Abstract
The limited repair potential of human articular cartilage contributes to development of debilitating osteoarthritis and remains a great clinical challenge. This has led to evolution of cartilage treatment strategies from palliative to either reconstructive or reparative methods in an attempt to delay or “bridge the gap” to joint replacement. Further development of tissue engineering-based cartilage repair methods have been pursued to provide a more functional biological tissue. Currently, tissue engineering of articular cartilage has three cornerstones; a cell population capable of proliferation and differentiation into mature chondrocytes, a scaffold that can host these cells, provide a suitable environment for cellular functioning and serve as a sustained-release delivery vehicle of chondrogenic growth factors and thirdly, signaling molecules and growth factors that stimulate the cellular response and the production of a hyaline extracellular matrix (ECM). The aim of this review is to summarize advances in each of these three fields of tissue engineering with specific relevance to surgical techniques and technical notes.
Introduction
The limited repair potential of human articular cartilage contributes to development of debilitating osteoarthritis and is a great clinical challenge. Cartilage repair strategies have evolved from “palliative” methods (debridement and lavage, abrasion chondroplasty) to what might be called the 3 ”R” paradigm: reconstruction, repair and replacement. Reconstruction aims at restoring the contour of the articular surface by reattaching and fixing a chondral/osteochondral fragment or replacing it by osteochondral grafts, whether autogenous, allogenous [1] or synthetic fillers. Repair entails formation of a biological regenerative tissue that fills the defect either by marrow stimulation techniques or by tissue engineering. Replacement by metal prostheses is reserved as a last resort given the finite durability of prostheses as well as the increasing life span and activity levels of the general population. Joint replacement therefore remains an option that is optimal in low demand, older patients with advanced osteoarthritis, or in individuals with less biological healing potential. These shortcomings have led to further development of tissue engineering-based repair methods to provide a more functional biological tissue. Currently, tissue engineering of articular cartilage has three cornerstones; a cell population capable of proliferation and differentiation into mature chondrocytes, a scaffold that can host these cells, provide a suitable environment for cellular functioning and serve as a sustained-release delivery vehicle of chondrogenic growth factors and thirdly, signaling molecules and growth factors that stimulate the cellular response and the production of a hyaline extracellular matrix (ECM). The aim of this review is to highlight the clinical applications of these three fields of tissue engineering with specific relevance to surgical techniques and technical notes.
Tissue Engineering Components
1. Cells
Autologous chondrocytes were the first cells to be introduced in the clinical setting after in vitro culture expansion in what was later called 1st generation autologous chondrocyte implantation (ACI) [2]. Second generation ACI emerged to avoid the drawbacks of the previous technique but still led to implantation of de-differentiated chondrocytes. Chondrocytes may “de-differentiate” or lose their characteristic morphology after in vitro mono –layer culture expansion or in vivo implantation, developing into spindle shaped fibroblast-like cells that secrete fibrous tissue or fibrocartilage. Preserving the chondrogenic phenotype is intended to prevent this process and ultimately maintain stable chondrocyte shape and function. 3rd generation ACI entails the use of newer 3D implants some of which are mechanically developed to preserve a stable chondrogenic phenotype. In addition, new cell lineages in this technique include the use of phenotypically stable characterized chondrocytes by selecting a chondrocyte sub-population that possesses a specific marker profile predictive of the capability to form stable hyaline cartilage [3]. As a recent modification of 3nd generation ACI, minced articular cartilage utilizes allogenous juvenile chondrocytes or autologous chondrocytes directly after harvest without culture expansion in a single stage procedure.
Since chondrocytes have a limited proliferative and intrinsic repair capacity as well as a tendency to de-differentiate in vitro, other cell sources were investigated, particularly adult mesenchymal stem cells (MSCs). Many studies have compared chondrogenic ability of different MSCs sources, of which bone marrow derived mesenchymal stem cells (BM-MSCs) have shown superior results [4–6]. BM-MSCs have the advantage of being readily available in larger quantities, easier to isolate without significant donor site morbidity, easier to expand in vitro compared to chondrocytes and have the potential to differentiate into chondrocytes in response to chondrogenic signals [7]. This led to the introduction of culture expanded bone marrow - mesenchymal stem cells (BM-MSCs) implantation in the clinical setting to treat cartilage defects [8]. Autologous matrix-induced chondrogenesis (AMIC) is a recent technique that utilizes local, unprocessed BM-MSCs recruited from the microfracture site onto a scaffold implanted in situ [9]. Following AMIC, minimally processed BM-MSCs have been more recently introduced in the clinical setting via the bone marrow aspirate concentrate (BMAC) technique which utilizes a bench side, single-stage procedure in the operating room to isolate and concentrate the MSCs resident in the nucleated cell portion of the bone marrow and re-implant them. Allogeneic MSCs represent an appealing ‘off-shelf’ option for cartilage repair since they are immune-privileged with minimal immunogenicity [10,11]. In addition to the traditional harvest site from the iliac crest, new sites for BM-MSCs harvest have been introduced.
2. Scaffolds and Synthetics
Scaffolds are natural substances with both chondro-inductive and conductive properties, designed to permit gradual controlled resorption with simultaneous cellular and tissue ingrowth. An ideal scaffold should be biocompatible, resorbable, mechanically stable to supply temporary support to the implanted cells, and should have sufficient porosity and interconnectivity to allow cell migration as well as passage of nutrients and waste products. Natural scaffolds used clinically can be classified as protein-based or carbohydrate-based. Protein-based scaffolds include collagen membranes or gels, fibrin glue (FG) and platelet rich plasma (PRP) while carbohydrate-based scaffolds include hyaluronic acid, alginate, agarose and chitosan. Collagen and hyaluronan-based scaffolds are currently the most used in the clinical setting, since their components are normal constituents of articular cartilage.
Collagen is a tri-helical protein that contains ligands which enable cell adhesion, migration and differentiation [12]. The presence of functional groups along its backbone also facilitates interaction with local or loaded growth factors, although release kinetics from collagen have been shown to be inferior to other scaffolds, particularly hyaluronan [13].
Hyaluronan scaffolds are the tissue engineered product of benzylic esterification of hyaluronic acid, consisting of a network of 20-µm thick fibers with variable porosity. Studies have shown that chondrocytes are able to re-differentiate (regain chondrogenic morphology and function) in hyaluronic acid and retain their phenotype even after long periods of in vitro monolayer culture [14–17]. Hyaluronic acid is highly biocompatible and fully resorbable in 3 months with controllable degradation rates. However, degradation products of hyaluronan can cause chondrolysis [18].
Alginate is a negatively charged polysaccharide derived from seaweed and made up of mannuronic and guluronic acid residues. In the presence of calcium, alginate chains cross-link. When cells are dropped in calcium chloride solution with alginate, 3-dimensional beads are formed that can be implanted into cartilage defects. Despite some concerns over its biocompatibility, it has been shown that articular chondrocytes do not de-differentiate in alginate [19].
Chitosan is composed of poly-glucosamine. Its degradation products include chondroitin sulphate, dermatan sulphate, hyaluronic acid, keratin sulphate and glycosylated type II collagen, which are all non-toxic compounds involved in the synthesis of articular cartilage [20]. The high positive charge of chitosan allows water-insoluble complexes to form with a variety of negatively charged substances. Therefore, negatively charged growth factors can be loaded and delivered from this scaffold [21].
Fibrin glue has also been used as a scaffold for cultured chondrocytes in a number of clinical studies [22,23]. Fibrin is a protein involved in the clotting of blood. It is formed by polymerization of fibrinogen in the presence of thrombin. The excellent adhesive properties, ease of application via injection, biocompatability and biodegradability of fibrin glue make it a very appealing scaffold in ACI. However, the mechanical properties of fibrin glue are poor [24]. In addition, it is potentially immunogenic [25]. Therefore, fibrin glue has been mainly used clinically to secure other tissue-engineered cartilage implants.
Platelet-rich plasma (PRP) is a scaffold that is gaining increasing popularity recently. Prepared from the platelet-rich portion of plasma after centrifugation and minimal processing, PRP has numerous advantages; it is injectable and easily applied, fairly adhesive, non immunogenic, rich in chondrogenic growth factors (TGF- β, IGF) and supplies a sustained release of these factors due to its controlled biodegradation [26,27]. Yet, like fibrin glue, PRP has poor structural properties.
