14 As such, early docetaxel administration in combination with ADT is an option in 2017 in patients with high-volume mHSPC who are fit for chemotherapy.15
Prior work from the Southwest Oncology Group (SWOG) 9346 trial, a phase III study that compared continuous versus intermittent androgen blockade in treatment-naïve, hormone-sensitive disease, revealed that patients who achieved a prostate-specific antigen (PSA) ≤ 0.2 ng/mL at 7 months had a significantly longer overall survival compared with patients who did not achieve this milestone.16 Similarly, in that same study, patients whose PSA declined to between 0.3 and 4 ng/mL had improved outcomes compared with those whose PSA remained > 4 ng/mL at 7 months after ADT initiation. Thus, failure to achieve a PSA ≤ 0.2 ng/mL may identify patients who might benefit from earlier intensification of therapy. In addition to enhancing clinical discussions and improving patient morale, the availability of a biomarker that could detect clinical benefit (or lack thereof) earlier than our current longer term time-to-event end points such as time to progression or overall survival would aid in trial design and drug development.
The current treatment armamentarium for metastatic prostate cancer offers multiple nonchemotherapeutic agents that improve survival, and given the results of the CHAARTED and STAMPEDE trials, we have witnessed a transition to using docetaxel earlier in the metastatic disease course over the past few years. We sought to evaluate whether achieving an optimal 7-month PSA ≤ 0.2 ng/mL after treatment initiation remained prognostic of superior overall survival when docetaxel was added to ADT for initial mHSPC treatment using the CHAARTED database of patients with mHSPC with its prospectively cataloged clinical outcomes.
METHODS
With institutional review board and ethics committee approval, we interrogated the CHAARTED Eastern Cooperative Oncology Group 3805 database (ClinicalTrials.gov identifier: NCT00309985) of 790 patients with mHSPC enrolled from July 2006 to December 2012. Inclusion in this planned secondary analysis of on-therapy PSA changes required the following: at least 7 months of follow-up after ADT initiation (if ADT started before random assignment) or after random assignment (if ADT started after random assignment) and PSA levels at 7 months from ADT initiation. Data on baseline clinical and demographic characteristics, such as age at ADT initiation, race, Gleason score, baseline PSA, disease volume, presence of visceral disease, prior local therapy, and presence of bone pain or weight loss at baseline, were procured. Patients were allowed to start hormonal therapy up to 120 days before random assignment.
Statistical Analyses
Patient data from the original 2015 published analysis4 were used. Landmark survival analyses were performed at 7 months after ADT initiation. For patients who started hormonal therapy before random assignment, overall survival was defined as the time from 7 months after ADT initiation to death or date when last known to be alive. For patients who started hormonal therapy after random assignment, overall survival was defined as the time from 7 months after randomization to death or date last known to be alive in follow-up. Landmark survival analyses were performed at 7 months after ADT initiation. The SWOG 9346 PSA cut points of ≤ 0.2, > 0.2 to 4, and > 4 ng/mL were used a priori.16 Patients who experienced progression or received non-protocol therapy (NPT) before 7 months were included in the 7-month PSA > 4 ng/mL group.
The Fisher’s exact test and Kruskal-Wallis test were used to evaluate the associations between baseline patient characteristics and PSA status at 7 months. Multivariable logistic regression models with stepwise selection were constructed to predict 7-month PSA ≤ 0.2 using the significant covariates from the univariable analysis. Multivariable proportional hazards regression models stratified by stratification factors at random assignment were developed for overall survival using stepwise selection. All variables associated with PSA ≤ 0.2 ng/mL were included in the model selection, and treatment arm was included in the final model regardless of significance.
