Abstract
In patients undergoing surgical resection of a metastatic brain tumor, whole brain radiation therapy reduces the risk of recurrence and neurologic death. Focal radiation has the potential to mitigate neurocognitive side effects. We present an institutional experience of postoperative radiosurgery for the treatment of brain metastases. A retrospective review of a prospectively maintained institutional radiosurgery database was performed for the years 2005–2015 identifying all adult patients treated with postoperative radiosurgery to the tumor bed. Primary endpoints include local recurrence and postoperative LMD. Kaplan-Meier curves and Cox regression were used to evaluate time to local recurrence and postoperative LMD. Ninety-one patients received adjuvant focal radiation for a brain metastasis. Median radiographic follow-up among patients who had not developed a local failure was 9 months. Of the 91 patients, 20 (22%) developed local recurrence and 32 (35%) experienced postoperative LMD. Freedom from local recurrence and LMD at 1 year was 84% and 69%, respectively. In multivariable models, predictors of local failure included the presence of more than one brain metastasis (HR=2.65, p = 0.04) with a preoperative tumor diameter of >3 cm (HR=4.16, p = 0.06) trending toward significance. There was a trend to a higher risk of LMD with > 1 tumor (HR 2.07, p = 0.06) and breast cancer (HR 2.37, p=0.07). More than one metastasis is an independent predictor of local and leptomeningeal failure following postoperative radiosurgery. The high rate of LMD was likely related to the liberal definition of LMD to include focal dural recurrences.
Keywords: brain metastasis, radiation, radiosurgery, local failure, leptomeningeal disease
Introduction
Brain metastases are the most common central nervous system malignancy with an estimated prevalence among cancer patients of 10%.[1–3] The incidence of brain metastases is increasing, likely due to closer surveillance, improved control of systemic cancer, and prolonged survival.[4] Their presence portends a poor prognosis with survival generally measured in weeks-months.[2] Current National Comprehensive Cancer Network guidelines recommend a combination of surgery and radiation for the treatment of resectable lesions with reasonable systemic treatment options.[5]
Following two landmark trials by Patchell et al., surgical resection followed by whole brain radiation therapy (WBRT) became the most widely accepted paradigm for the treatment of solitary or oligometastases.[6, 7] The addition of WBRT to surgical resection reduced tumor recurrence and was associated with a lower rate of neurologic death.[7] However, WBRT subjects a large volume of normal tissue to therapeutic radiation doses and has been associated with deleterious neurocognitive sequela.[8–10] As treatment has become more individualized, there has been an emphasis on balancing treatment efficacy against neurotoxicity, especially in patients with a good prognosis.[4]
The equivalent overall survival and superior neurocognitive outcomes of stereotactic radiosurgery (SRS) alone versus WBRT plus SRS for the treatment of intact brain metastases[8, 11, 12] has led some physicians to prefer focal radiation as first line for the adjuvant treatment of resected brain metastases. We present an institutional experience of postoperative radiosurgery for the treatment of brain metastases with a focus on local failure and leptomeningeal disease.
Materials and Methods
Patient Selection
A retrospective review of a prospectively maintained radiosurgery database was performed at a single academic institution for the years 2005–2015 identifying all patients treated with postoperative radiosurgery. Given the concern for cognitive impairment following whole brain radiation therapy (WBRT), the preferred modality for adjuvant radiation at this institution is radiosurgery. Thus, all adult patients undergoing surgical resection of a brain metastasis were treated with radiosurgery to the surgical cavity in the absence of extenuating circumstances (i.e. unexpected death, innumerable metastases, change in goals of care, loss to follow up). Inclusion criteria: adult patient with a newly diagnosed intracranial metastasis treated with surgical resection (gross total or subtotal resection) followed by postoperative radiosurgery (single or hypofractionated treatment regimen) to the tumor bed. Exclusion criteria: age < 18 years, radiosensitive tumor pathology (small cell lung cancer and lymphoma), prior WBRT, biopsy as only surgical intervention, pathology consistent with primary brain tumor, lack of radiographic follow up, and lack of clinical follow up. The records of included patients were reviewed and the following data was collected: age, sex, pathology of intracranial metastasis, number of metastases, size of index metastasis (tumor undergoing resection and postoperative radiation), location of index metastasis, presence of preoperative leptomeningeal involvement, date of surgery, surgical result (gross total vs subtotal resection) date of radiosurgery, radiosurgery dose and schedule, adverse events, radiographic follow up, and clinical follow up. This study was approved by the University of Alabama at Birmingham Institutional Review Board.