Synthetics, on the other hand, are an artificial alternative to biological repair via scaffolds. Compared to natural scaffolds, they have the advantage of being biomechanically able to withstand weight bearing forces through controlling their mechanical properties. In addition, in vivo synthetic polymer degradation and growth factor release kinetics can be readily modified and improved to control degradation and release of growth factors [28]. Synthetics are also inexpensive, minimally immunogenic and implanted via a single stage arthroscopic or mini-arthrotomy press-fit technique. Synthetics also have the potential use as “back-fill” for donor sites in osteochondral autologous transfers thus minimizing morbidity, hematoma formation and arthrofibrosis. Examples of available synthetics are poly-lactic acid (PLA), poly-glycolic acid (PGA) and poly lactic co-glycolic acid (PLGA). Such polymers have shown to enhance proteoglycan production, chondrocyte proliferation, differentiation and maturation [29–31]. The TrueFit Plug (OsteoBiologics/Smith & Nephew, Andover, MA) is a biphasic poly[D,L-lactide]/glycolide and calcium sulfate polymer that is used in the treatment of small, isolated, full thickness osteochondral defects. It undergoes staged resorption over a 12 to 36-month period allowing for bone and soft tissue remodeling and replacement. Chondromimetic (Orthomimetics, Cambridge, UK) is similarly a porous biphasic synthetic composed of calcium phosphate, collagen, and glycosaminoglycans. Other synthetics include polyvinyl alcohol-hydrogel (SaluCartilage – Salumedica, Smyrna, GA and Carticept Medical Inc – Alpharetta, GA) and biphasic polyurethrane cylinders (ABS ChondroCushion – Advanced Bio-Surfaces, Minnetonka, MN) [32]. Potential disadvantages might arise from lack of porosity of some synthetics that hinders tissue ingrowth and replacement. Other disadvantages include loosening and dislodgment [33] as well as toxic byproducts with pH changes causing inflammation and cell death [34].
3. Growth factors
Several growth factors are involved in proliferation and differentiation of chondrogenic progenitor cells. The TGF super-family includes Transforming growth factor-β (TGF-β) and bone morphogenetic proteins (BMPs), TGF-β1 has been found to induce MSC proliferation, chondrogenic differentiation, ECM production, and inhibit terminal differentiation of MSCS into hypertrophic chondrocytes [35,36]. BMPs (especially BMP -4, -6 and 7) have a chondrogenic effect, increasing collagen type II and proteoglycan production [7,37]. Both BMP- 2 and BMP-7 (Osteogenic Protein-1 OP-1) have been reproduced by recombinant DNA technology and approved for clinical use. Insulin-like growth factor-1 (IGF-I) is a mitogen that has been shown to increase proteoglycan and collagen type II production [38]. Fibroblast growth factor (FGF) family encodes a total of 22 different members, of which FGF-2 is the most widely investigated. FGF-2 induces cell proliferation and chondrogenic differentiation [39,40]. Platelet-derived growth factor (PDGF), released from the α-secretory granules of platelets, is a key chemo-attractant and an up-regulator of MSC differentiation and ECM production [35,41,42].
Despite the promising results of in vitro application of growth factors, in vivo clinical application has not been as successful. This is due to the short half lives of these growth factors which necessitate a sustained delivery system that ensures supplying therapeutic doses of these growth factors over prolonged periods to negate the need for their repeated local administration for maintenance of effective levels.
Clinical Approach and Decision Making
The approach to cartilage lesions should be tailored to every individual. Factors contributing to decision making are both patient specific and lesion specific: Patient-specific factors include: age (physiological rather than chronological age), body mass index (BMI), activity levels/functional demands, systemic inflammatory and immunosuppressive disorders, the intrinsic healing capacity and ability to comply with rehabilitation. Lesion-specific factors include defect etiology, size, site, containment, and condition of the surrounding cartilage [43].
Surgical Techniques
Preparation of Recipient Site
In any cartilage repair technique, it is essential to prepare the defect bed to receive the implant. The lesion is debrided with a ring curette or a low profile drill, achieving sharp vertical walls and removing all fissures, abnormal cartilage and fibrous tissue in the base including the calcified layer of cartilage till the level of the subchondral bone. In ACI, care should be taken so as not to violate the subchondral bone to avoid bleeding with subsequent entry of marrow cells. Marrow stimulation techniques (subchondral bone drilling and microfracture) as well as MSCs implantation rely mainly on bone marrow elements, so perforations are made in the subchondral bone. However, it was found that violation of subchondral bone per se – in any cartilage repair procedure- leads to more enchondral ossification [44]. In addition, high oxygen tension might impede chondrogenesis of BM-MSCs [45,46]. That is why we do not recommend violating the subchondral bone plate in BM-MSCs implantation. If any bleeding is encountered from the subchondral bone it can be controlled via local application of epinephrine, thrombin or fibrin glue. The defect is measured with an arthroscopic graded probe and templated using sterile paper or aluminum that is subsequently used to size and prepare the graft if it is not in an injectable gel form.
New Generation Autologous Chondrocyte Implantation (ACI)
2nd generation ACI
Principle
1st generation ACI implants chondrocytes in suspension. The cell-suspension is injected under a sutured periosteal flap or collagen membrane sealed with fibrin glue. Drawbacks include a two-stage procedure, morbidity to donor cartilage and to the anteromedial tibial periosteum as well as the recipient cartilaginous rim from suturing the flap. Other limitations include longer recovery due to the second stage arthrotomy, potential cell leakage, uneven cell distribution within the defect, graft failure and delamination as well as periosteal flap hypertrophy requiring re-operation to remove the excess soft tissue [47]. To avoid these complications and to allow an even distribution and settlement of cells in the defect as well as easier fixation, 2nd generation ACI emerged. This method relied upon seeding the culture expanded chondrocytes on various membranes (matrices) that could be fixed with fibrin glue without the need of sutures, thus facilitating arthroscopic implantation. The primary matrix used was a collagen membrane and this technique was named Matrix-Induced Autologous Chondrocyte Implantation (MACI). Other common commercially available scaffolds are summarized in Table 1.
Table 1.
Commonly used scaffolds in 2nd generation ACI:
| Product | Composition | Cells seeding on scaffold |
Form (gel/membrane/cylinder) | Method of implantation (Mini-open/Arth) |
Method of Fixation |
|---|---|---|---|---|---|
| Matrix-induced autologous chondrocyte implant (MACI)* | porcine-derived type I/III collagen bilayer patch | 3 – 4 days before implantation | Bilayer Membrane: rough surface with open weave for cellular invasion and attachment, smooth compact barrier surface | Arthroscopic | Fibrin Glue at base |
| Cartilage Regeneration System, CaReS** | type I collagen gel | 2 weeks before implantation | Gel | Mini-open | Fibrin Glue at base |
| Atelocollagen | bovine type 1 collagen gel - antigenic proteins (telopeptides) removed minimizing immunogenicity | 3 weeks before implantation | Gel | Mini-open | Periosteal Flap Cover |
| Hyalograft C *** | benzylic ester of hyaluronic acid | 3 weeks before implantation | Membrane | Arthroscopic | Self adhesive – N/A |
| Bioseed C **** | polymer-based scaffold of polyglycolic/polylactic acid (polyglactin, vicryl) and polydioxanone fleece with a fibrin gel to evenly distribute cells | 3 weeks before implantation | Fleece | Arthroscopic | Fibrin Glue |
| Fibrin Glue ***** | Protein based fibrinogen and thrombin gels that polymerize on mixing | Upon implantation | Gel | Arthroscopic | N/A |
(Genzyme Biosurgery, Cambridge, MA / Chondro-Gide; Geistlich Biomaterials, Wolhusen, Switzerland / Matricel, Hezoenrath, Germany / Verigen, Leverkusen, Germany) /
(Arthro Kinetics, Esslingen, Germany) /
(HYAFF 11, Fidia Advanced Biopolymers Laboratories, Padova, Italy) /
(Biotissue AG, Freiburg, Germany) /
(Tissucol, Baxter, Austria)
Technique
Harvest of autologous cartilage is carried out arthroscopically using a gauge or ring curette from healthy, non weight-bearing areas of the knee; usually the superomedial or superolateral edge of the femoral condyle, the lateral intercondylar notch or the lateral or medial femoral trochlea. 2–3 small pieces (150–300g) of partial or full thickness cartilage are extracted. Similar to arthroscopic meniscectomy, leaving the end of the biopsy attached to the subchondral bone makes it easy to grasp and tear off with a grasper. In vitro expansion aims at reaching 1–2 × 106 cells/cm2 of defect [48].
Open technique
Reserved for arthroscopically inaccessible lesions (patella, trochlea, posterior femoral condyle) and scaffolds that cannot be implanted arthroscopically, the open technique follows the same principles as 1st generation ACI except for suturing a cell-loaded scaffold to the rim of the cartilage defect instead of injecting a cell suspension under a periosteal flap.
Arthroscopic technique
MACI and Collagen Membranes
After defect preparation and measurement, the flow of saline to the knee is stopped and the knee drained. The collagen membrane with pre-implanted cells is cut to fit the defect size. Two mini anchors with 5-0 absorbable sutures are placed at opposing sides of the rim of the defect. The sutures are then passed through the MACI membrane. The membrane is guided down the sutures to the cartilage defect by an inserter through a special cannula or passer. The membrane is placed such that the cell-loaded surface faces the subchondral bone and the surface is spread out using a tamper. Fibrin glue is then applied to the base of the defect under the membrane. For additional fixation, bioabsorbable pins can be used. The inserter holds the graft in place with firm pressure for 5 minutes till it is completely adherent to the underlying surface. Excess glue is removed and the 2 sutures are tied over the implant by arthroscopic knot tying. Saline flow is then restored and the stability of the implant confirmed by passing the joint through range of motion [49,50] (Fig. 1).
