Abstract
Background
Among patients undergoing non-cardiac surgery, major vascular surgery is associated with a high risk of perioperative myocardial infarction (MI). Currently there are no perioperative MI risk calculators accounting for intraoperative and postoperative risk factors in vascular surgery patients. We aimed to investigate specific risk factors for perioperative MI after major open vascular surgery to determine which patients are at highest risk of MI, and the association of perioperative MI with perioperative transfusion.
Methods
This statewide, retrospective cohort study analyzed risk factors for perioperative MI in major open vascular surgery between July 2012 and December 2015 using the Michigan Surgical Quality Collaborative, a multicenter quality collaborative. Patients were identified using current procedure terminology codes including open abdominal aortic aneurysm repairs (oAAA), aortobifemoral bypasses (AFB) and lower extremity bypasses (LEB). Rates of myocardial infarction were described for each procedure. A priori, preoperative, intraoperative and postoperative variables were evaluated using univariate, and multivariable statistics after adjusting for intraoperative factors including: anesthesia type, intraoperative blood loss, intraoperative transfusion and intraoperative vasopressor medications.
Results
A total of 3,689 patients underwent major open vascular surgery, including 375 oAAA, 392 AFB, and 2,922 LEB procedures. The overall incidence of MI was 2.4%, varying from 1.8% for aortobifemoral bypass, 2.4% for lower extremity bypass and 3.7% for open abdominal aortic aneurysm repair. Although preoperative risk factors for myocardial infarction included age, American Society of Anesthesiologists score, diabetes, coronary artery disease, congestive heart failure, use of beta-blocker, lower preoperative hematocrit and surgical priority (urgent/emergent cases), after adjusting for intraoperative risk factors all preoperative risk factors were not significant with the exception of surgical priority. After adjusting for intraoperative factors, only surgical priority (OR=1.70, 95% CI [1.01–2.85], p<.001) and postoperative transfusion (OR = 2.65, 95% CI [1.59–4.44], p<.001) was associated with myocardial infarction and higher nadir hematocrit was inversely associated with myocardial infarction (OR = 0.89, 95% CI [.85–.94], p<.001).
Conclusions
Among vascular surgery patients undergoing major open vascular surgery, surgical priority was the only preoperative risk factors were independently associated with MI, and only postoperative variables such as nadir hematocrit and postoperative transfusion were associated with MI. This suggests minimizing intraoperative blood loss and prioritizing early intraoperative transfusion may be potential targets for process improvement.
Keywords: Perioperative myocardial infarction, major open vascular surgery, Michigan Surgical Quality Collaborative (MSQC), transfusion, postoperative transfusion, intraoperative transfusion
1.1 INTRODUCTION
Among patients undergoing non-cardiac surgery, vascular surgery is a leading cardiac risk factor.(1) Acute myocardial infarction (MI) following major vascular surgery has been estimated to range from 1.6–6.6%.(2–4) The Revised Cardiac Risk Index (RCRI) is one of the most widely used preoperative risk calculators, but there is ongoing debate regarding the accuracy of this tool in assessing cardiac risk in vascular surgery patients.(1,3,5) The Vascular Quality Initiative (VQI) Cardiac Risk Index (CRI) is a more recent vascular-specific MI prediction tool for the preoperative period, however, for both the RCRI and the VQI CRI, little is known regarding risk factors in the intraoperative and postoperative period.(3,5) Since many patients will be considered moderate to high risk for surgery, strategies to minimize risk within the operating room and thereafter are lacking.
Intraoperative blood loss and postoperative blood transfusion are common in vascular surgery and have been identified as a predictor for poor outcomes following major vascular surgery.(6) Postoperative blood transfusion has been associated with a three to six-fold increase in postoperative mortality in vascular surgery patients.(7,8) Currently, there is conflicting literature describing the cardiac consequences of blood transfusion in vascular surgery patients, resulting in a variation of clinical practice.(9–13) To date, there are no evidence-based guidelines to determine appropriate transfusion thresholds in the perioperative vascular surgery patient.