RESULTS
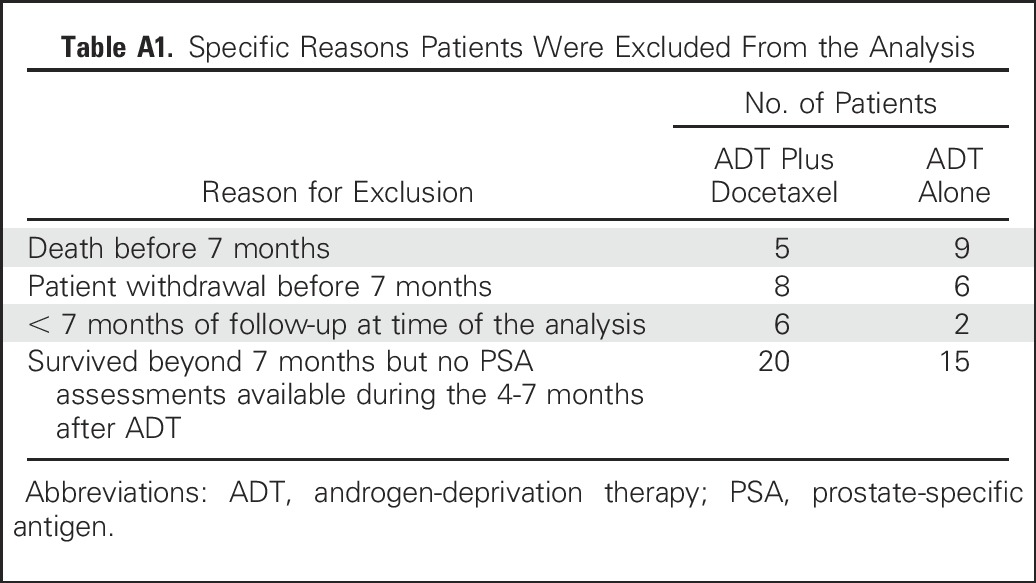
Of the 790 patients enrolled onto the CHAARTED study, 719 patients were eligible for this landmark analysis; 358 patients were treated with ADT plus docetaxel, and 361 patients were treated with ADT alone (Table 1). Patients were excluded if they had less than 7 months of follow-up after starting ADT (n = 36) or no PSA assessments during the 4 to 7 months after ADT initiation (n = 35; Appendix Table A1, online only). Patients who experienced progression before 7 months were included in the 7-month PSA > 4 ng/mL group. Patients who did not experience progression and did not have PSA assessments during months 4 to 7 were excluded from the analysis. The protocol did not specify a time frame for starting ADT after random assignment. From the database, 92 of 96 patients who began ADT after random assignment started it within 10 days of random assignment. Median follow-up, which started after 7 months of ADT, was 23.1 months (range, 0.2 to 72.8 months).
Table 1.
Patient Characteristics by 7-Month PSA Level

Among all patients, median overall survival was significantly longer if PSA at 7 months reached ≤ 0.2 ng/mL compared with > 4 ng/mL (P < .001; Table 2). Across the three different cut points identified in SWOG 9346, the lower the PSA at 7 months, the longer was the survival. Median overall survival by 7-month PSA (all patients; Fig 1A and Table 2) was 60.4 months with PSA ≤ 0.2 ng/mL, 51.9 months with PSA of 0.3 to 4 ng/mL, and 22.2 months with PSA > 4 ng/mL. This trend held true across each of the study arms with ADT plus docetaxel (Fig 1B) or ADT alone (Fig 1C). The proportion of patients with visceral disease was greatest among the patients with a 7-month PSA > 4.0 ng/mL (20.9%; 50 of 239 patients) and significantly lower in the groups with PSA ≤ 0.2 (12%; 32 of 266 patients) and PSA of 0.2 to 4 ng/mL (14.1%; 30 of 213 patients; P = .02).
Table 2.