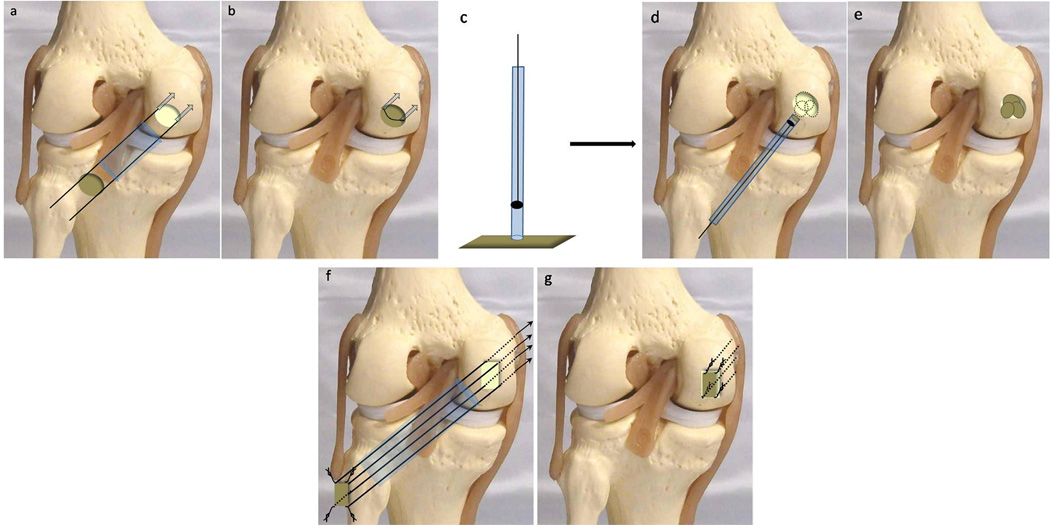
Figure 1. Autologous Chondrocyte Implantation (ACI).
Collagen membranes: a. Two mini anchors (dotted arrows) with absorbable sutures are placed at opposing sides of the defect and the sutures passed through the collagen membrane. The membrane is guided down the sutures through a cannula (blue tube) to the defect. b. The membrane is placed, fibrin glue is applied to the base and sutures are tied over. Hyaluronan grafts: c. Delivery device with sharp edge is placed on the cell-loaded hyaluronic acid patch embedding it into the tip. d. The delivery device is introduced to overlie the defect and the graft is pushed out and positioned within the defect. e. Patches overlapped to create a complete coverage of large defects in an overlapping “mosaic” pattern. Polymer fleece: f. The 4 corners of the defect are drilled with guide wires through the cannula in an inside-out technique. The scaffold is armed at its corners with resorbable sutures and mounted on the corresponding guide wires. g. The threads are pulled into the joint by the guide wires leading the implant through the cannula into the joint.
Steinwachs [51] described a modification of this technique where culture expanded chondrocytes are loaded on the collagen membrane intra-operatively after cutting and sizing the membrane to match the defect size, in contrast to traditional cell-seeding days or weeks before implantation. This is intended to reduce damage inflicted through repeated membrane manipulation during sizing and implantation with subsequent loss of viable chondrocytes. It also allows a more even distribution of cells as well as application of higher cellular concentrations to the collagen surface which potentiates the repair response.
Hyaluronan -based scaffolds (Hyalograft C)
For arthroscopic implantation, a 0.9-mm K- wire is inserted into the center of the defect and perpendicular to its plane. A cannulated drill (6.5 to 8.5 mm diameter) with a safety stop passes over the guide wire and reams the cartilage to a depth of only 2 mm thus avoiding penetration of the subchondral bone, while creating a well-defined area for graft placement. The fluid influx is turned off to ensure a dry joint. A delivery device (6.5 to 8.5 mm diameter) with a sharp edge is then placed on the cell-loaded hyaluronic acid patch cutting and embedding it into the tip of the delivery device which is then introduced through the arthroscopic portal to overlie the defect. The graft is then pushed out of the delivery device and precisely positioned within the defect where it adheres to the subchondral bone. In larger lesions, patches can be overlapped to create a complete coverage of the defect in an overlapping “mosaic” pattern which, unlike the usual osteochondral graft technique, does not leave uncovered spaces. In addition, applying fibrin glue to the margin of the defect helps improve implant stability [52] (Fig. 1).
Polymer fleece (Bioseed C)
For arthroscopic implantation, the defect is debrided to a rectangular shape and measured. An 8-mm cannula is introduced through standard arthroscopic portal and the 4 corners of the defect are drilled with guide wires through the cannula in an inside-out technique. The scaffold is armed at its corners with a double-knot loop using resorbable sutures. One three-fold knot approximately 1–2 cm from the edge secures the sling and an additional knot approximately 1cm further outwards anchors the sling to the corresponding guide wire and serves as a pulley. The threads are then pulled into the joint by the guide wires, through the femoral bone, leading the implant through the cannula into the joint. The 3-fold knots act as anchors that seize within the subchondral bone and secure the scaffold in place. The pulley slings are cut close to the skin exit and removed. Tibial defects can be similarly fixed with an outside-in technique using an ACL drill guide and a suture retriever with finally tying the threads over the bony bridge on the anteromedial proximal tibia. This technique ensures rigid fixation of the implant allowing early knee mobilization without concerns regarding graft detachment. However, it is technically demanding. Coordinated tension on all 4 threads is essential to avoid twisting of the threads and the implant, which might cause confusion. Different color threads may be beneficial to resolve this problem [53,54]. This technique also requires the maintenance of sutures in the scaffold without “cutting-through”, which -unlike the strong polymer fleece, may not be feasible in inherently mechanically weak scaffolds (Fig. 1).
3rd generation ACI
Principle
It was found that in vitro expanded chondrocytes cultured on 3D matrices regain their chondrogenic phenotype after in vivo implantation [55,56]. In addition, mechanical stimulation of chondrocytes in 3D culture can promote maintenance of the chondrogenic phenotype enabling the production of a stable mature hyaline matrix [57,58]. To avoid chondrocyte de-differentiation and phenotype loss, 3rd generation ACI evolved with new 3D cartilage constructs. Techniques to mechanically condition these 3D constructs in vitro prior to implantation were also developed to improve the material properties of the cell/scaffold implant. Other modalities of 3rd generation ACI included the use of phenotypically stable characterized chondrocytes. These chondrocytes were selected based on the quantitative gene expression of a selection of positive and negative markers developed to predict chondrocyte ability to form hyaline cartilage in vivo [3].
Technique
NeoCart (Histogenics, Waltham, MA)
This is a 3-dimensional bovine collagen type I matrix seeded with autologous chondrocytes processed in a hydrostatic bioreactor for a minimum of 7 days. Construct development takes about 6 weeks and the final construct is trimmed to match the defect. Via a mini-arthrotomy, a collagen/PEG (polyethylene glycol) biologic adhesive is placed in a “sandwich” technique; after coating the underlying prepared subchondral bone, NeoCart is positioned and gentle pressure is applied to ensure fixation of the implant. Finally, the bio-adhesive is spread over the implant and adjacent cartilage to secure the NeoCart implant [59].
Alginate cultured 3-Dimensional Grafts
Cartipatch (TBF Banque de tissues, France)
This is a hydrogel scaffold composed of agarose and alginate seeded with autologous chondrocytes. The implant can be manipulated at 37°C into complex shapes that solidify at ≃ 25°C thus providing excellent handling properties. The procedure is performed through a mini-arthrotomy. The debrided defect is drilled to a depth of 4 mm using drill bits with depths and sizes corresponding to the available hydrogel plugs (10 mm, 14 mm and 18 mm). One or more trial components are fitted into the pre-drilled holes. Stability is tested by passing the knee through a range of motion. The cell-loaded agarose-alginate hydrogels are then delivered to the defect and gently squeezed in with forceps. After completely covering the defect, stability of the plugs is tested again. One to 6 implants can be inserted in a mosaic pattern [60].
Alginate beads
This implant uses allogeneic chondrocytes that are harvested from donors’ knees within 24 hours of death. Chondrocytes are culture-expanded and mixed with alginate to form beads over a 2 week period. Through a mini arthrotomy, the defect is covered by a sutured periosteal flap. The chondrocyte cultured alginate beads are then packed under the periosteal flap until the defect is filled. Fibrin glue is then injected into the defect to fill the spaces between the beads. The periosteal flap does not need to be watertight since the 3-D structure of the beads prevents leakage [61].
In the future, allogeneic chondrocytes can be seeded on alginate with the potential of establishing a “chondrocyte bank” ready for “off-shelf” use in a single-stage procedure. Alginate-cultured allogeneic chondrocytes have been shown to retain viability after cryopreservation and prolonged storage [62].
Minced Articular Cartilage
Principle
This procedure is a single-stage modification of 3nd generation ACI currently in the early phases of clinical utilization. After harvest from the knee and without culture expansion, autologous cartilage is “minced” into small fragments intra operatively and loaded onto a scaffold. Alternatively, allogeneic juvenile cartilage from cadaver knees is processed similarly and loaded in advance to be available “off-shelf”. This was developed on the basis that chondrocytes from young individuals have a higher anabolic activity and potential for neo-cartilage formation without terminal chondrocyte hypertrophy when compared to adult cells [63]. The cartilage fragments are a source of viable chondrocytes that migrate into the surrounding scaffold or fibrin glue to produce extracellular matrix. Mincing a small amount of tissue, approximately one-tenth the size of the original defect, creates enough chondrocytes to treat a relatively large defect. It has been demonstrated that the smaller the fragment size, the more the repair tissue growth and that highest cellular activity was noted at the edge of the minced tissue [64]. Finer mincing increases the total surface area and the total number of exposed chondrocytes. This potentiates cell migration from the matrix and also helps fixation.