Therefore, identifying perioperative factors that increase risk of MI in an already vulnerable population is critical. As such, we aimed to investigate specific risk factors for intra- and postoperative MI after major open vascular surgery to determine which patients are at highest risk of MI, and the association of perioperative MI with intra- and postoperative transfusion.
1.2 MATERIALS AND METHODS
1.2.1 Data source
We performed a statewide, retrospective observational cohort study using the Michigan Surgical Quality Collaborative (MSQC),(14) a statewide quality collaborative of Michigan Hospitals. The collaborative encompasses 72 participating hospitals and includes data on 14 procedures from within the disciplines of general surgery, vascular surgery, and obstetrics and gynecology. Current procedure terminology (CPT) codes include 306 unique procedures. The sampling technique involves trained Surgical Clinical Quality Reviewers (SCQRs) using a hybrid approach. Cases are first drawn from all potential cases meeting criteria and then stratified into procedural groups. A simple random sampling then occurs to select the cases for data retrieval. Once these cases are retrieved, the SCQR performs a comprehensive chart review including review of preoperative H&P, operative reports, progress notes, laboratory results, radiology studies/reports and discharge summaries. The MSQC database is then populated by the SCQR using the pertinent perioperative information. Access to MSQC database was granted through a standard institution review board (IRB) and the MSQC compliance office after completion of a data user agreement. All patient information was de-identified and patient consent was not required.
1.2.2 Patient selection
All patients undergoing major open vascular surgery at participating MSQC hospitals from July 2012 to December 2015 were included. The procedures were identified using CPT codes (Appendix 1) and included open abdominal aortic aneurysm repair (oAAA), aortobifemoral bypass (AFB) and infrainguinal lower extremity bypass (LEB). Urgent, emergent and elective cases were included. Patients undergoing endovascular abdominal aortic aneurysm repair (EVAR), extra-anatomic bypass (i.e. axillary to femoral bypass) and visceral bypasses were not included. Patient demographics were examined, including: age, gender, race, and comorbidities, use of beta-blocker, and smoking status. Additionally, surgical priority or whether a case was elective versus non-elective were also included. Aspirin and statin use was not documented in the MSQC dataset for the time period examined. Perioperative factors were also examined to identify predictors of perioperative MI including anesthesia type, packed red blood cell (PRBC) transfusion, estimated blood loss (EBL), use of intraoperative vasopressor medications, postoperative transfusion and nadir hematocrit.
1.2.3 Outcomes
The primary outcome was perioperative myocardial infarction (MI) after major open vascular surgery. Secondary outcomes included 30-day mortality and postoperative complications including superficial, deep and organ space surgical site infection (SSI), pneumonia, acute renal insufficiency, urinary tract infection (UTI), stroke/cerebrovascular accident (CVA), postoperative cardiac arrest, sepsis, severe sepsis, Clostridium difficile (C. difficile) or central-line associated bloodstream infections (CLASBI).
MSQC utilizes the universal American Heart Association (AHA) MI definition(15) to define perioperative MI, including a rise in a cardiac biomarker (preferably troponin) with at least one value above the 99th percentile upper reference limit and at least one of the following: symptoms of ischemia, new ST segment or T wave changes, new left bundle branch block, development of pathologic Q waves on ECG, imaging evidence of new regional wall motion abnormality or identification of an intracoronary thrombus by angiography or autopsy. The AHA does not differentiate between ST-elevation MI (STEMI) and non ST-elevation MI (NSTEMI) and thus, this granularity was not present in MSQC.
1.2.4 Statistical Analysis
The analysis was performed using an MSQC de-identified patient level dataset. Patient demographics, comorbidities and procedural details including surgery type, anesthesia type, use of intraoperative vasopressors, intra and postoperative blood transfusions, EBL and nadir hematocrit were all variables used in the analysis. These variables were compared between the group of patients without evidence of MI and the group of patients with perioperative MI. Continuous variables were evaluated with a Student t-test and Wilcoxon Rank Sum. Categorical variables were evaluated using X2 test. Univariate analysis was performed, examining the association of the primary outcome, MI, with pre, intra and postoperative factors. Using a stepwise logistic regression model to adjust for baseline differences, intra and postoperative factors including all variables with a p value < .10, were included in the multivariable analysis. Multivariable logistic regression was used to identify factors associated with perioperative MI. Finally, we performed Student t-test to analyze secondary outcomes including 30-day mortality and postoperative complications in patients with and without perioperative MI. Analyses were completed using STATA 14 (StataCorp LP, College Station, Texas) with an alpha level of .05.