Radiosurgery Technique
All patients received either Gamma Knife radiosurgery (model B or C) or linear accelerator (LINAC) radiosurgery with a VMAT technique. The modality of choice has transitioned gradually from predominantly Gamma Knife to predominantly LINAC over the last 2–3 years of included cases. Initially, larger cavities were preferentially treated with LINAC due to efficiency and the ability to hypofractionate treatment. The decision to treat patients with a single fraction or hypofractionated dose schedule was based upon maximum diameter greater than 3–4 cm. LINAC patients received either a single dose of radiation or five fractions of 5–6 Gy (total 25 Gy or 30 Gy); Gamma Knife was delivered as a single dose of radiation. Median dose single fraction treatment was 16 Gy (range 10 – 20 Gy; mean 15.9 Gy). The target volume in all cases was the cavity without an additional margin. GKS was typically prescribed to the 50% isodose line. LINAC treatment was prescribed to the isodose line that covered 99–100% of the target with typical hotspots of 30–80% beyond the prescription dose allowed. Dose heterogeneity was not penalized in the cost function of the LINAC plans. LINAC delivery was with 2–4 arcs with a 2400 MU/min flattening filter free mode utilizing TrueBeam STX and the HD-120 multi-leaf collimator with a central leaf resolution of 2.5 mm. LINAC patients were treated frameless with KV and cone beam CT (CBCT) image guidance for alignment; localization accuracy of KV and CBCT was within 1 mm. Additional details of the single isocenter VMAT planning and delivery have been published.[13] Radiosurgery timing depended on the volume of the post-operative cavity and plans to integrate radiosurgery with systemic therapy. Patients were generally treated based upon a treatment planning MRI performed postoperative day 1 (treatment within 2 weeks of surgery) or based upon MRI at one month follow-up (treatment generally within 5–6 weeks of surgery).
Radiographic Definitions
Intracranial metastases were initially diagnosed with contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) with the typical findings of a contrast enhancing lesion with surrounding vasogenic edema. The number of intracranial metastases were totaled at the time of radiosurgery. Size of the index tumor was reported at the maximum diameter on axial imaging in centimeters (cm). Location of the index metastasis was reported as supratentorial or infratentorial. The presence of preoperative leptomeningeal involvement was defined as any metastasis that contacts a leptomeningeal surface; this includes a superficial metastasis that abuts arachnoid and dura without obvious dural enhancement. Gross total resection was defined as lack of contrast enhancing mass on postoperative imaging.
Local failure was defined as the development or progression of nodular contrast enhancement within 5 millimeters (mm) of the index metastasis. Subsequent operative interventions within 5 mm of the index tumor cavity that resulted in pathologic specimens consistent with tumor were also considered local failure. Diffuse tumor cavity enhancement or nodular enhancement that resolved on subsequent imaging was considered treatment effect. Postoperative leptomeningeal disease (LMD) was defined as focal or diffuse leptomeningeal enhancement of the brain, spinal cord, or cauda equina, dural enhancement beyond 5 mm from the index metastasis, subependymal enhancement, and enhancement of cranial nerves.
Imaging, clinic notes, and radiology reports regarding local failure were reviewed by the lead author (PMF). In cases of ambiguity, the imaging, clinic notes, and radiology reports were then reviewed with senior authors (JMM and JBF) for a final determination. All cases of potential postoperative LMD (imaging, clinic notes, and radiology reports) were reviewed by three authors (PMF, JMM, and JBF) for final determination.
Follow up
Brain metastasis patients are routinely followed by a combination of oncology, radiation oncology, and neurosurgery with contrast enhanced MRIs obtained on postoperative day 1 and then at 3 month intervals. Date of last follow up was considered to be the last time a patient was formally evaluated by a physician in one of these three fields. Important to note, follow up was capitated at the time of WBRT with the first day of treatment considered the last day of follow up. Days from treatment to local failure or postoperative LMD was calculated from the date of surgical resection to the date of imaging diagnosis. Adverse events were defined by the Common Terminology Criteria for Adverse Events Version 4.0 and included grade 4 or 5 neurologic events, grade 3 or higher seizure, grade 4 or 5 dermatologic event, or grade 3 or higher wound complication.
Statistical Analysis
Descriptive statistics are presented for the overall sample in terms of frequencies and percentages for categorical variables and median, minimum, and maximum for continuous variables. The Kaplan-Meier approach was used to plot survival curve estimates for both days from surgery to Local Failure and LMD time-to-event outcomes. Bivariate associations between time-to-event outcomes and patient characteristics were assessed using log-rank tests for strata homogeneity. In addition to this, we used multivariable Cox regression in order to estimate the Hazard Ratios (HR) and 95% Confidence limits (95%CL) for the associations between covariates and outcomes. The multivariable model was informed by both a priori knowledge of existing relationships and stepwise model selection criteria using entry and stay thresholds of 0.25 and 0.15. Data management and statistical analysis was performed using SAS v9.4, where results were considered statistically significant if p-values were less than 0.05.