OS by PSA Status at 7 Months
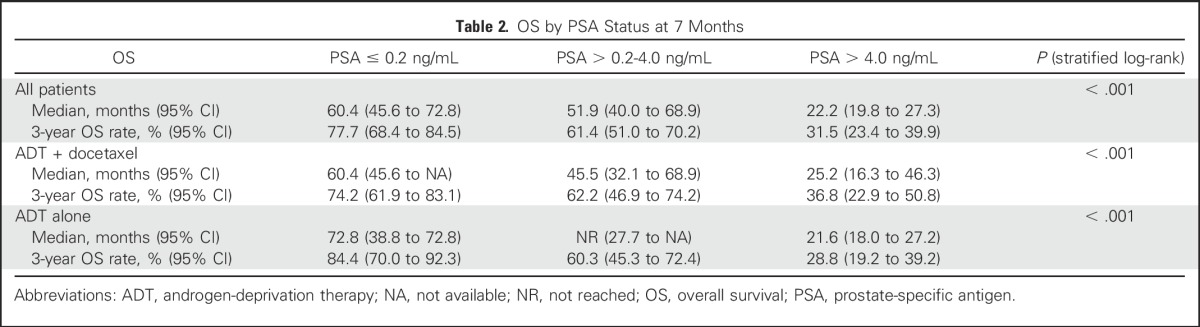
Fig 1.
Kaplan-Meier analysis of overall survival by prostate-specific antigen (PSA) level at 7 months in (A) patients on ADT alone, (B) patients on androgen-deprivation therapy (ADT) plus docetaxel, and (C) all patients. NPT, non-protocol therapy; NR, not reached; PD, progressive disease.
On multivariable analysis, achieving a PSA of ≤ 0.2 ng/mL at 7 months was more likely if patients had received docetaxel, had low-volume disease, had received prior local therapy, had started ADT after random assignment, and had lower baseline PSA levels (continuous variable; all P ≤ .01; Table 3).
Table 3.
Multivariable Logistic Regression Model Predicting a PSA ≤ 0.2 ng/mL at 7 Months

On multivariate analysis, longer overall survival was associated with PSA ≤ 0.2 ng/mL at 7 months, low-volume disease, and higher baseline PSA (all P < .01; Table 4) but not docetaxel use. Despite the latter statistical finding, there was a higher percentage of patients who achieved PSA ≤ 0.2 ng/mL in the docetaxel arms regardless of volume status (Table 5). Of the ADT plus docetaxel patients, 45.3% achieved a PSA ≤ 0.2 ng/mL at 7 months compared with 28.8% of patients on ADT alone. More importantly than whether PSA reaches this level is whether achieving this landmark correlates with improved survival. In an exploratory subset analysis evaluating how 7-month PSA correlated with overall survival, patients with high-volume disease had a worse median overall survival if they did not experience a 7-month PSA ≤ 0.2 ng/mL. Overall, patients with high-volume disease who received docetaxel had a higher median overall survival than patients on ADT alone, and patients who achieved a 7-month PSA ≤ 0.2 ng/mL had a longer median overall survival than those whose 7-month PSA remained > 0.2 ng/mL (Table 5). In patients with high-volume disease who received ADT alone, median overall survival was 40.1 months if their PSA hit the landmark, compared with 25.4 months if PSA remained > 0.2 ng/mL at 7 months. The patients with high-volume disease who received docetaxel had a median overall survival time of 60.4 months if 7-month PSA was ≤ 0.2 ng/mL and 45.4 months if PSA was > 0.2 ng/mL. The median overall survival of patients with low-volume disease had not been reached in the majority of subsets.
Table 4.
Multivariable Proportional Hazards Model for Risk of Death After 7 Months of ADT

Table 5.
Distribution of Median OS After 7 Months of ADT by Treatment Arm, Disease Volume, and PSA Status at 7 Months

DISCUSSION
In the wake of the overall survival benefit demonstrated by the modern CHAARTED and STAMPEDE studies, there is now substantial rationale to support the use of docetaxel with ADT in the hormone-sensitive state, especially in fit patients with high-volume metastatic prostate cancer. The SWOG 9346 study, which was conducted in the 1990s, tested continuous versus intermittent androgen blockade in patients with mHSPC and revealed that patients with 7-month PSA ≤ 0.2 ng/mL had significantly longer survival than patients who did not achieve this landmark.16 We sought to evaluate whether this potential on-therapy biomarker remained prognostic when docetaxel was added to ADT in the current treatment era in which multiple systemic agents are available that improve survival. CHAARTED also offered the first opportunity in the mHSPC setting to evaluate this landmark in the context of a randomized clinical trial in which one arm showed definite improved overall survival and to compare the percentage of patients who achieved an ideal 7-month PSA and survival associations between the different treatments.