1.3 RESULTS
In total there were 3,689 patients underwent major open vascular surgery, including 375 oAAA, 392 AFB, and 2,922 LEB procedures. The overall incidence of perioperative MI was 2.4% (N= 90), varying from 1.8% (N=7) for AFB, 2.4% (N=69) for LEB and 3.7% (N=14) for oAAA repair (Figure 1).
Figure 1.
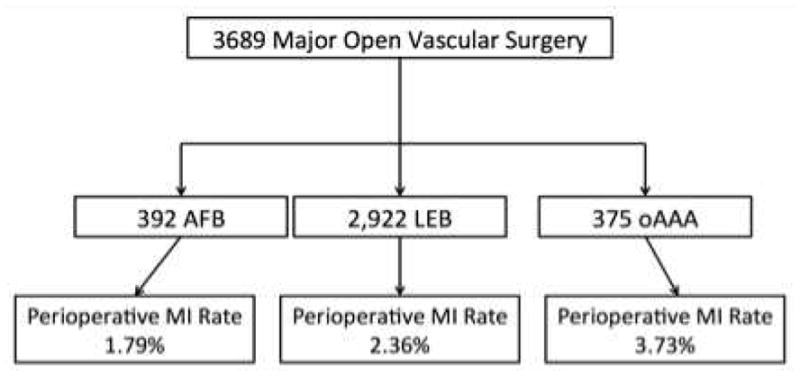
Flow diagram demonstrating the procedure breakdown and perioperative MI rate in each group.
AFB, aortobifemoral bypass; LEB, infrainguinal lower extremity bypass; oAAA, open abdominal aortic aneurysm repair; MI, myocardial infarction.
1.3.1 Patient characteristics
Patient baseline characteristics are summarized in Table I. The MI group of patients were older, had a higher percentage of women and patients with an American Society of Anesthesiologists (ASA) score ≥ 4. Patients in the perioperative MI group also had fewer patients with tobacco use in the last year, and were more likely to have a history of coronary artery disease, congestive heart failure, and diabetes and be on beta-blocker therapy. This group also had a lower pre- and postoperative hematocrit, and were more likely to a non-elective case (urgent or emergent) and have an EBL >2L, and intra and postoperative blood transfusions.
Table I.
Baseline characteristics, intra and postoperative factors of all major open vascular surgeries without perioperative myocardial infarction versus patients with perioperative myocardial infarction.
| No Myocardial Infarction (n=3599), No. (%) | Myocardial Infarction (n=90), No. (%) | P value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age*, years (SD)† | 65.8 (10.7) | 70.8 (11) | <.001 |
| Sex (male) | 2411 (67.0) | 51 (57) | .040 |
| Race (non black)‡ | 2844 (81.6) | 68 (76) | .214 |
| BMI§(SD)† | 27.6 (7.2) | 26.31 (4) | .322 |
| ASA (≥ 4) | 1016 (28.2) | 40 (44) | .001 |
| Decreased functional status[] | 387 (10.8) | 15 (17) | .072 |
| Tobacco (within 1 year) | 1952 (54.2) | 34 (38) | .002 |
| History of DVT|| | 488 (13.6) | 10 (11) | .494 |
| Diabetes | 1336 (37.1) | 45 (50) | .013 |
| Hypertension | 2988 (83.0) | 79 (88) | .234 |
| Coronary artery disease | 1693 (47.0) | 60 (67) | <.001 |
| Congestive Heart Failure | 53 (1.5) | 4 (4) | .024 |
| Chronic lung disease | 836 (23.2) | 24 (27) | .446 |
| Dialysis | 142 (4.0) | 6 (7) | .194 |
| Urinary tract infection | 65 (1.8) | 4 (4) | .068 |
| Beta blocker¶ | 1841 (54.1) | 58 (69) | .007 |
| Preoperative Transfusion | 115 (3.2) | 6 (7) | .068 |
| Preoperative Hematocrit# (SD)† | 38.2 (6.5) | 35 (7) | <.001 |
| Surgical Priority (Urgent or emergent cases) | 1124 (31.2) | 46 (51) | <.001 |
| Intraoperative Factors | |||
| Anesthesia Type (General) | 3347 (93.0) | 82 (91) | .490 |
| Intraoperative Vasopressors | 2428 (67.5) | 65 (72) | .341 |
| Intraoperative Blood Transfusions | 628 (17.6) | 34 (38) | <.001 |
| EBL (>2L) | 241 (6.7) | 16 (18) | <.001 |
| Postoperative Factors | |||
| Postoperative Transfusion | 533 (14.8) | 46 (51) | <.001 |
| Nadir Hematocrit (SD)† | 28.3 (6.2) | 23.01 (5) | <.001 |
Data missing on 3 individuals.