Results
One hundred and seventy-seven patients were screened and 91 patients with a newly diagnosed brain metastasis treated with surgical resection and postoperative radiosurgery were included. Median radiographic follow-up among patients who had not developed a local failure was 9 months. The most common primary pathology was non-small cell lung cancer (42.86%) followed by melanoma (18.68%) and breast cancer (12.09%). The median number of intracranial metastases was 1 (range 1–19) with a median diameter of 3.6 cm (range 1.4–7.3 cm). The index tumor was located in the supratentorial compartment in 68.13% of cases and preoperative leptomeningeal involvement was present in 64.84%. The Gamma Knife (64.44%) was used more often than the LINAC and a single fraction dose schedule (72.22%) was most commonly employed. A single patient developed LMD following resection and prior to radiosurgery; this patient was treated with WBRT. (Table 1 and 2)
Table 1.
Patient Characteristics
| N=91 | % | ||
|---|---|---|---|
| Sex | Male | 41 | 45.05 |
| Female | 50 | 54.95 | |
|
| |||
| Pathology | Non-Small Cell Lung Cancer | 39 | 42.86 |
| Melanoma | 17 | 18.68 | |
| Breast Cancer | 11 | 12.09 | |
| Renal Cell Carcinoma | 8 | 8.79 | |
| Colon Cancer | 3 | 3.3 | |
| Other | 10 | 10.99 | |
| Unknown | 3 | 3.3 | |
|
| |||
| Number of Intracranial Metastases | Median (Min, Max) | 1 | (1, 19) |
| Number Category | Single | 64 | 70.33 |
| > 1 | 27 | 29.67 | |
|
| |||
| Diameter of Index Metastasis (cm) | Median (Min, Max) | 3.6 | (1.4, 7.3) |
| Diameter Category | > 3 cm | 61 | 67.03 |
| ≤ 3 cm | 30 | 32.97 | |
|
| |||
| Tumor Location | Supratentorial | 62 | 68.13 |
| Infratentorial | 29 | 31.87 | |
|
| |||
| Time from Surgery to Radiosurgery* | > 30 days | 52 | 57.78 |
| ≤ 30days | 38 | 42.22 | |
|
| |||
| Type of Radiosurgery* | Gamma Knife | 58 | 64.44 |
| LINAC | 32 | 35.56 | |
|
| |||
| Dose Schedule* | Single Fraction | 65 | 72.22 |
| > 1 Fraction | 25 | 27.78 | |
|
| |||
| Surgical Result | Gross Total Resection | 70 | 76.92 |
| Subtotal Resection | 21 | 23.08 | |
|
| |||
| Local Failure | Yes | 20 | 21.98 |
| No | 71 | 78.02 | |
| 12-Month Survival Rate | 76 | 83.52 | |
|
| |||
| LMD Failure | Yes | 32 | 35.16 |
| No | 59 | 64.84 | |
| 12-Month Survival Rate | 63 | 69.23 | |
a single patient suffered LMD after resection and before radiosurgery; patient treated with WBRT
LMD – leptomeningeal disease; LINAC – linear accelerator
Table 2.
Excluded Patients
| Exclusion criteria | Number of patients |
|---|---|
| - Prior WBRT | 36 |
| - Images unavailable | 31 |
| - Small cell lung cancer | 6 |
| - Use of adjuvant treatment* | 4 |
| - More than 1 resection prior to SRS | 3 |
| - Prior resection and SRS | 2 |
| - Lymphoma | 1 |
| - Prior cyst aspiration | 1 |
| - Concurrent meningioma resection | 1 |
| - Pediatric patient | 1 |
Adjuvant therapy included brachytherapy (3 patients) and Gliadel wafers (1 patient)
Local Failure
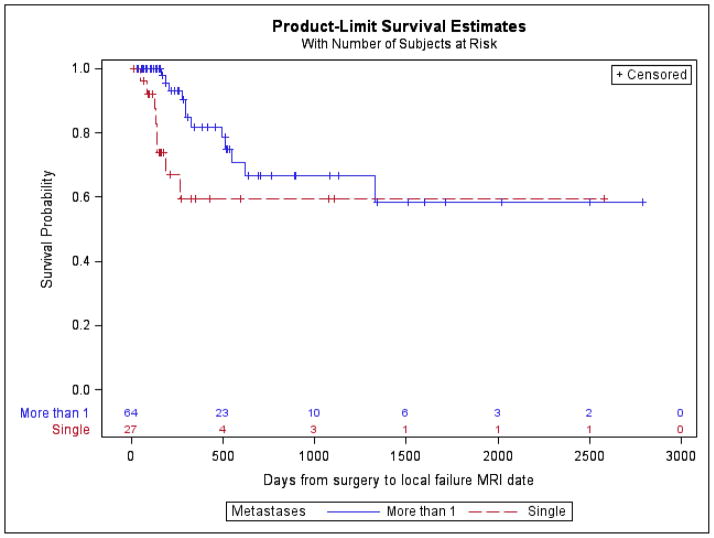
Local failure occurred in 20 (21.98%) patients. Freedom from local failure at 1 year was 83.52%. Statistically significant bivariate associations of local failure included the presence of more than one brain metastasis (log-rank P-value = 0.03) and a preoperative axial tumor diameter of >3 cm (log-rank P-value = 0.04, log-rank). Both the number of brain metastases and tumor diameter category were included in the multivariable model. We observed that the presence of more than one brain metastases (HR=2.65; 95%CL: 1.07, 6.59; p = 0.04) was associated with an increased risk of local failure. Preoperative tumor diameter of >3 cm (HR=4.16; 95%CL: 0.96, 18.02; p = 0.06) trended toward increased risk of local failure. No significant differences were identified with respect to tumor pathology, tumor location (supratentorial vs infratentorial), time from surgery to radiosurgery (>30 days vs < 30 days), type of radiosurgery (GK vs LINAC), dose schedule (single vs more than 1), or extent of surgical resection (GTR vs STR). (Table 3)
Table 3.