Technique
The Cartilage Autograft Implantation System (CAIS; DePuy Mitek, Raynham, MA)
This system involves a harvester and a disperser. The harvester consists of a stainless steel tube with a controlled cutting tip connected to a retraction system to allow engagement with the cartilage. With the aid of surgical suction, the disperser directs the minced cartilage mixed with irrigation fluid onto the scaffold which is a resorbable copolymer foam of polycaprolactone (PCL) and polyglycolic acid (PGA), reinforced with a polydioxanone (PDS) mesh. The tissue is minced and dispersed onto the scaffold which is attached to the bottom compartment of the disperser. After confirmation of even distribution of the minced cartilage, fibrin glue is used to stabilize the fragments on the scaffold. Through a mini-arthrotomy, a slightly oversized template of the defect is made and the scaffold trimmed accordingly. The minced cartilage implant is then placed into the defect and fixed with resorbable polydioxanone (PDS) staples (U-shaped) applied circumferentially to the defect rim [65] (Fig. 2).
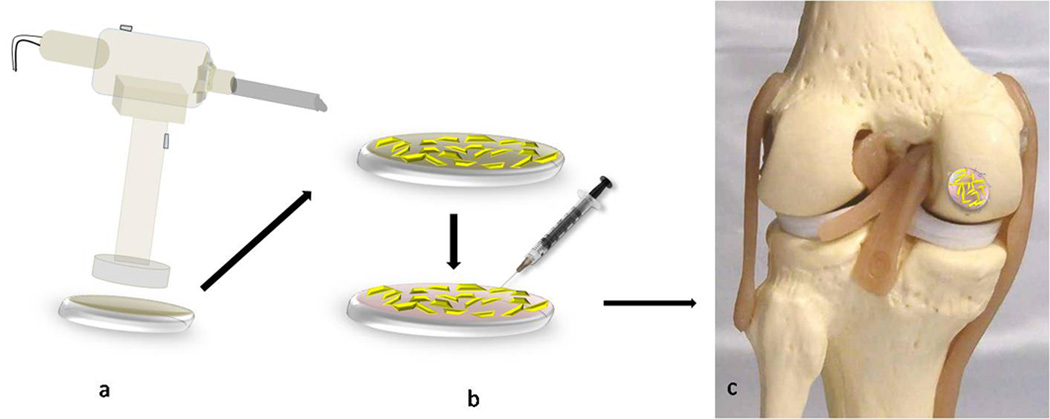
Figure 2. Minced Articular Cartilage.
a. The harvester-disperser instrument with PCL/PGA scaffold attached to the bottom. b. Even distribution of the minced cartilage is followed by fibrin glue injection to stabilize the fragments on the scaffold. c. Minced cartilage implant is placed into the defect and fixed with PDS U-shaped staples.
DeNovo Natural Tissue (NT) graft (Zimmer, Warsaw, IN/ISTO, St Louis, MO)
This implant is composed of allogeneic juvenile minced cartilage processed under optimized biologic conditions. It is implanted in a single stage arthroscopic procedure where the chondral defect is sized and the DeNovo cultured cell membrane is cut accordingly and implanted in the defect. The adhesive is then injected onto the defect between the implant and the subchondral bone, fixing the implant to the base of the lesion [65].
Bone Marrow Mesenchymal Stem Cell (BM-MSCs) Tissue Engineering Techniques
Bone Marrow Aspiration
During bone marrow aspiration, certain technicalities have to be attended to in order to avoid obtaining a diluted marrow aspirate with inferior numbers of MSCs. This would affect MSCs in vitro expansion or in situ implantation and ultimately the clinical outcome. Bone marrow can be aspirated from either the posterior or anterior iliac crests. Since most commonly the procedure is formed during knee arthroscopy with the patient in the supine position, the anterior approach is preferred. The anterior superior iliac spine is palpated and the inner and outer tables of the iliac crest are held between the thumb and index fingers to help aim the needle to be in line with the iliac wing. The bone marrow aspiration needle (11 to 18G × 100 mm) is inserted percutaneously and gently set into bone by applying firm forward pressure or gently tapping with a mallet on the needle handle. The needle is proceeded 3–4 cm deep into the spongy bone with the needle bevel facing the 12 o’clock position. After a maximum of 5 ml marrow is aspirated, the surgeon then turns the needle (guided by the direction of the needle handle) 90° clockwise so that the bevel is facing the 3 o’clock position. Another 5 ml are aspirated. This procedure is repeated till the needle bevel moves through the 6 and 9 o’clock positions. Upon returning to the 12 o’clock position, the surgeon then withdraws the needle 1cm from within the iliac crest and repeats the previously mentioned steps. Another option is to totally withdraw the needle out of the bone (but not the skin) and insert it through another perforation in the iliac crest. With this technique, up to 50–60 ml of bone marrow can be obtained from a single iliac wing without diluting the bone marrow cellular elements [66].
Since bone marrow of long bones as the femur contains MSCs [67], Elvenes et al [68] described a method for harvesting BM-MSCs from microfracture holes during knee arthroscopy and characterized the cells in vitro confirming their chondrogenic potential. In what they described as a “one-hole technique”, a microfracture hole is created in the middle of the cartilage defect using the microfracture awl. A 14-gauge needle with syringe is introduced into the microfracture hole, and 4–5 ml bone marrow is aspirated. If necessary, a hammer is used to drive the needle further into the subchondral marrow to better aspirate the sample. Although this method has the advantage of bypassing the iliac crest and reducing its morbidity while confining all the interventional procedures to the affected knee, it has some limitations. Firstly, a smaller amount of marrow can be retrieved from the knee relative to the iliac crest without significant dilution. Secondly, marrow aspiration after all holes are done, results in a more diluted sample in addition to leakage into the joint and mixing with the fluid from the arthroscopy pump. Thirdly, violating the subchondral bone through microfracture has been shown to have a strong negative effect on subsequent cartilage repair strategies [69]. However, this could be overcome by a “one-hole” microfracture in a non weight bearing area of the knee similar to harvest sites used for ACI limiting morbidity at the defect site.
Autologous Culture Expanded BM-MSCs Implantation
Principle
To decrease donor site morbidity associated with chondrocyte harvest and due to their abundance and chondrogenic potential, BM-MSC implantation was introduced by Wakitani et al [8] in a method similar to the ACI open technique.
Technique
1–2 days before implantation, culture expanded BM-MSCs are embedded in acid soluble bovine or porcine type I collagen then placed onto a collagen sheet from porcine tendon and gelated. After preparing the defect through a mini-arthrotomy, the implant was placed into the defect and a periosteal patch was sutured to the cartilage rim without the need for fibrin glue.
In a trial of developing a 2nd generation BM-MSC implantation using a cell/scaffold/growth factor implant, the authors of this article conducted a clinical feasibility study by implanting BM-MSCs on platelet-rich fibrin glue to treat articular cartilage defects (unpublished data). After harvest and in vitro culture, autologous BM-MSCs were mixed intra operatively with equal volumes of PRP and Fibrin Glue (Platelet-rich-Fibrin Glue) that was allowed to gel. This ensured even distribution of cells onto the scaffold and easy implantation. Although intended to be placed without a periosteal covering, the addition of PRP to Fibrin Glue resulted in decreasing the adhesive properties of the fibrin glue. Therefore, the implant was covered with a sutured periosteal flap via a mini-arthrotomy to ensure secure fixation and avoid dislodgment of the BM-MSCs/PR-FG implant in the early post operative rehabilitation period. In a case report, PRP was successfully used for fixation of a loose chondral fragment of the medial femoral condyle and to potentiate its healing to the subchondral bone [70]. However, the chondral fragment still needed additional fixation with resorbable pins.
Technical notes
Periosteum harvesting and suturing for BM-MSC implantation is a technically demanding procedure same as in ACI. The periosteal flap should be harvested with 1–2 mm over-sizing to avoid the potential shrinkage of periosteum. Once harvested, the outer layer should be marked to avoid confusion during suturing of the flap with the inner (cambium) layer intended to face the subchondral bone. The thickness of the periosteal flap is critical as thinner flaps were found to yield better results through avoiding flap protrusion beyond the cartilage surface with subsequent delamination [71]. Therefore, any excess subcutaneous tissue or fat should be trimmed off the flap. Finally, the sutures should be placed 2–3 mm apart and the knots should be placed on the periosteal side to minimize pressure induced damage to the healthy cartilage [48].