(SD) indicates contiuous variables with summary measure of mean (standard deviation) and P value from Student t-test. Data is coded as “unknown” in 114 individuals.
Data missing on 2,338 individuals.
Data missing in 24 individuals.
Data missing on 14 individuals.
Data missing on 204 individuals.
Data missing on 61 individuals.
Data missing on 181 individuals.
Categorical vairables are summarized by N (%), and P values are calculated from the X2 test. ASA, American Society of Anesthesiologists; BMI, body mass index; DVT, deep venous thrombosis; EBL, estimated blood loss.
1.3.2 Outcomes
Although preoperative risk factors for MI included age, female sex, ASA, diabetes, coronary artery disease, congestive heart failure, beta-blocker usage, preoperative hematocrit and non-elective cases (Table II), after adjusting for intraoperative risk factors, all preoperative risk factors were not significant with the exception of surgical priority (Table III). The only risk factor associated with higher perioperative MI after adjusting for intraoperative factors was surgical priority (OR=1.70, 95% CI [1.01–2.85], p<.001) and postoperative transfusion (OR = 2.65, 95% CI [1.59–4.65], p<.001). Higher nadir hematocrit (OR = 0.89, 95% CI [0.85–0.94], p <.001) was inversely associated with perioperative MI after adjustment.
Table II.
Univariate analysis of predictors of perioperative MI (pre, intra and postoperative).
| OR (95% CI) | P value | |
|---|---|---|
| Baseline Characteristics | ||
| Age* | 1.04 (1.02–1.07) | <.001 |
| Sex (male) | 0.64 (0.42–0.98) | .042 |
| Race (non black)† | 0.73 (0.44–1.20) | <.001 |
| ASA (>=4) | 2.03 (1.33–3.10) | <.001 |
| Decreased functional status‡ | 1.67 (0.95–2.94) | .075 |
| Tobacco (within 1 year) | 0.51 (0.33–0.79) | <.002 |
| Diabetes | 1.69 (1.11–2.57) | .014 |
| Hypertension | 1.47 (0.78–2.78) | .237 |
| Coronary artery disease | 2.25 (1.45–3.51) | <.001 |
| Congestive Heart Failure | 3.11 (1.10–8.79) | .032 |
| Chronic lung disease | 1.20 (0.75–1.93) | .447 |
| Dialysis | 1.74 (0.75–4.05) | .199 |
| Peripheral vascular disease | 1.64 (0.95–2.83) | .076 |
| Beta blocker§ | 1.89 (1.18–3.02) | .008 |
| Preoperative Transfusion | 2.16 (0.93–5.06) | .075 |
| Preoperative Hematocrit[] | 0.94 (0.91–0.97) | <.001 |
| Surgical Priority (Urgent or emergent cases) | 2.30 (1.51–3.50) | <.001 |
| Intraoperative Factors | ||
| Anesthesia Type (General) | .77 (.37–1.61) | .491 |
| Intraoperative Vasopressors | 1.25 (0.79–2.00) | .342 |
| Intraoperative Blood Transfusions | 2.87 (1.86–4.44) | <.001 |
| EBL (>2L) | 3.01 (1.73–5.25) | <.001 |
| Postoperative Factors | ||
| Postoperative Transfusion | 6.01 (3.94–9.18) | <.001 |
| Higher Nadir Hematocrit|| | 0.86 (0.82–0.89) | <.001 |
Data missing on 3 individuals.