Multivariable model estimates for Local Failure and Postoperative LMD risk
| Local Failure Risk | HR | LCL | UCL | P-value | |
|---|---|---|---|---|---|
| Metastases | > 1 vs. Single | 2.65 | 1.07 | 6.59 | 0.0360 |
| Diameter of Index Tumor | > 3cm vs. ≤ 3cm | 4.16 | 0.96 | 18.02 | 0.0566 |
| Leptomeningeal disease Risk | HR | LCL | UCL | P-value | |
|---|---|---|---|---|---|
| Metastases | > 1 vs. Single | 2.07 | 0.98 | 4.36 | 0.0573 |
| Surgery to Radiosurgery | > 30 days vs. ≤ 30 days | 0.59 | 0.28 | 1.21 | 0.1477 |
| Cancer Type | Breast vs. Other | 2.37 | 0.93 | 6.07 | 0.0716 |
HR-Hazard Ratio; LCL=95% lower confidence limit; UCL-95% upper confidence limit; Multivariable models were based on a priori knowledge and stepwise selection criterion
Postoperative Leptomeningeal Disease
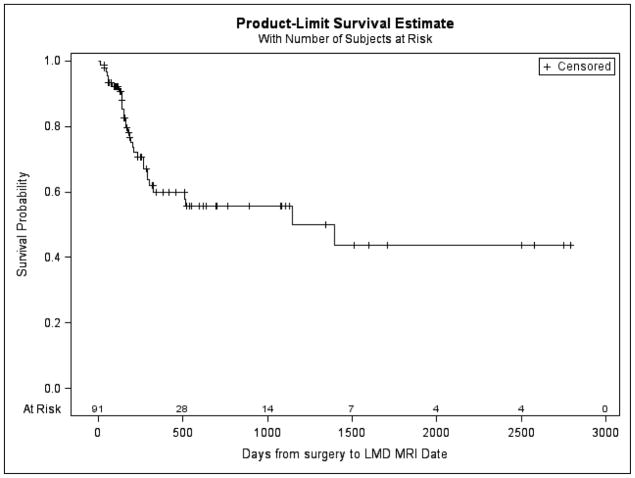
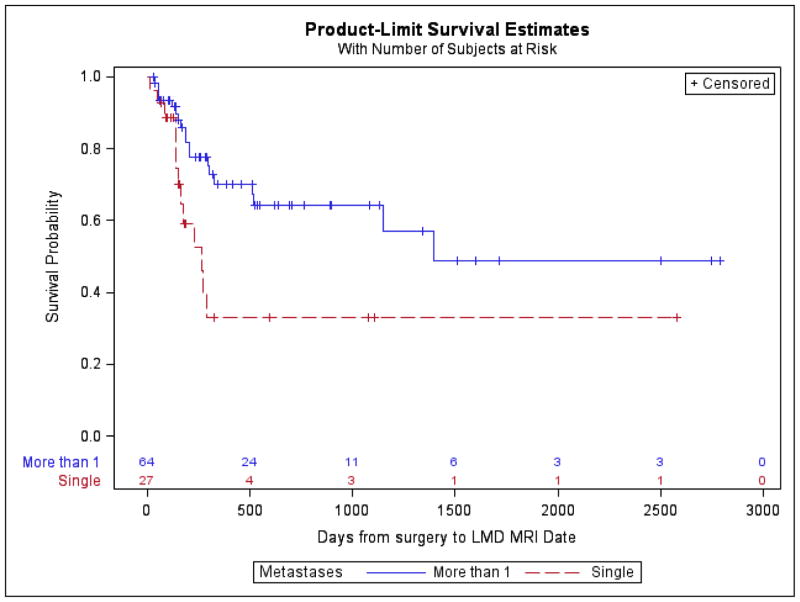
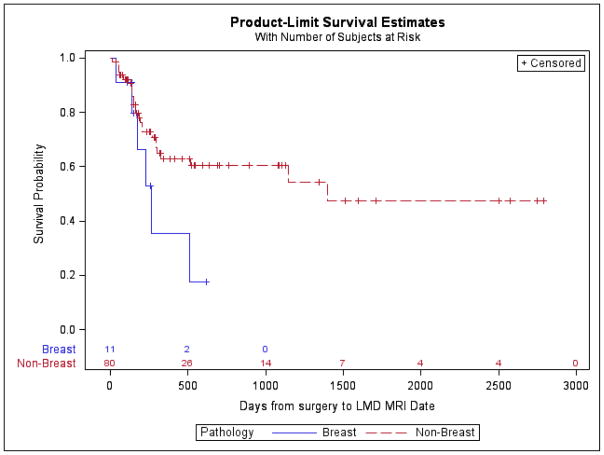
Postoperative LMD occurred in 32 (35.16%) patients. Freedom from postoperative LMD at 1 year was 69.23%. Postoperative LMD was more likely in the presence of more than one brain metastasis (log-rank P-value = 0.01) and more likely with a breast cancer primary (log-rank P-value= 0.07), though the latter did not reach statistical significance. The multivariable Cox regression model included the following indicator variables: single metastasis, surgery to radiosurgery >30 days, and breast cancer. In the multivariable model, we observed a trend toward a higher risk of LMD for patients with > 1 tumor (HR 2.07; 95%CL: 0.98, 4.36; p = 0.06) and breast cancer (HR 2.37; 95%CL: 0.93, 6.07; p=0.07). Patients with surgery to radiosurgery ≤30 days had lower risk of LMD but not statistically significant (HR 0.59; 95%CL: 0.28, 1.21; p=0.15). No significant differences were identified with respect to tumor location (supratentorial vs infratentorial), time from surgery to radiosurgery (>30 days vs < 30 days), type of radiosurgery (GK vs LINAC), dose schedule (single vs more than 1), or extent of surgical resection (GTR vs STR). (Table 3)
Adverse Events
Fifteen adverse events occurred in 12 patients. Four patients experienced a wound complication; three of these required operative intervention and were considered grade 4, whereas one required only hospitalization (grade 3). All four patients with a wound complication were treated with single fraction SRS with doses ranging from 15–18 Gy. Five patients underwent a total of 6 subsequent operations (grade 4) for progressive imaging changes (4 radiation necrosis, 2 radiation necrosis + tumor recurrence). Of the 3 patients requiring 4 interventions for radiation necrosis, all were treated with a single fraction with doses ranging from 16–18 Gy. Of the 2 patients requiring intervention for radiation necrosis + tumor recurrence, 1 was treated with a 15 Gy in a single fraction while the other received 20 Gy in 4 fractions before treatment was stopped. Two patients were hospitalized for seizures (grade 3). Both patients were treated with single fraction SRS with 16 and 18 Gy. Two patients were hospitalized for symptomatic cerebral edema (grade 3), and one patient underwent a craniotomy for an intracranial hemorrhage (ICH) (grade 4). One patient hospitalized with symptomatic cerebral edema was treated with 15 Gy in a single fraction; the other patient was treated with 20 Gy in 4 fractions before treatment was stopped. The patient requiring craniotomy for ICH had metastatic melanoma that was treated with single fraction SRS with 17 Gy. (Table 4)