We hypothesized that achieving a 7-month PSA ≤ 0.2 ng/mL would remain prognostic for improved overall survival when docetaxel chemotherapy is added to ADT at the time of mHSPC. Using a more contemporary data set obtained from the CHAARTED study, we observed that achieving a PSA ≤ 0.2 ng/mL at 7 months correlated with longer overall survival with ADT in patients with mHSPC, whether administered alone or with docetaxel. Further, when we incorporated the use of docetaxel into the analysis, its combination with ADT increased the likelihood of achieving a lower PSA and subsequent improved survival. Thus, while achieving a PSA ≤ 0.2 ng/mL is prognostic, it remains a post-treatment variable and cannot direct upfront who should receive early docetaxel. Although we have shown that early docetaxel administration increases the likelihood of attaining this optimal biomarker state, whether the agents proven to prolong overall survival when added to ADT (ie, abiraterone acetate or docetaxel) should be reserved only for patients who do not achieve a 7-month PSA ≤ 0.2 ng/mL has not yet been determined. In theory this tiered strategy might avoid therapy for some, however, it may also miss the possible benefit of attacking the cancer clones at an earlier stage when they are more vulnerable and the associated survival benefits. Indeed, a phase II study of patients with mHSPC whose PSA did not decrease to ≤ 4 ng/mL after 4 to 6 months of initial ADT showed that the addition of abiraterone acetate with prednisone can induce a PSA reduction to ≤ 0.2 ng/mL in some patients.17
The recently reported findings of the LATITUDE and STAMPEDE phase III studies revealed a similar benefit to administering abiraterone acetate earlier at the diagnosis of hormone-sensitive metastatic disease.1,2 It will be interesting to see whether attaining a PSA ≤ 0.2 ng/mL remains prognostic in these studies and in what time frame. Teasing out when to use docetaxel in light of the recent positive abiraterone studies and the upcoming enzalutamide studies (if positive) is the subject of much ongoing discussion in the field, and ultimate recommendations will likely take into account toxicity and administration profiles, quality of life, and financial repercussions.
Although PSA is an easy, inexpensive, and long-established clinical test, predictive pretreatment biomarkers are needed. By interrogating the tumor genetics (RNA and DNA), germline DNA, and circulating proteins and androgens from patients enrolled onto CHAARTED, we may identify more sophisticated biomarkers that are predictive of benefit with early docetaxel for patients and warrant its toxicity.
There are limitations inherent in any retrospective analysis. It is likely that the CHAARTED study as a whole was subject to selection bias in terms of which patients were referred for a study that included chemotherapy. This theory is possibly supported by the younger median age in the CHAARTED study (63 years) versus the LATITUDE and STAMPEDE (abiraterone arm) studies (68 and 67 years, respectively). The analysis was also limited to patients with at least 7 months of follow-up after ADT initiation and patients who had a 7-month PSA level available. These criteria may have biased the results somewhat toward patients with a better risk profile because they survived at least 7 months after ADT and/or were not lost to follow-up in that same time frame. However, only 36 patients of the 790 patients enrolled on CHAARTED were excluded for these reasons. In addition, patients with rapidly progressive disease do not need an intermediate clinical end point because their disease or other comorbidities will already be evident.
In conclusion, attaining a PSA ≤ 0.2 ng/mL at 7 months on ADT remains prognostic for improved overall survival in the more modern era, and the addition of docetaxel to androgen blockade increases the likelihood of achieving that milestone. Given the long times to an overall survival end point from the time of diagnosis of mHSPC, identification of an intermediate clinical end point for drug development is crucial to move the field forward in more tangible time frames. Our work suggests that 7-month PSA ≤ 0.2 ng/mL is worthy of prospective study as an intermediate clinical end point, and if proven, it could be a reasonable surrogate for overall survival in future trials. In addition, further prospective study is needed to investigate whether intensification of therapy in patients who do not reach a 7-month PSA ≤ 0.2 ng/mL is warranted and whether it will improve clinical outcomes. Future work modeling PSA dynamics, degree of decline, and different PSA time points and their association with improved clinical outcomes is planned. On-therapy biomarkers that are associated with improved outcomes are critical to improving patient mindset and to optimally identify patients for testing alternative therapies when the ideal prognostic metric is not achieved.