Data is coded as “unknown” in 114 individuals.
Data missing in 24 individuals.
Data missing on 204 individuals.
Data missing on 61 individuals.
Data missing on 181 individuals.
OR, odds ratio; ASA, American Society of Anesthesiologists; EBL, estimated blood loss.
Table III.
Multivariable analysis of predictors of perioperative MI (pre, intra and postoperative).
| OR (95% CI) | P value | |
|---|---|---|
| Baseline Characteristics | ||
| Age | 1.02 (1.00–1.04) | .120 |
| Sex (male) | 0.91 (0.56–1.47) | .696 |
| Race (non black) | 1.03 (0.58–1.83) | .907 |
| ASA Score (>=4) | 1.14 (0.69–1.87) | .603 |
| Decreased functional status | 1.03 (0.55–1.94) | .920 |
| Tobacco (within 1 year) | 0.71 (0.42–1.20) | .202 |
| Diabetes | 1.46 (0.89–2.41) | .137 |
| Coronary artery disease | 1.52 (0.89–2.60) | .129 |
| Congestive Heart Failure | 0.60 (0.13–2.81) | .520 |
| Peripheral vascular disease | 1.94 (0.99–3.81) | .054 |
| Beta blocker | 1.47 (0.86–2.52) | .162 |
| Preoperative Transfusion | 0.96 (0.37–2.50) | .935 |
| Preoperative Hematocrit | 1.01 (0.97–1.06) | .509 |
| Surgical Priority (Urgent or emergent cases) | 1.70 (1.01–2.85) | .044 |
| Intraoperative Factors | ||
| Anesthesia Type (General) | 0.74 (0.32–1.73) | .489 |
| Intraoperative Vasopressors | 1.07 (0.63–1.81) | .811 |
| Intraoperative Blood Transfusions | 1.06 (0.59–1.89) | .846 |
| EBL (>2L) | 2.12 (0.97–4.65) | .061 |
| Postoperative Factors | ||
| Postoperative Transfusion | 2.65 (1.59–4.44) | <.001 |
| Higher Nadir Hematocrit | 0.89 (0.85–0.94) | <.001 |
Number of observations in logistic regression (n=3151). OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; EBL, estimated blood loss.
1.3.3 Complications
Table IV summarizes overall 30-day complications and mortality in patients with and without perioperative MI. Death, pneumonia, acute renal insufficiency, stroke/CVA, postoperative cardiac arrest, sepsis, severe sepsis and central-line associated bloodstream infection were all more common in the perioperative MI group. The three most common complications seen in the MI group were acute renal insufficiency (22.2% vs. 3.1%, p < .001), pneumonia (20.0% vs. 2.7%, p < .001), and postoperative cardiac arrest (12% vs. 0.9%, p < .001). Uncommon complications, which were still significantly higher in the MI group, were CLABSI (1% vs. 0.1%, p = .001), stroke/CVA (2% vs. 0.5%, p = .035) and severe sepsis (10% vs. 2.2%, p < .001). Death was significantly higher in the MI group (32% vs. 3.8%, p < .001). To further delineate complications and mortality we performed a subgroup analysis to evaluate these outcomes in all the non-elective cases and elective cases (Appendix 2). The only difference we found during the sub-group analysis was stroke and CLASBI were no longer significantly different in the non-elective cases between the patients with MI compared to the patients without MI. For the elective cases, the sub-group analysis demonstrated that stroke and severe sepsis were no longer significantly different between the patients with and without MI. Death and all other complications were significantly different between patients with and without MI for both non-elective and elective cases.
Table IV.