Table 4.
Summary of Adverse Events
| Adverse Event | Grade | Number of events* |
|---|---|---|
| Requiring hospitalization | ||
| - Symptomatic cerebral edema | 3 | 2 |
| - Seizures | 3 | 2 |
| - Wound complication | 3 | 1 |
|
| ||
| Requiring operative intervention | ||
| - Radiation necrosis | 4 | 4 |
| - Wound complication | 4 | 3 |
| - Radiation necrosis + tumor progression | 4 | 2 |
| - Intracerebral hemorrhage | 4 | 1 |
15 adverse events in 12 patients
Discussion
Postoperative radiation is regarded as standard of care following surgical resection of a brain metastasis.[14] The addition of postoperative WBRT improves intracranial tumor control but fails to improve overall survival or duration of functional independence,[14] while exposing a large volume of normal brain to therapeutic radiation doses. Due to the potential for neurocognitive decline as a result of WBRT, many institutions have transitioned to a more focal postoperative radiation treatment paradigm consisting of stereotactic radiosurgery to the tumor bed. The current study evaluates the efficacy of postoperative radiosurgery to the tumor bed with respect to local failure and postoperative leptomeningeal disease.
Local Failure
The benefit of postoperative stereotactic radiosurgery for improving local control following surgical resection was recently confirmed in a prospective randomized study of observation versus radiosurgery for completely resected brain metastases.[15] In the current series, freedom from local failure at 1 year approached 84%, a rate comparable to other contemporary series of postoperative SRS.[16–21] While some authors have reported improved local control with the addition of a margin around the resection bed,[22] it is notable that the current series targeted only the resection cavity. The presence of more than one brain metastasis at diagnosis was identified as an independent predictor of local failure (HR=2.65, p = 0.04), while a preoperative tumor diameter of > 3 cm trended toward significance in multivariable modeling (HR=4.16, p = 0.06). Ojerholm et al. identified a similar tumor diameter cutoff of ≥ 3 cm with a HR of 3.7, notably however, they did not investigate the impact of the overall number of brain metastases. This 3 cm threshold has also been reported by others,[15, 16, 23] and has been attributed to the technical difficulty of treating large tumors and more aggressive tumor biology.[16]
The number of intracranial metastases has traditionally dictated the decision to pursue focal versus whole brain radiation treatment, with multiple metastases favoring the latter. However, a growing experience with radiosurgery for the treatment of multiple metastases has identified a greater number of brain metastases as a risk factor for local failure,[24] as in the current series, and distant brain failure[19, 25]. It should be noted that this finding is not consistent across studies, and some authors report that tumor volume, not number of metastases, dictates the risk of local and distant brain failure.[26–28] The explanation for increasing numbers of intracranial metastases as a prognostic marker for local control is not obvious, but likely reflects inherently aggressive tumor biology.