Unconstrained chondral lesions lack a peripheral rim of cartilage which opposes a challenge when suturing the periosteum or collagen membrane. This could be encountered if the lesion encroaches onto the intercondylar notch. Suturing to the intercondylar synovium can solve this issue, although it places the graft at risk during early knee motion as the synovium glides. Another unconstrained site would be peripheral condylar lesions. The first option to overcome this issue is to take “bone sutures” using a cutting needle after drilling holes in the peripheral bony rim with a K-wire. The second option is to use mini-suture anchors with resorbable sutures [48].
Deep osteochondral lesions (> 8 mm deep) pose a challenge since they consume the cellular elements in the repair of the bony part of the defect. The “sandwich technique” was described to address this problem by autologous or allogenous bone grafting of the defect to the level of the subchondral bone and placing two periosteal patches; one on top of the bone graft in level with the subchondral bone and another at the level of the cartilage defect with the cell/scaffold “sandwiched” in between the two layers [72] (Fig. 3).
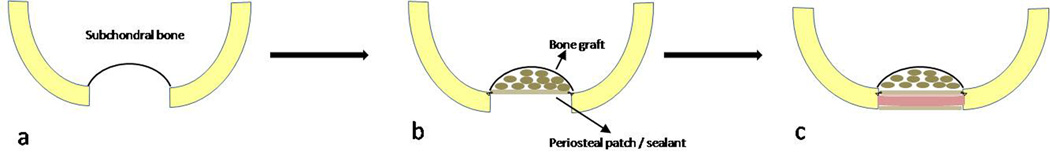
Figure 3. Sandwich technique.
a. An osteochondral defect extending deep into the subchondral bone b. Bone grafting to the level of the subchondral bone and placing a periosteal patch or sealant in level with the bone surface. c. A second periosteal patch or sealant layer is placed at the level of the cartilage surface with the cell/scaffold “sandwiched” in between the two layers.
Autologous matrix-induced chondrogenesis (AMIC)
Principle
Autologous matrix-induced chondrogenesis (AMIC) is a modification of the microfracture technique. It is a simple, single stage procedure without in vitro culture of MSCs. Bone marrow from microfracture holes is used to saturate a collagen membrane in situ with bone marrow derived cells and subsequently implant the membrane in the cartilage defect.
Technique
Using a mini arthrotomy, the microfractured defect is covered with a collagen I/III matrix for about 5 minutes. The matrix is then removed after obvious bone marrow saturation and trimmed to fit the defect size and fixed with fibrin glue. To prevent delamination, laying the membrane edges over the rim of the cartilage should be avoided. The knee is held in an extended position for 5 min before testing the stability of the implant through a range of motion [9] (Fig. 4).
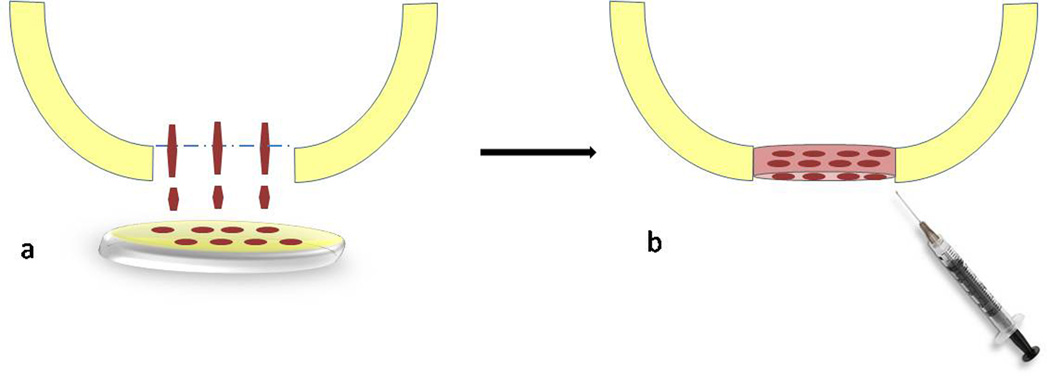
Figure 4. Autologous Matrix-Induces Chondrogenesis (AMIC).
a. The microfractured defect is covered with a collagen membrane for 5 minutes. b. After bone marrow saturation, the matrix is trimmed and fixed with fibrin glue
Other marrow stimulated, scaffold-based techniques: Chitosan BST-Car Gel
Principle
BST Car Gel (Biosyntech, Quebec, Canada) is a chitosan/glycerol copolymer hydrogel that is mixed with blood and injected into a chondral defect following microfracture forming a semi-solid gel that helps in retaining the mesenchymal blood clot with its cellular elements and growth factors in addition to potentiating chondrogenesis [73].
Technique
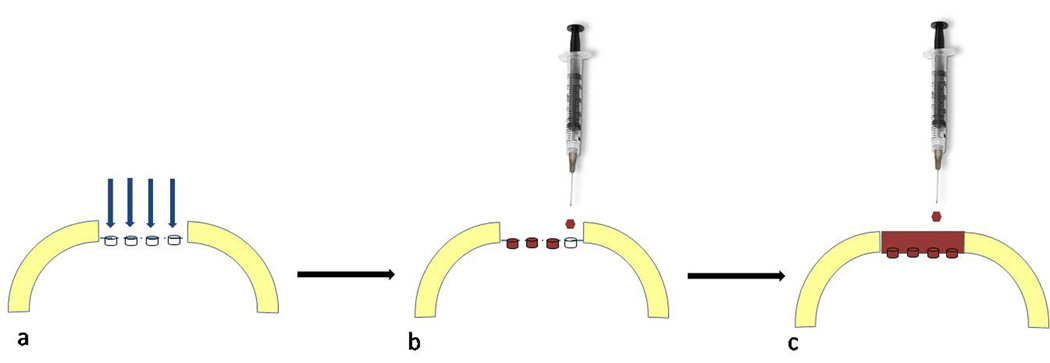
BST-Car Gel can be administered through an arthroscopic technique. After the cartilage defect is microfractured, the fluid is suctioned to create a dry field. Positioning of the leg is critical so that the plane of the lesion remains parallel to the floor and facing upwards to avoid displacement of the gel. To eliminate needle positioning delays, the delivery needle is directed perpendicular and central to the lesion before the rapidly clotting BST-Car Gel/blood mixture is prepared. Five ml of peripheral blood is then drawn from the patient and inserted into a sterile vial containing 6 small mixing beads and the mixture is vigorously shaken for 10 seconds. The mixture is then delivered in a drop-wise fashion into each of the microfractured holes, and then onto the surrounding defect taking care not to “overfill”. The leg is immobilized for 15 minutes to allow for solidification of the clot [74] (Fig. 5).
Figure 5. Chitosan/Blood Clot.
a. The cartilage defect is microfractured. b. The delivery needle is directed perpendicular and central to the lesion and the BST-CarGel/blood mixture is delivered in a drop-wise fashion into each microfracture hole c. and then onto the surrounding defect taking care not to “overfill”.
One-step Bone Marrow-derived Cell Transplantation – Bone Marrow Aspirate Concentrate (BMAC)
Principle
To avoid the aforementioned limitations of BM-MSCs and AMIC, a single-stage arthroscopic procedure was recently described to repair cartilage lesions by implanting bone marrow-derived cells that are minimally processed intra operatively using a device (Smart PReP; BMAC; Harvest Technologies Corp, Plymouth, MA) that extracts and concentrates the mononuclear cell layer of bone marrow which contains MSCs: Bone Marrow Aspirate Concentrate - BMAC) [75]. This was based on studies that showed that a concentrator device produces bone marrow-derived cells with similar or greater functional activity compared with the traditional method using Ficoll isolation [76].
Technique
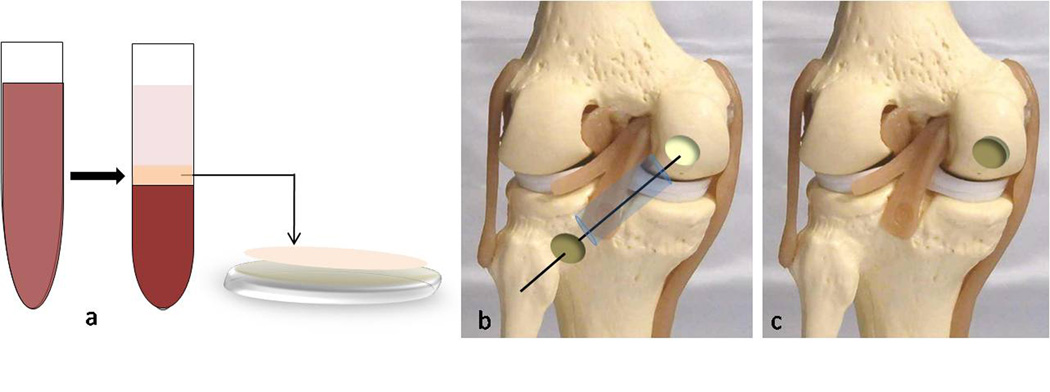
PRP was autogenously prepared from a peripheral venous blood sample one day before surgery (Vivostat System1, Vivolution A/S, Birkeroed, Denmark). On the day of surgery, the defect is prepared, arthroscopic fluid influx is stopped and the joint drained. The BMAC is implanted on a combined mixture of platelet gel (PRP) and collagen powder or hyaluronic acid membrane and then inserted through the arthroscopic portal closer to the lesion through a cannula with a sliding positioner. When it reaches the margin of the lesion, the implant is positioned with the help of a probe and adheres due to its PRP content. The cannula is then removed and the implant fitted into the lesion using a flattened probe. A layer of PRP is then applied to the surface of the implant for additional fixation and growth factor release. The joint is passed through a range of motion to ensure implant stability [75] (Fig. 6).