30-day mortality and postoperative complications in patients with and without perioperative MI.
| No Myocardial Infarction (n=3599), No. (%) | Myocardial Infarction (n=90), No. (%) | P value | |
|---|---|---|---|
| Death* | 136 (3.8) | 28 (32) | <.001 |
| Superficial SSI | 177 (4.9) | 6 (7) | .450 |
| Deep SSI | 104 (2.9) | 2 (2) | .708 |
| Organspace SSI | 26 (0.7) | 1 (1) | .669 |
| Pneumonia | 98 (2.7) | 18 (20) | <.001 |
| Acute renal insufficiency | 111 (3.1) | 20 (22) | <.001 |
| Urinary tract infection | 65 (1.8) | 4 (4) | .068 |
| Stroke/CVA | 19 (0.5) | 2 (2) | .035 |
| Postoperative cardiac arrest | 31 (0.9) | 11 (12) | <.001 |
| Sepsis | 85 (2.4) | 9 (10) | <.001 |
| Severe sepsis | 80 (2.2) | 9 (10) | <.001 |
| C-difficile | 33 (0.9) | 1 (1) | .849 |
| CLABSI | 2 (0.1) | 1 (1) | .001 |
Data missing in 19 individuals.
N (%) is used as a summary measure. P values are calculated from the X2 test. SSI, surgical site infection; CVA, cerebrovascular accident; C. difficile, Clostridium difficile; CLABSI, central-line associated bloodstream infections.
1.4 DISCUSSION
We found that the overall rate of perioperative MI in patients undergoing vascular surgery was 2.4%, with the highest incidence in patients undergoing oAAA repair (3.7%). Additionally, we found that the risk of perioperative MI was associated with non-elective surgical priority and postoperative blood transfusion, while having a higher nadir hematocrit level was inversely associated with postoperative MI. Lastly, patients who suffered perioperative MI had higher complication rates and mortality, underscoring the necessity of preventing this uncommon but morbid complication.
1.4.1 Understanding risk before & after surgery
There has been substantial effort to create accurate, efficient and effective preoperative risk assessment tools to aid clinicians in risk stratification for their vascular surgery patients.(3,5,16) Most of these tools look exclusively at preoperative variables such as patient comorbidities and history of coronary revascularization. While these tools help guide decision-making preoperatively, there is no tool available to surgeons to be used in the perioperative period. Understanding the effects of perioperative transfusion and anemia on outcomes will allow surgeons to provide evidence guided, patient-specific care. Our study demonstrated the importance of these variables association with perioperative MI. This suggests that intraoperative resuscitation may play a role in determining perioperative risk of MI.
1.4.2 Timely intraoperative resuscitation
This study demonstrates the importance of perioperative anemia. Although some studies have demonstrated that intraoperative anemia is associated with adverse outcomes, the effect of transfusion is less clear.(17) Our data suggest that intraoperative transfusion is not associated with perioperative MI. In contrast, it appears that postoperative transfusion and anemia (nadir hematocrit) are not associated with perioperative MI. Although a causal relationship cannot be established, this data suggests that appropriate transfusion in the operating room does not increase the risk of MI. Based upon these results, it is imperative to facilitate prompt and adequate intraoperative communication between the surgical and anesthesia teams to ensure execution of optimal intraoperative transfusion. Based on this finding and our experience, we believe that prompt transfusion while in the controlled setting of the operating room is necessary. Once a patient is in the postoperative period, there is potential for delayed resuscitation and worse outcomes.
Importantly, we found that postoperative transfusion was associated with a higher risk of perioperative MI. Patients requiring postoperative transfusion may represent a subset of higher acuity patients that continue to require transfusion due to ongoing bleeding or shock. According to Obi et al, the postoperative transfusion may be a more complex phenomenon including dramatic fluid shifts and potential peak releases of stress hormones.(11) It is also unclear whether these are patients experiencing an acute coronary syndrome versus demand ischemia in the setting of known coronary disease.(12,13)
1.4.3 Implications on clinical decision-making
This study highlights the importance of strategic intraoperative transfusion in patients undergoing major vascular operations. As such, this raises the question as to what the optimal transfusion threshold should be for patients identified as high risk for perioperative MI. A study from Abdelsattar et al on the variation in transfusion practices and the effect on outcomes after non-cardiac surgery found that postoperative transfusions after non-cardiac surgery were associated with increased adverse postoperative outcomes, with the exception of postoperative MI.(18) While this does contradict our result that postoperative blood transfusion is associated with MI, it supports our finding that higher postoperative hematocrit is associated with a lower rate of MI. There are several key differences in the two analyses, which could explain these disparate findings. Importantly; our data reflects a higher risk population as compared to the study by Abdelsattar et al, as the previous study sample included only 25.0% of vascular surgery procedures and included more than 50.0% of the procedures from gastrointestinal surgery, including laparoscopic cholecystectomy and appendectomy. These cohorts represent vastly different risk of MI at baseline. Secondly, Abdelsattar and colleagues designed their study to examine the effect of postoperative transfusion on outcomes of surgery. We have examined a much broader question of identifying intraoperative and postoperative risk factors for MI in a vascular surgery specific context. In the absence of a randomized control trial of liberal and restrictive transfusion strategies among patients undergoing major vascular surgery, this data suggests that intraoperative transfusion is critical to mitigating risk of MI and late transfusion appears to increase the risk of MI.