Leptomeningeal Disease
Neoplastic involvement of the leptomeninges and subarachnoid space is thought to occur in 4–15% of all patients with solid tumors, however, the true incidence is largely unknown since the condition is clinically underdiagnosed.[29, 30] With improved systemic cancer treatment, the rate of parenchymal and leptomeningeal metastases has increased, since the neoplastic cells take refuge behind the blood-brain and cerebrospinal fluid (CSF)-brain barriers.[30] Among solid tumors, the most frequently identified primary sites are lung, breast, and melanoma,[29, 30] coinciding with the most frequent primary sites in the current series. The diagnosis of LMD is challenging and often relies on a combination of clinical signs and symptoms, CSF analysis, and imaging.[31] Given the retrospective nature of the current series, the diagnosis of LMD relied heavily on clinical documentation and image interpretation; CSF analysis was rarely performed. In an effort to improve the sensitivity of the diagnosis, a liberal definition of LMD was adapted from Freilich et al.[31] to include focal dural enhancement. A 2014 publication evaluating postoperative radiosurgery have also applied a LMD definition that included focal enhancement of the dura.[32] Survival among the 26 patients diagnosed with LMD ranged from 1.1 to 36.7 months,[32] indicating wide prognostic variability within the diagnosis. While most clinicians would agree that a focal dural deposit of tumor therapeutically and prognostically differs from diffuse involvement of the leptomeninges, this distinction requires additional investigation.
Postoperative LMD occurred in 35% of patients in the current series, with the vast majority being diagnosed within the first year after surgery. While this rate exceeds what is commonly reported in contemporary series, it is comparable to a 1999 surgical series that experienced a rate of 33%.[33] A 2014 publication by the Emory group found an overall postoperative LMD rate of 17% but a rate of 31% in patients treated with postoperative radiosurgery as opposed to WBRT.[18] Their definition of LMD was quite similar to our own: “leptomeningeal disease was defined as new abnormal leptomeningeal enhancement of the brain, spinal cord, or cauda equina and focal or diffuse enhancement of the dura or subarachnoid space”.[18] Additionally, they too identified the presence of more than one intracranial metastasis at diagnosis as a risk factor for postoperative LMD.[18] This finding was later corroborated by the same group in a 2016 multi-institution publication that included 180 patients,[19] and has been reported by others.[34, 35] Multiple metastases has also been identified as a risk factor for distant brain failure,[19, 25] suggesting a more aggressive clinical course in these patients likely inherent to the tumor itself.
Other frequently cited risk factors for the development of postoperative LMD include breast cancer primary[17, 32, 36] and infratentorial tumor location[17, 33]. While infratentorial tumor location was not predictive in the current series (data not shown), there was a strong trend toward increased risk of LMD with a breast cancer primary; increasing rates of LMD in breast cancer is thought to be related to a CNS predilection of HER-2 positive cells, poor CNS penetration by trastuzumab, and improved visceral disease control.[30] In our patient sample there was a non-significant difference in LMD survival curves between infratentorial and supratentorial tumor location (log-rank P-value=0.83), when considered for consideration in the full model, it did not meet the entry threshold criteria to be entered into the multivariable model. Given breast cancer’s predilection for leptomeningeal involvement, it is difficult to determine whether postoperative LMD diagnosed in the presence of a breast cancer primary represents new tumor deposition or a consequence of tumor spillage with subsequent dissemination. This distinction is critical when attempting to prevent postoperative LMD, as tumor spillage related LMD could potentially be reduced by neoadjuvant radiation or en bloc resection[35].
