Familial HCM is caused by mutations in genes encoding sarcomere proteins. Among HCM-linked disease genes, cardiac TnT mutations are associated with a high incidence of arrhythmic cardiac death (1), but the underlying mechanism has remained elusive. Studies in mice suggest that increased myofilament Ca buffering caused by pathogenic TnT mutations such as TnT-I79N generates susceptibility to ventricular arrhythmias by Ca-dependent AP remodeling (2). As cardiac electrophysiological properties of mice differ substantially from humans, here we used cardiomyocytes (CM) derived from human induced pluripotent stem cells (hiPSC) to study the effect of increasing myofilament Ca sensitivity on cytosolic Ca buffering and cardiac action potential (AP).
We first generated hiPSCs lines from dermal fibroblasts of three healthy donors using standard approaches. One of the three hiPSC lines was edited using CRISPR/Cas9 and a heterozygous Ca-sensitizing TnT-I79N mutation introduced. Hence, this line was isogenic to the I79N line. The other two lines were named population controls. We then used the matrigel mattress method (3) to generate single rod-shaped CMs for each iPSC line and studied TnT-I79N protein levels, cytosolic Ca buffering and electrophysiology.
Analysis by nano-liquid chromatography mass spectrometry indicated that 43% of total TnT protein was mutant in I79N hiPSC-CM. Cytosolic Ca buffering was quantified in voltage clamped hiPSC-CMs as reported in (4). Cytosolic Ca binding affinity was significantly higher (=lower Kd values) in I79N compared to both isogenic control and population control hiPSC-CMs (Figure 1A). Furthermore, application of the Ca sensitizer EMD57033 (3 μM), which increases Ca sensitivity comparable to I79N (2), significantly lowered Kd values of population control hiPSC-CMs (Figure 1A). Maximal binding capacity Bmax, an estimate of total cytoplasmic Ca binding sites, was not altered by I79N or EMD treatment (data not shown).
Figure 1.
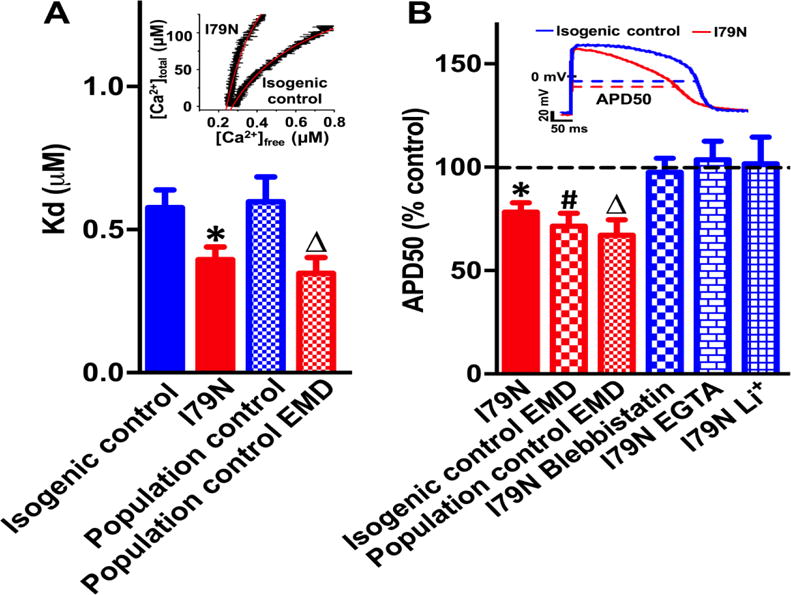
(A) Representative cytosolic Ca buffering curves (inset) and average Kd data. (B) Representative APs (inset) and average APD50 change in response to genetic or pharmacological interventions. Values are mean ± SEM. N=12-17 CM per group, P<0.05 versus isogenic (*,#) or population (Δ) control.
We then asked if increasing myofilament Ca buffering alters the human AP. APs were recorded in current clamp mode without exogenous Ca buffers added to intracellular solutions and analyzed as described in (5) during steady-state pacing at 0.5 Hz. Resting membrane potential, AP amplitude and AP upstroke velocity were comparable in all groups (data not shown). Compared to isogenic controls, APs of I79N hiPSC-CM were more triangulated (i.e., shorter early repolarization (APD50) without changes in late repolarization (APD90), Figure 1B) and exhibited beat-to-beat AP instability (Average ratio of interquartile range to median APD50: I79N 0.12±0.02 vs. isogenic control 0.07±0.01, n=17-21, P<0.05). Increasing buffering with 3 μM EMD had the same triangulating effect in isogenic and population control hiPSC-CMs (Figure 1B). Afterdepolarizations were not observed. Pretreatment with the Ca-desensitizer blebbistatin (3 μM) or excess cytosolic buffering (by adding 14 mM EGTA to intracellular solutions) prevented AP triangulation (Figure 1B). These results provide strong evidence that increased myofilament Ca buffering causes AP triangulation and AP instability.
How could increased myofilament Ca buffering alter the human cardiac AP? Any increased Ca buffering should reduce intracellular free [Ca]. We indeed found that I79N hiPSC-CM had lower peak Ca transients (data not shown), analogous to results obtained in I79N mice (2). As cellular Ca removal by NCX generates inward currents, lowering [Ca] would decrease inward NCX current and therefore shorten the AP, especially during early repolarization when intracellular [Ca] reaches its peak. To test this hypothesis, NCX-mediated Ca extrusion was blocked by rapidly replacing extracellular Na+ with Li+. NCX block with Li+ eliminated the AP differences between I79N and isogenic control CMs (Figure 1B).
Taken together, our results indicate that myofilament-dependent increases in cytosolic Ca buffering shortens early repolarization via a Ca-dependent, NCX-mediated mechanism, resulting in AP triangulation and AP instability, both of which are well-established predictors of increased arrhythmia risk (5). Our results not only provide novel insight into the regulation of the human cardiac AP by myofilament Ca buffering, but also suggest a new mechanism of how a pathogenic TnT mutation causes pro-arrhythmic AP changes in human CMs.
Acknowledgments
Disclosure of funding:
The work was supported in part by grants from the US National Institutes of Health (R01HL71670, R01HL128044, R01HL124935 to BCK, R01 HL128683 to JRP). S.P. was supported by NIGMS T32 GM07347 through the Vanderbilt Medical-Scientist Training Program and NHLBI F30 HL131179.
Abbreviations
Non-standard abbreviations
- HiPSCs
Human-induced pluripotent stem cells
- HCM
Hypertrophic cardiomyopathy
- Ca
Calcium
- CM
Cardiomyocytes
- NCX
Sodium Calcium Exchanger
- AP
Action potential
- APD50
90, Action potential duration at 50 and 90% repolarization
- SR
Sarcoplasmic reticulum
- Troponin T
TnT
Footnotes
There are no relationships with industry. The authors declare no financial conflict of interest
References
- 1.Varnava AM, et al. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–4. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 2.Schober T, et al. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circulation research. 2012;111:170–9. doi: 10.1161/CIRCRESAHA.112.270041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feaster TK, et al. Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation research. 2015;117:995–1000. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang HS, et al. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. Journal of molecular and cellular cardiology. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–13. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]