Figure 6. Bone Marrow Aspirate Concentrate (BMAC).
a. In the operating room, the mononuclear layer of the bone marrow (BMAC) is separated (middle zone) and implanted on a combined mixture of platelet gel and collagen powder or hyaluronic acid membrane. b. The cell/scaffold implant is inserted through the arthroscopic portal through a cannula with a sliding positioner or on a guide wire drilled through the centre of the defect. c. The implant is positioned with the help of a probe and adheres due to its PRP content. An additional layer of PRP is then applied to the surface for further fixation.
Tissue engineered synthetic osteochondral plugs
Technique
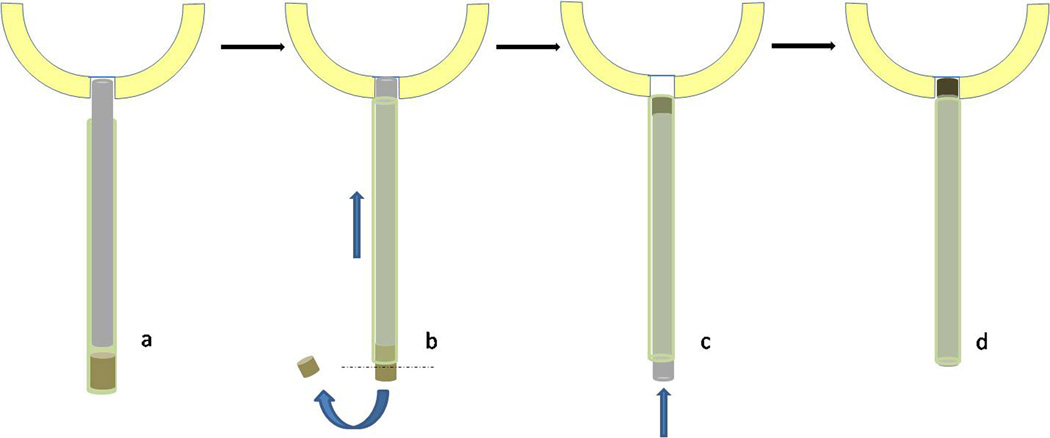
All tissue engineered osteochondral synthetics are implanted in a similar manner to osteochondral autologous transplantation. TruFit Bone Graft Substitute is an example of such synthetics. It is a cylindrical plug supplied in different diameters (5, 7, 9, and 11-mm) preloaded on a special delivery device with an outer sleeve that can be introduced arthroscopically. After preparation of the defect, an obturator with a drill sleeve is inserted perpendicular to the lesion and driven to a depth of 5–15 mm using a mallet, as measured by depth markings on the sleeve. Once at the appropriate depth, the obturator is removed, the drill is inserted into the sleeve and the lesion is drilled to the desired depth marked when the drill stop contacts the sleeve. The next step is to size the implant height to the appropriate drilled depth. A measuring tamp is inserted into the sleeve of the delivery device until it contacts the preloaded implant. The measuring tamp is then placed into the drilled defect so that it contacts the base of the defect. The outer sleeve is slid over the tamp until it is flush against the cartilage surface. This automatically pushes part of the osteochondral plug out of the sleeve. The remaining height of the osteochondral plug inside the sleeve exactly matches the depth of the drilled defect. A disposable knife is then used to cut the protruding extra portion of the implant. The implant is then inserted into the defect by manually advancing the tamp into the delivery device. This process is repeated in a “mosaicplasty-like” fashion with subsequent TruFit plugs until the bony defect is filled [77,78] (Fig. 7).
Figure 7. Osteochondral plug (TruFit).
a. The cylindrical osteochondral plug (brown) is preloaded on a delivery device with an outer sleeve (green cylinder) and a measuring tamp (grey). The assembly is inserted perpendicular to the lesion until the measuring tamp contacts the base of the defect. b. The outer sleeve is slid over the tamp (blue straight arrow) until it is flush against the cartilage surface. This pushes the excess portion of the osteochondral plug out of the sleeve which is subsequently cut (dashed line). c. The sleeve is reversed and the implant is inserted into the defect by manually advancing the tamp within the delivery device.
Discussion
In summary, tissue engineering cartilage repair techniques can be classified as: cell only repair (1st generation ACI), scaffold only repair, cell/scaffold repair (2nd and 3rd ACI, minced articular cartilage, 1st generation BM-MSCS implantation, AMIC), cell/scaffold/growth factor repair (2nd generation BM-MSCs/PR-FG implantation and BMAC/PRP implantation).
Each of the above mentioned methods has its advantages and limitations. Although with good long term results especially in large sized defects, 1st and 2nd generation ACI remain a two-stage procedure with potential donor site morbidity. Not all the scaffolds used completely recreate the 3-dimensional (3D) structure of cartilage. Chondrocytes cultured in monolayer in this technique were found to lose their phenotype and de-differentiate on re-implantation with the production of fibrocartilage [79]. Despite the improvement in scaffolds for 3rd generation ACI, most of the procedures for these constructs reverted to a two-stage procedure with a 2nd mini-arthrotomy, the drawbacks of which were previously mentioned. Minced articular cartilage procedures are attractive as they are single-stage, consist of natural chondral tissue inserted on a scaffold that can potentially be loaded with growth factors and placed with arthroscopic techniques. However, the damage induced by “mincing” cartilage can induce chondrocyte death and the amount of cells processed through this technique may be insufficient to produce a high quality ECM. Although chondrocytes are considered immuno-privileged since they reside deep within the extra cellular matrix [80], yet as with any allogeneic tissue, concerns of potential immunogenicity remain an issue. Although with no morbidity to potential donor sites of native cartilage of the knee, BM-MSCs implantation is still a difficult two-stage procedure. Suturing periosteum or collagen membrane to the healthy rim of articular cartilage is technically demanding and was found to induce local damage at suture sites that progressed to osteoarthritis-like changes [81]. As with 1st generation ACI, periosteal hypertrophy in BM-MSC implantation is a potential problem that may lead to mechanical symptoms and require revision surgery to trim the excess soft tissue. Another concern with the use of BM-MSCs mentioned in preclinical studies is the terminal differentiation and hypertrophy of BM-MSCs - derived chondrocytes. Enchondral ossification of the repair cartilage ensues with thinning and loss of the cartilaginous tissue and its replacement by subchondral bone [82]. Although the AMIC procedure stabilizes marrow elements from microfracture on the scaffold, it still fails to sufficiently concentrate the cellular elements of the microfracture mesenchymal clot with inferior reparative response. In addition to all the AMIC limitations, chitosan-based techniques have the following drawbacks: Because chitosan necessitates interaction with blood to clot, patients with abnormal coagulation profiles or those using anticoagulants and NSAIDs are not candidates for this technique. In addition, if the lesion is unintentionally “over filled’, cell leakage and loss of the implant are eminent. Minimal manipulation of the knee and leg is essential during closing and early rehabilitation as the implant is relatively unstable [74]. While BMAC is a single-stage procedure with minimal cell manipulation, a drawback is that it has inferior numbers of cells than culture expansion which may pose a cellular challenge in the repair of large sized defects. In addition, BMAC does not contain a pure MSC population. Other mononuclear cells (monocytes, lymphocytes) may interfere with chondrogenesis via release of cytokines, inflammatory mediators [83] or angiogenics [84]. While synthetic osteochondral plugs avoid donor site morbidity and supply an “off-shelf” implant for a single-stage arthroscopic procedure, it has some of the drawbacks of autologous or allogenous osteochondral transfers. Formation of fibrous tissue in between the plugs renders the repair mechanically weak and predisposes to failure. In addition, absence of cellular elements and growth factors is expected to yield inferior quality cartilaginous tissue. A recent report of 2 cases treated with synthetic osteochondral plugs reported the formation of fibrous tissue with giant cell reaction and no evidence of viable tissue necessitating revision surgery [85]. It is important to note that most of these procedures (BM-MSCs implantation, BMAC, AMIC, Chitosan-based scaffolds) have not been approved for clinical application in the United States. While synthetic osteochondral plugs have been approved for treatment of osteochondral defects in Europe, they have been only FDA approved as a “back-fill” for osteochondral donor sites in autologous osteochondral transfers.