Appendix
Table A1.
Specific Reasons Patients Were Excluded From the Analysis
Footnotes
Sanofi provided docetaxel for early use, and financial grant support was provided by the National Cancer Institute Cancer Therapy Evaluation Program and the Eastern Cooperative Oncology Group (ECOG)–American College of Radiology Imaging Network (ACRIN). This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group co-chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following grant numbers: CA180820, CA180794, CA180795, CA180790, CA180802, CA180821, CA180833, CA180847, CA180853, CA180867, CA180888, CA180801, and CA189829.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Clinical trial information: NCT00309985.
AUTHOR CONTRIBUTIONS
Conception and design: Lauren C. Harshman, Yu-Hui Chen, Maha Hussain, Christopher J. Sweeney
Financial support: Christopher J. Sweeney
Administrative support: Christopher J. Sweeney
Provision of study materials or patients: Nicholas J. Vogelzang, Joel Picus, Christopher J. Sweeney
Collection and assembly of data: Yu-Hui Chen, Christopher J. Sweeney
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Seven-Month Prostate-Specific Antigen Is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated With Androgen Deprivation With Or Without Docetaxel
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Lauren C. Harshman
Consulting or Advisory Role: Dendreon, Medivation/Astellas, Pfizer, National Comprehensive Cancer Network, Genentech, Theragene, KEW Group, Corvus Pharmaceuticals, Merck, Applied Clinical Education, Physician Education Resource
Research Funding: Medivation/Astellas (Inst), Bayer (Inst), Sotio (Inst), Genentech (Inst), Dendreon/Valient (Inst), Bristol-Myers Squibb (Inst), Takeda (Inst), Merck (Inst), Janssen Oncology (Inst)
Travel, Accommodations, Expenses: Bayer
Yu-Hui Chen
Employment: Constellation Pharmaceuticals (I)
Glenn Liu
Leadership: AIQ Solutions
Stock or Other Ownership: AIQ Solutions
Consulting or Advisory Role: Sanofi
Research Funding: Johnson & Johnson (Inst), Novartis (Inst), Millennium (Inst), Cellectar (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: US 9161720 B2: Quantitative Evaluation of Total Tumor Burden Using Functional and Anatomic Imaging
Other Relationship: AIQ Solutions
Michael A. Carducci
Consulting or Advisory Role: Astellas Pharma, Churchill Pharmaceuticals, Abbvie, Genentech, Pfizer
Research Funding: Bristol-Myers Squibb (Inst), Pfizer (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
David Jarrard
Patents, Royalties, Other Intellectual Property: Biomarker patent pending
Robert Dreicer
Consulting or Advisory Role: Astellas Pharma, Asana Biosciences, Exelixis, AstraZeneca, Bristol-Myers Squibb, Exelixis, Genentech, EMD Serono, Genzyme
Research Funding: Genentech (Inst), Asana Biosciences (Inst)
Noah Hahn
Consulting or Advisory Role: Bristol-Myers Squibb, Oncogenex, AstraZeneca/MedImmune, Pieris Pharmaceuticals, Inovio Pharmaceuticals, Genentech, Health Advances, Merck, Rexahn Pharmaceuticals, Seattle Genetics
Research Funding: Novartis (Inst), Genentech (Inst), Mirati Therapeutics (Inst), Oncogenex (Inst), Merck (Inst), Heat Biologics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Principa Biopharma (Inst), Merck (Inst), Acerta Pharma (Inst)
Travel, Accommodations, Expenses: Merck, Seattle Genetics, AstraZeneca, Genentech, Bristol-Myers Squibb, Pieris Pharmaceuticals
Jorge A. Garcia
Consulting or Advisory Role: Sanofi, Pfizer, Bayer, Eisai, Exelixis, Medivation, Genentech
Speakers' Bureau: Bayer, Sanofi, Medivation/Astellas, Genentech
Research Funding: Pfizer (Inst), Astellas Pharma (Inst), Orion Pharma GmbH (Inst), Bayer (Inst), Janssen Oncology (Inst), Genentech (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: Pfizer, Bayer, Sanofi, Exelixis, Eisai, Medivation/Astellas, Genentech
Maha Hussain
Honoraria: Sanofi, Onclive