Future Directions
As clinicians move away from postoperative WBRT and toward a more focal treatment paradigm, rigorous prospective investigation is needed to guide therapy. While the results for stereotactic radiosurgery in intact metastases are encouraging,[8, 11] the role of SRS in the postoperative setting is not well defined. Our institutional experience suggests high rates of LMD using postoperative SRS, especially when multiple intracranial metastases are present at diagnosis. This data provides insight on the safety and efficacy of this treatment paradigm in a real-world setting (i.e. outside the rigorous inclusion/exclusion criteria of a clinical trial) and serves as a historical control for new techniques to be compared. The results of the recently completed NCCTG-N107C, a phase III trial comparing postoperative SRS to WBRT, were recently published in Lancet Oncology. This serves as the first head to head trial of SRS versus WBRT following the surgical resection of a brain metastasis and revealed equivalent survival but superior cognitive function and quality of life with SRS.[37] Neoadjuvant radiosurgery is another exciting topic of investigation with the potential to improve tumor targeting, reduce the effective radiation dose, and decrease viable tumor spillage during surgical resection. This strategy has previously been reported as safe and effective by the Carolina and Emory groups[19, 38] and is the focus of a recently completed phase I trial at the University of Alabama at Birmingham. Of particular relevance to the current study was the significantly lower rate of postoperative LMD with the use neoadjuvant radiosurgery as compared to postoperative radiosurgery.[19]
With regard to the diagnosis of LMD, a uniform and consistently applied definition is needed to allow for comparison across studies and to facilitate inter-physician communication. Leptomeningeal disease is a fatal form of central nervous system metastasis that can present as a focal nodule, a diffuse, non-adherent process, or a combination of the two. Treatment is tailored to the morphology of the metastasis and generally involves a combination of radiotherapy, systemic chemotherapy, and intrathecal chemotherapy, with the latter primarily utilized in the diffuse subtype.[30] The prognostic implications of focal versus diffuse leptomeningeal involvement requires special attention in future research.
Limitations
The current study is limited primarily by its retrospective nature and lack of a comparison group treated with WBRT. However, the goal of the study was not to compare results of SRS to WBRT but rather to investigate the rates of local failure and LMD following postoperative SRS. The study’s broad inclusion criteria support the generalizability of the results but allows for variability in clinical features and treatment decisions. The treatment of systemic cancer and brain metastases have evolved substantially over the 10 years included in the study introducing therapeutic heterogeneity among patients. Radiosurgery treatment volumes were not available and thus were not analyzed; axial tumor diameter was used as a proxy for tumor size. The current study focused on pre-resection risk factors and axial tumor diameter was felt to reflect clinical practice. Although the diagnosis of LMD was reviewed by a minimum of three authors (PMF, JMM, and JBF), there still exists a component of ambiguity in the radiographic diagnosis of this entity. The authors chose to utilize a liberal definition, to include focal dural deposits, in an effort to maximize the sensitivity of the diagnosis; prognostic implications of this liberal diagnosis remain to be determined. Despite the aforementioned limitations, the study provides a detailed examination of risk factors for local failure and postoperative LMD in patients undergoing postoperative SRS at a single institution.
Conclusions
More than one intracranial metastasis at the time of diagnosis is an independent predictor of local and leptomeningeal failure following postoperative radiosurgery. The greater number of intracranial metastases is felt to be an indication of aggressive tumor biology. The high rate of LMD was likely related to the liberal definition of LMD to include focal dural recurrences, and the clinical significance of limited dural dissemination requires additional study.
Supplementary Material
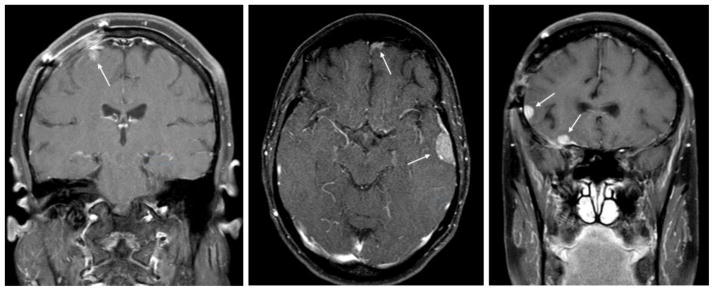
Figure 1.
Three example patients diagnosed with postoperative leptomeningeal disease (LMD). Patients were selected to demonstrate the liberal definition of LMD that includes focal involvement. Arrows highlight areas of LMD.
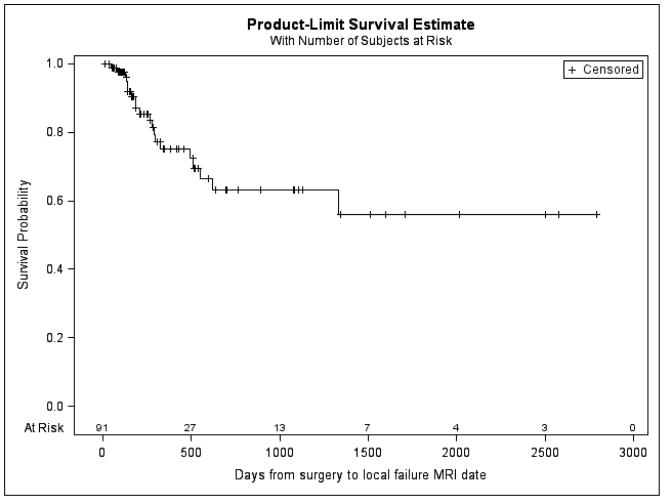
Figure 2.
Overall local failure rate. Freedom from local failure at 1 year was 83.52%.
Figure 3.
Local failure rate with respect to number of brain metastases. Presence of more than one brain metastasis is significantly associated with local failure (log-rank P-value = 0.03).