The major challenge facing tissue engineering technologies for cartilage repair is to find a method that proves significant superiority over current repair strategies. Until today, results of various studies have been both equivocal and controversial and do not show any of these methods to be superior to one another [86]. In addition, there is no preference among currently available scaffolds or cell types. While marrow stimulation techniques –namely microfracture- have shown to provide satisfactory long term results especially in small sized defects (< 2–3 cm2) [87], other studies have shown deterioration as early as 18 months post operatively, especially in older patients (> 40 years) [88] and athletes [89]. Comparison of short term clinical results ACI to microfracture did not show significant difference [90] despite the repair tissue in ACI being structurally superior [3]. This may influence long term results. Furthermore, newer generations ACI were not found to be superior to conventional 1st generation ACI [91]. A recent clinical study also found no difference in outcome following ACI versus BM-MSCs implantation [92]. A preclinical in vivo study compared microfracture versus AMIC and showed no improvement of repair tissue between both treatments. However, the use of AMIC reinforced with cultured chondrocytes improved the repair tissue [93]. This emphasizes the role of the high density cellular component in cartilage repair strategies. AMIC and BMAC are relatively recent techniques and clinical results are still in the early phase. However, a clinical study recently reported the 1 year results of AMIC versus BMAC. While a difference in cell concentration and differentiation potential was found to be superior in the BMAC group, no significant difference could be detected in the short term clinical outcome scores and MRI at 1 year [94].
All the aforementioned methods seem to promote chondrocyte differentiation and formation of a predominant fibrocartilage [95–98]. Although ACI shows superior histology with more hyaline cartilage formation [3], none of the current treatment modalities has demonstrated a histological ideal repair that mimics the characteristic complex zonal architecture of hyaline articular cartilage with the desired optimal clinical long term results.
Obstacles and Future Perspectives
Many factors play an important role in developing the optimal cartilage repair solution. Major cell-based issues include a number of obstacles: First, to provide the appropriate biophysical stimuli for chondrogenic differentiation, including mechanical stimuli and low oxygen tension that have shown to significantly impact in vitro and in vivo chondrogenesis. Secondly, to prevent causes of potential death (necrosis or apoptosis) of implanted cells especially along the margins of the defect via mechanical damage from trimming, or from the release of inflammatory cytokines, degenerative enzymes or oxidative stress, all of which create an unfavorable environment for repair tissue integration [99]. Thirdly, to enhance the secretion of extracellular matrix from implanted cells for better integration of the repair tissue with surrounding cartilage. Fourthly, to prevent chondrocyte de-differentiation or chondroprogenitor cell hypertrophy during in vitro culture or after in vivo implantation by maintaining a phenotypically stable cell. Chondroprogenitor cells include chondroblasts (juvenile chondrocytes) and stem cells derived from various tissues such as bone marrow, adipose tissue, muscle, umbilical cord or embryonic stem cells. A possible approach would be characterizing, isolating and culture expanding phenotypically stable cell populations. Another alternative would be the in vivo implantation of undifferentiated cells in the hope of in situ differentiation through the native chondrogenic environment and subsequent better integration.
In scaffolds, ongoing challenges include fixation and maintenance within the defect, controlling the rate of degradation, sustained delivery of growth factors, and promoting tissue maturation. Nanotechnology is a rapidly emerging area in tissue engineering that is likely to form the basis of scaffold production for cartilage repair in the near future. It involves the production of scaffolds on a nano-scale, thereby simulating the structure and porosity of the normal hyaline ECM. This leads to subsequent improvement in contact surface area and physiochemical properties such as cell adhesion and growth factor adsorption [100].
As for growth factors, the question remains regarding the ultimate choice, combination and dosage of various growth factors for in vivo clinical applications of cartilage repair. Until these growth factors are clinically available and safely delivered, this question remains unanswered [34].
Gene therapy has a potential role in the future clinical approach to tissue engineering of cartilage. By delivering genes or complementary DNA (cDNA) encoding production of chondrogenic growth factors into implanted cells (chondrocytes or MSCs), these cells can produce the desired growth factors in vivo without the need for exogenous supplementation overcoming the obstacles of short half-lives and sub-therapeutic doses. A vector is used to carry the cDNA. Two methods are available for transfection; direct and indirect. The direct method involves injecting the vector directly into the joint to transfect native cells. However, it is difficult to transfect cells in-vivo. This may result in low levels of growth factor expression as well as expression in undesired adjacent tissues due to non specific cellular uptake of genes [101]. Indirect transfer involves transfection of the tissue-engineered cells or scaffold by either incorporating growth factor genes into gene-modified cells [102,103] or loading the scaffold with growth factor cDNA [104,105]. Indirect transfection is considered a safer, more controlled option. Viral vectors that have been used in the preclinical setting include adenovirus (AV), adeno-associated virus (AAV) and lentivirus [106].
Finally, the perfect tissue engineering solution for cartilage repair must remain a simple, cost-effective, single-staged, all-arthroscopic procedure with minimal donor site morbidity and peri-operative complications. The ideal construct should contain optimized cells, scaffolds and growth factors that are either loaded at the time of graft manufacturing -with subsequent preservation- or during the implantation procedure.
References
- 1.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999 Mar;360:159–168. [PubMed] [Google Scholar]
- 2.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008 Feb;36(2):235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 4.Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther. 2006;1:345–364. doi: 10.2174/157488806778226803. [DOI] [PubMed] [Google Scholar]
- 5.Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322–331. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 6.Vidal MA, Robinson SO, Lopez MJ, Paulsen DB, Borkhsenious O, Johnson JR, Moore RM, Gimble JM. Comparison of chondrogenic potential in equine mesenchymal stromal cells derived from adipose tissue and bone marrow. Vet Surg. 2008;37:713–724. doi: 10.1111/j.1532-950X.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wakitani S, Imoto K, Yamamato T, Saito M, Murata N, Yuneda M. Human autologous culture expanded bone marrow mesenchymal stem cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis and Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 9.Kramer J, Böhrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006 Mar;63(5):616–626. doi: 10.1007/s00018-005-5527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine SM, Peter S, Martin BJ, Barry F, McIntosh KR. Mesenchymal stem cells: stealth and expression. Cancer J. 2001;7(Suppl 2):76–82. [PubMed] [Google Scholar]
- 11.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002 Mar 5;59(3):573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 14.Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, Kastenbauer E, Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172–181. doi: 10.1002/(sici)1097-4636(199811)42:2<172::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Brun P, Abatangelo G, Radice M, Zacchi V, Guidolin D, Daga Gordini D, Cortivo R. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337–346. doi: 10.1002/(sici)1097-4636(19990905)46:3<337::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Solchaga LA, Yoo JU, Lundberg M, Dennis JE, Huibregtse BA, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers in the treatment of osteochondral defects. J Orthop Res. 2000;18:773–780. doi: 10.1002/jor.1100180515. [DOI] [PubMed] [Google Scholar]
- 17.Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, Duca M, Pavesio A, Facchini A. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–1195. doi: 10.1016/s0142-9612(01)00236-8. [DOI] [PubMed] [Google Scholar]
- 18.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosacchrarides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. 2000;43:1165–1174. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Häuselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, Thonar EJ. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(Pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 20.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 21.Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release. 2003;91:365–374. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- 22.Visna P, Pasa L, Hart R, Kocis J, Cizmár I, Adler J. Treatment of deep chondral defects of the knee using autologous chondrocytes cultured on a support. Results after one year. Acta Chir Orthop Traumatol Cech. 2003;70:356–362. [PubMed] [Google Scholar]
- 23.Visna P, Pasa L, Cizmár I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques. A randomized controlled study. Acta Chir Belg. 2004;104:709–714. doi: 10.1080/00015458.2004.11679648. [DOI] [PubMed] [Google Scholar]
- 24.van Susante JL, Buma P, Schuman L, Homminga GN, van den Berg WB, Veth RP. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials. 1999;20:1167–1175. doi: 10.1016/s0142-9612(97)00190-7. [DOI] [PubMed] [Google Scholar]
- 25.Kawabe N, Yoshinao M. The repair of full-thickness articular cartilage defects. Immune responses to reparative tissue formed by allogeneic growth plate chondrocyte implants. Clin Orthop Relat Res. 1991;268:279–293. [PubMed] [Google Scholar]
- 26.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004 Apr;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Rep Reg. 2008;16:356–363. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006 Sep;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 29.Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D. Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995 Sep;29(9):1147–1154. doi: 10.1002/jbm.820290915. [DOI] [PubMed] [Google Scholar]
- 30.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology Sports Med Arthrosc. 2008 Dec;16(4):196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 33.Falez F, Sciarretta F. Treatment of osteochondral symptomatic defects of the knee with SaluCartilage. J Bone Joint Surg Br. 2005;87(suppl II):202. [Google Scholar]
- 34.Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today's research, tomorrow's practice? J Bone Joint Surg Br. 2009 May;91(5):565–576. doi: 10.1302/0301-620X.91B5.21832. [DOI] [PubMed] [Google Scholar]
- 35.Cassiede P, Dennis JE, Ma F, Caplan AI. Osteochondrogenic potential of marrow mesenchymal progenitor cells exposed to TGF-beta 1 or PDGF-BB as assayed in vivo and in vitro. J Bone Miner Res. 1996;11:1264–1273. doi: 10.1002/jbmr.5650110911. [DOI] [PubMed] [Google Scholar]