Research Funding: Genentech (Inst), Pfizer (Inst), Bayer (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Title: Systems and Methods for Tissue Imaging, 3676 Our File: Serial No.: UM-14437/US-1/PRO 60/923,385 UM-14437/US-2/ORD 12/101,753US 8,185,186 (US patent No.), EP 08745653.9 (EP application No.) CA 2683805 (Canadian application No., pending), US 13/362,500 (US application No., pending), continuation application of US 8,185,186; Title: Method of Treating Cancer, Docket No.: Serial No.: 224990/10-016P2/311733 61/481/671, application filed on 5/2/2011
Travel, Accommodations, Expenses: Sanofi
Daniel Shevrin
Honoraria: Sanofi
Speakers' Bureau: Bayer
Mario Eisenberger
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Sanofi, Pfizer
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi, Pfizer
Manish Kohli
No relationship to disclose
Elizabeth R. Plimack
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Acceleron Pharma, Genentech, AstraZeneca/MedImmune, Synergene, Acceleron Pharma, Eli Lilly, Horizon Pharma, Pfizer, Inovio Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), Acceleron Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Eli Lilly (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst), Peloton Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No.: 14/588,503, filed 1/2/2015 (Inst), US Patent No.: 15/226,474 filed 7/1/2015 (Inst)
Matthew Cooney
Speakers' Bureau: Potomac Center for Medical Education
Nicholas J. Vogelzang
Stock or Other Ownership: Caris Life Sciences
Honoraria: UpToDate, Pfizer
Consulting or Advisory Role: Amgen, Cerulean Pharma, Pfizer, Bayer, Genentech, Churchill Pharmaceuticals, Heron, AstraZeneca, Caris Life Sciences, Fujifilm, Tolero Pharmaceuticals
Speakers' Bureau: Bayer, Sanofi, Genentech, Bristol-Myers Squibb, Exelixis
Research Funding: US Oncology (Inst), Viamet Pharmaceuticals (Inst), Endocyte (Inst), Merck (Inst), Kintor (Inst)
Travel, Accommodations, Expenses: Genentech, US Oncology, Pfizer, Bayer/Onyx, Exelixis, AstraZeneca/MedImmune
Joel Picus
No relationship to disclose
Robert Dipaola
No relationship to disclose
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech, AstraZeneca, Pfizer
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Sotio (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix, Parthenolide, Dimethylaminoparthenolide; Exelixis: Abiraterone plus cabozantinib combination
REFERENCES
- 1.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 2.James ND, de Bono JS, Spears MR, et al. : Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 377:338-351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James ND, Sydes MR, Clarke NW, et al. : Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163-1177, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney CJ, Chen YH, Carducci M, et al. : Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 373:737-746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono JS, Oudard S, Ozguroglu M, et al. : Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376:1147-1154, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, et al. : Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411-422, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, et al. : Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213-223, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Tannock IF, de Wit R, Berry WR, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502-1512, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gravis G, Boher JM, Joly F, et al. : Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: Impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol 70:256-262, 2016 [DOI] [PubMed] [Google Scholar]
- 14. Sweeney C, Chen YH, Liu G, et al: Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial. Ann Oncol 27:720PD, 2016 (suppl 6) [Google Scholar]
- 15. National Comprehensive Cancer Network: NCCN Guidelines Version 2.2017: Prostate Cancer. https://www.nccn.org/professionals/physician_gls/default.aspx#prostate. [DOI] [PubMed]
- 16.Hussain M, Tangen CM, Higano C, et al. : Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 24:3984-3990, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Flaig TW, Plets M, Hussain MHA, et al. : Abiraterone acetate for metastatic prostate cancer in patients with suboptimal biochemical response to hormone induction. JAMA Oncol 3:e170231, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]