1.4.4 Limitations
Limitations of our study include the retrospective nature of analysis. Unfortunately, key variables such as preoperative aspirin, statin medication use, preoperative cardiac stress tests, preoperative coronary revascularization and the differentiation of STEMI versus NSTEMI were not included in our analysis because they are not currently captured by MSQC. These factors have previously been identified as modifiable risk fators.(19) Within the state of Michigan aspirin use exceeds 80% among patients undergoing major vascular surgery, however patient specific data was not available. Another key limitation is the lack of granular intraoperative details including overall operative times, potential-prolonged periods of hypotension, blood pressure lability and timing of intraoperative transfusion in relation to acute intraoperative blood loss. The ability to merge surgical and anesthesia intraoperative details will be an invaluable quality collaborative resource in the future that could address these gaps, within the state of Michigan, collaborative efforts are underway to develop a combined dataset. Additionally, our study is limited by the possibility of missing data, although this was rare. Potential for errors in data entry are also unlikely given the robust mechanisms in place to ensure data reliability at MSQC including, data input by trained and validated SCQRs and routine audits for data integrity. As previously described, there are mechanisms in place within the MSQC to regulate and detect erroneous entries.(14)
1.5 CONCLUSION
In summary, surgical priority was the only preoperative risk factor independently associated with MI after accounting for intraoperative and postoperative factors. However, postoperative transfusion was associated with MI and a higher nadir hematocrit was inversely associated with perioperative MI. Taken together, this data suggests that preoperative risk stratification based on comorbidities may not successfully predict patients at the highest risk of perioperative MI. However, minimizing excessive operative blood loss, avoiding physiologic stress and optimizing intraoperative transfusion may mitigate risk of MI.
Supplementary Material
Acknowledgments
Funding: This work and Danielle C. Sutzko is supported by the National Institutes of Health [T32 NIH HL076123 Vascular Surgery Training grant].
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eagle KA, Rihal CS, Mickel MC, Holmes DR, Foster ED, Gersh BJ, et al. Cardiac Risk of Noncardiac Surgery. Circulation [Internet] 1997 Sep 16;96(6):1882–LP-1887. doi: 10.1161/01.cir.96.6.1882. Available from: http://circ.ahajournals.org/content/96/6/1882.abstract. [DOI] [PubMed] [Google Scholar]
- 2.Simons JP, Baril DT, Goodney PP, Bertges DJ, Robinson WP, Cronenwett JL, et al. The effect of postoperative myocardial ischemia on long-term survival after vascular surgery. J Vasc Surg [Internet] 2013 Dec;58(6):1600–8. doi: 10.1016/j.jvs.2013.06.062. Available from: http://www.sciencedirect.com/science/article/pii/S0741521413012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertges DJ, Neal D, Schanzer A, Scali ST, Goodney PP, Eldrup-Jorgensen J, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg United States. 2016 Nov;64(5):1411–1421.e4. doi: 10.1016/j.jvs.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet (London, England). England. 2005 Dec;366(9501):1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and Prospective Validation of a Simple Index for Prediction of Cardiac Risk of Major Noncardiac Surgery. Circulation [Internet] 1999 Sep 7;100(10):1043–LP-1049. doi: 10.1161/01.cir.100.10.1043. Available from: http://circ.ahajournals.org/content/100/10/1043.abstract. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Barbieri A, Politi L, Di Girolamo A, Malagoli A, Grimaldi T, et al. Perioperative Red Blood Cell Transfusion and Outcome in Stable Patients after Elective Major Vascular Surgery. Eur J Vasc Endovasc Surg [Internet] Elsevier Ltd. 2009;37(3):311–8. doi: 10.1016/j.ejvs.2008.12.002. Available from: http://dx.doi.org/10.1016/j.ejvs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 7.O’Keeffe SD, Davenport DL, Minion DJ, Sorial EE, Endean ED, Xenos ES. Blood transfusion is associated with increased morbidity and mortality after lower extremity revascularization. J Vasc Surg [Internet] Elsevier Inc. 2010;51(3):616–621. e3. doi: 10.1016/j.jvs.2009.10.045. Available from: http://dx.doi.org/10.1016/j.jvs.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Valentijn TM, Hoeks SE, Bakker EJ, Van De Luijtgaarden KM, Verhagen HJ, Stolker RJ, et al. The impact of perioperative red blood cell transfusions on postoperative outcomes in vascular surgery patients. Ann Vasc Surg [Internet] Elsevier Inc. 2015;29(3):511–9. doi: 10.1016/j.avsg.2014.08.021. Available from: http://dx.doi.org/10.1016/j.avsg.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 9.SVR, JGJ, RAH, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA [Internet] 2004 Oct 6;292(13):1555–62. doi: 10.1001/jama.292.13.1555. Available from: http://dx.doi.org/10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 10.Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;2016(10):2012–3. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obi AT, Park YJ, Bove P, Cuff R, Kazmers A, Gurm HS, et al. The association of perioperative transfusion with 30-day morbidity and mortality in patients undergoing major vascular surgery. J Vasc Surg [Internet] Elsevier. 2015;61(4):1000–1009. e1. doi: 10.1016/j.jvs.2014.10.106. Available from: http://dx.doi.org/10.1016/j.jvs.2014.10.106. [DOI] [PubMed] [Google Scholar]
- 12.Amin M, Fergusson D, Aziz A, Wilson K, Coyle D, Hébert P. The cost of allogeneic red blood cells – a systematic review. Transfus Med [Internet] Blackwell Science Ltd. 2003;13(5):275–86. doi: 10.1046/j.1365-3148.2003.00454.x. Available from: http://dx.doi.org/10.1046/j.1365-3148.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 13.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. N Engl J Med [Internet] 6. Vol. 340. Massachusetts Medical Society; 1999. Feb 11, A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care; pp. 409–17. Available from: http://dx.doi.org/10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 14.Michigan Surgical Quality Collaborative. Blue Cross Blue Shield of Michigan and Blue Care Network (BCBSM/BCN) [Internet] [cited 2017 Mar 14]. Available from: www.msqc.org.
- 15.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. United States. 2012 Oct;126(16):2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 16.Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381–7. doi: 10.1161/CIRCULATIONAHA.110.015701. [DOI] [PubMed] [Google Scholar]
- 17.Hogervorst EK, Rosseel PMJ, van de Watering LMG, Brand A, Bentala M, van der Bom JG, et al. Intraoperative Anemia and Single Red Blood Cell Transfusion During Cardiac Surgery: An Assessment of Postoperative Outcome Including Patients Refusing Blood Transfusion. J Cardiothorac Vasc Anesth United States. 2016 Apr;30(2):363–72. doi: 10.1053/j.jvca.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Abdelsattar ZM, Hendren S, Wong SL, Campbell DAJ, Henke P. Variation in Transfusion Practices and the Effect on Outcomes After Noncardiac Surgery. Ann Surg United States. 2015 Jul;262(1):1–6. doi: 10.1097/SLA.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 19.Henke PK, Zamora-Berridi G, Englesbe MJ, Cai S, Brooks L, McKeown E, et al. A case-cohort study of postoperative myocardial infarction: impact of anemia and cardioprotective medications. Surgery United States. 2014 Oct;156(4):1018–1026. doi: 10.1016/j.surg.2014.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