- 36.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-β1 to human mesenchymal stem cells improves cartilage repair. Gene Therapy. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411–418. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 38.Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signalling. J Bone Miner Res. 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 39.Cuevas P, Burgos J, Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun. 1988;156:611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- 40.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005 May;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 41.Kieswetter K, Schwartz Z, Alderete M, Dean DD, Boyan BD. Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation. Endocrine. 1997;6:257–264. doi: 10.1007/BF02820501. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis and cartilage. 2006;14(5):403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Williams RJ, Iii, Brophy RH. Cartilage repair procedures: clinical approach and decision making. Instr Course Lect. 2008;57:553–561. [PubMed] [Google Scholar]
- 44.Blanke M, Carl HD, Klinger P, Swoboda B, Hennig F, Gelse K. Transplanted Chondrocytes Inhibit Endochondral Ossification Within Cartilage Repair Tissue. Calcif Tissue Int. 2009 Sep;10 doi: 10.1007/s00223-009-9288-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Coyle CH, Izzo NJ, Chu CR. Sustained hypoxia enhances chondrocyte matrix synthesis. J Orthop Res. 2009 Jun;27(6):793–799. doi: 10.1002/jor.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zscharnack M, Poesel C, Galle J, Bader A. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel Cells Tissues Organs. 2009;190(2):81–93. doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]
- 47.Wood JJ, Malek MA, Frassica FJ, et al. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88:503–507. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
- 48.Brittberg M. Autologous chondrocyte implantation-technique and long-term follow-up. Injury. 2008 Apr;39(Suppl 1):S40–S49. doi: 10.1016/j.injury.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 49.Abelow SP, Guillen P, Ramos T. Arthroscopic technique for matrix induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Op Tech Orthop. 2006;16:257–261. [Google Scholar]
- 50.Krishnan SP, Skinner JA, Carrington J, et al. Collagen-covered autologous chondrocyte implantation for osteochondritis dissecans of the knee. J Bone Joint Surg Br. 2006;88:203–205. doi: 10.1302/0301-620X.88B2.17009. [DOI] [PubMed] [Google Scholar]
- 51.Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009 Feb;25(2):208–211. doi: 10.1016/j.arthro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006 Nov;34(11):1763–1773. doi: 10.1177/0363546506288853. [DOI] [PubMed] [Google Scholar]
- 53.Erggelet C, Sittinger M, Lahm A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy. 2003 Jan;19(1):108–110. doi: 10.1053/jars.2003.50025. [DOI] [PubMed] [Google Scholar]
- 54.Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9:41. doi: 10.1186/ar2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 56.Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P. Reexpression of cartilage specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 57.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590–596. doi: 10.1016/S0736-0266(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 58.Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Hong J, Kandel RA. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. J Bone Joint Surg Am. 2003;85-A(suppl 2):101–105. doi: 10.2106/00004623-200300002-00013. [DOI] [PubMed] [Google Scholar]
- 59.Crawford DC, Heveran CM, Cannon WD, Jr, Foo LF, Potter HG. An Autologous Cartilage Tissue Implant NeoCart for Treatment of Grade III Chondral Injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009 Jul;37(7):1334–1343. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- 60.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008 May;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 61.Almqvist KF, Dhollander AA, Verdonk PC, Forsyth R, Verdonk R, Verbruggen G. Treatment of Cartilage Defects in the Knee Using Alginate Beads Containing Human Mature Allogenic Chondrocytes. Am J Sports Med. 2009 Jun;19 doi: 10.1177/0363546509335463. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Almqvist KF, Wang L, Broddelez C, Veys EM, Verbruggen G. Biological freezing of human articular chondrocytes. Osteoarthritis Cartilage. 2001 May;9(4):341–350. doi: 10.1053/joca.2000.0394. [DOI] [PubMed] [Google Scholar]
- 63.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391(suppl):S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 65.McCormick F, Yanke A, Provencher MT, Cole BJ. Minced articular cartilage--basic science, surgical technique, and clinical application. Sports Med Arthrosc. 2008 Dec;16(4):217–220. doi: 10.1097/JSA.0b013e31818e0e4a. [DOI] [PubMed] [Google Scholar]
- 66.Kitchel SH, Wang MY, Lauryssen CL. Techniques for aspirating bone marrow for use in spinal surgery. Neurosurgery. 2005 Oct;57(4 Suppl):286–289. doi: 10.1227/01.neu.0000176412.17360.95. [DOI] [PubMed] [Google Scholar]
- 67.Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21(2):190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 68.Elvenes J, Knutsen G, Johansen O, Moe BT, Martinez I. Development of a new method to harvest chondroprogenitor cells from underneath cartilage defects in the knees. J Orthop Sci. 2009 Jul;14(4):410–417. doi: 10.1007/s00776-009-1349-4. [DOI] [PubMed] [Google Scholar]
- 69.Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009 May;37(5):902–908. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- 70.Sánchez M, Azofra J, Anitua E, Andía I, Padilla S, Santisteban J, Mujika I. Plasma rich in growth factors to treat an articular cartilage avulsion: a case report. Med Sci Sports Exerc. 2003 Oct;35(10):1648–1652. doi: 10.1249/01.MSS.0000089344.44434.50. [DOI] [PubMed] [Google Scholar]
- 71.Driesang IM, Hunziker EB. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res. 2000 Nov;18(6):909–911. doi: 10.1002/jor.1100180609. [DOI] [PubMed] [Google Scholar]
- 72.Peterson L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin. 2003;8(2):291–303. doi: 10.1016/s1083-7515(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 73.Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, Buschmann MD. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005 Dec;87(12):2671–2686. doi: 10.2106/JBJS.D.02536. [DOI] [PubMed] [Google Scholar]
- 74.Shive MS, Hoemann CD, Restrepo A, Hurtig MB, Duval N, Ranger P, Stanish W, Buschmann MD. BST-CarGel: In Situ ChondroInduction for Cartilage RepairOper Tech Orthop. 2006;16:271–278. [Google Scholar]
- 75.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step Bone Marrow-derived Cell Transplantation in Talar Osteochondral Lesions. Clin Orthop Relat Res. 2009 May;:16. doi: 10.1007/s11999-009-0885-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, Jacobson MS, Heeschen C. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–1069. [PubMed] [Google Scholar]
- 77.Trukor Plus. Surgical Technique Guide. Smith & Nephew Endoscopy. 2006 [Google Scholar]
- 78.Kerker JT, Leo AJ, Sgaglione NA. Cartilage repair: synthetics and scaffolds: basic science, surgical techniques, and clinical outcomes. Sports Med Arthrosc. 2008 Dec;16(4):208–216. doi: 10.1097/JSA.0b013e31818cdbaa. [DOI] [PubMed] [Google Scholar]
- 79.Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84:571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 80.Friedlaender GE. Immune responses to osteochondral allografts: current knowledge and future directions. Clin Orthop. 1983;174:58–68. [PubMed] [Google Scholar]
- 81.Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008 Sep;16(9):1067–1073. doi: 10.1016/j.joca.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal Cell-Based Repair of Large, Full-Thickness Defects of Articular Cartilage. J Bone Joint Surg Am. 1994 Apr;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 83.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009 Mar;60(3):801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, Li G, Fu FH, Huard J. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009 Jan;60(1):155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sgaglione NA, Florence AS. Bone graft substitute plug failure with giant cell reaction in the treatment of osteochondral lesions of the distal femur: a report of 2 cases with operative revision. Arthroscopy. 2009 Jul;25(7):815–819. doi: 10.1016/j.arthro.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 86.Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005 Oct;87(10):2232–2239. doi: 10.2106/JBJS.D.02904. [DOI] [PubMed] [Google Scholar]
- 87.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003 May-Jun;19(5):477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 88.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006 Nov;14(11):1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006 Sep;34(9):1413–1418. doi: 10.1177/0363546506288240. [DOI] [PubMed] [Google Scholar]
- 90.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007 Oct;89(10):2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 91.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg [Br] 2005;87-B:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 92.Nejadnik H, Hui JHP, Choong PF, TAI BC. Is stem cell a good source for autologous chondrocyte implantation? A comparative study. Presented at the 8th World Congress of the International Cartilage Repair Society (ICRS); May 2009; Miami Florida. [Google Scholar]
- 93.Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005 Aug;13(8):655–664. doi: 10.1016/j.joca.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Volpi P, de Girolamo L, Cervellin M, Bait C, Galli M, Schoenhuber H. Treatment of chondral defects with AMIC technique (Autologous Matrix Induced Chondrogenesis) compared to AMIC enhanced by concentrated bone marrow. Presented at the 8th World Congress of the International Cartilage Repair Society (ICRS); May 2009; Miami Florida. [Google Scholar]
- 95.Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005 Apr;13(3):213–221. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 96.Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, Smailys A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005 Sep;21(9):1066–1075. doi: 10.1016/j.arthro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 97.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 98.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007 Jan-Feb;1(1):74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 99.Archer CW, Redman S, Khan I, Bishop J, Richardson K. Enhancing tissue integration in cartilage repair procedures. J Anat. 2006;209:481–493. doi: 10.1111/j.1469-7580.2006.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 101.Evans CH, Robbins PD, Ghivizzani SC, et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 102.Gelse K, von der MK, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430–441. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- 103.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 104.Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, Zhang J. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2006;27:1095–1103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 105.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Therapy. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 106.Caplan AI. Mesenchymal stem cells and gene therapy. Clin Orthop. 2000;(379 Suppl):67–70. doi: 10.1097/00003086-200010001-00010. [DOI] [PubMed] [Google Scholar]