Figure 4.
Overall postoperative leptomeningeal disease rate. Freedom from postoperative LMD at 1 year was 69.23%.
Figure 5.
Postoperative leptomeningeal disease rate with respect to number of brain metastases. Presence of more than one brain metastasis is significantly associated with postoperative leptomeningeal disease (log-rank P-value = 0.01).
Figure 6.
Postoperative leptomeningeal disease rate with respect to tumor pathology. Breast cancer primary is non-significantly associated with postoperative leptomeningeal disease (log-rank P-value= 0.07).
Highlights.
Postoperative radiosurgery for brain metastasis treatment is evaluated
Postoperative radiosurgery is associated with a high rate of LMD
Presence of >1 brain metastasis is a risk factor for local failure and LMD
Implications of focal versus diffuse leptomeningeal failure require further investigation
Acknowledgments
Funding: The National Institute of Health provided financial support in the form of T32 CA091078 funding. The sponsor had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203–22. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 4.Lin X, DeAngelis LM. Treatment of Brain Metastases. J Clin Oncol. 2015;33:3475–84. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabors LB, Portnow J, Ammirati M, Baehring J, Brem H, Brown P, et al. Central Nervous System Cancers, Version 1.2015. J Natl Compr Canc Netw. 2015;13:1191–202. doi: 10.6004/jnccn.2015.0148. [DOI] [PubMed] [Google Scholar]
- 6.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 7.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 8.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–96. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 10.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–93. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 12.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401–9. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark GM, Popple RA, Prendergast BM, Spencer SA, Thomas EM, Stewart JG, et al. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol. 2012;2:306–13. doi: 10.1016/j.prro.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Tsao MN, Rades D, Wirth A, Lo SS, Danielson BL, Gaspar LE, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–25. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1040–8. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brennan C, Yang TJ, Hilden P, Zhang Z, Chan K, Yamada Y, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–6. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojerholm E, Lee JY, Thawani JP, Miller D, O’Rourke DM, Dorsey JF, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(Suppl):75–83. doi: 10.3171/2014.6.GKS14708. [DOI] [PubMed] [Google Scholar]
- 18.Patel KR, Prabhu RS, Kandula S, Oliver DE, Kim S, Hadjipanayis C, et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol. 2014;120:657–63. doi: 10.1007/s11060-014-1601-4. [DOI] [PubMed] [Google Scholar]
- 19.Patel KR, Burri SH, Asher AL, Crocker IR, Fraser RW, Zhang C, et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases: A Multi-institutional Analysis. Neurosurgery. 2016;79:279–85. doi: 10.1227/NEU.0000000000001096. [DOI] [PubMed] [Google Scholar]
- 20.Do L, Pezner R, Radany E, Liu A, Staud C, Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys. 2009;73:486–91. doi: 10.1016/j.ijrobp.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 21.Rwigema JC, Wegner RE, Mintz AH, Paravati AJ, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery to the resection cavity of brain metastases: a retrospective analysis and literature review. Stereotact Funct Neurosurg. 2011;89:329–37. doi: 10.1159/000330387. [DOI] [PubMed] [Google Scholar]
- 22.Choi CY, Chang SD, Gibbs IC, Adler JR, Harsh GRt, Lieberson RE, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys. 2012;84:336–42. doi: 10.1016/j.ijrobp.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Jensen CA, Chan MD, McCoy TP, Bourland JD, deGuzman AF, Ellis TL, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg. 2011;114:1585–91. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greto D, Scoccianti S, Compagnucci A, Arilli C, Casati M, Francolini G, et al. Gamma Knife Radiosurgery in the management of single and multiple brain metastases. Clin Neurol Neurosurg. 2016;141:43–7. doi: 10.1016/j.clineuro.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Ling DC, Vargo JA, Wegner RE, Flickinger JC, Burton SA, Engh J, et al. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery. 2015;76:150–6. doi: 10.1227/NEU.0000000000000584. discussion 6–7; quiz 7. [DOI] [PubMed] [Google Scholar]
- 26.Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, et al. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery. J Neurosurg. 2013;119:1139–44. doi: 10.3171/2013.7.JNS13431. [DOI] [PubMed] [Google Scholar]
- 27.Likhacheva A, Pinnix CC, Parikh NR, Allen PK, McAleer MF, Chiu MS, et al. Predictors of survival in contemporary practice after initial radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:656–61. doi: 10.1016/j.ijrobp.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 28.Bhatnagar AK, Flickinger JC, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol. 2005;75:85–99. doi: 10.1007/s11060-004-8101-x. [DOI] [PubMed] [Google Scholar]
- 30.Mack F, Baumert BG, Schafer N, Hattingen E, Scheffler B, Herrlinger U, et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;43:83–91. doi: 10.1016/j.ctrv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38:51–7. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 32.Atalar B, Modlin LA, Choi CY, Adler JR, Gibbs IC, Chang SD, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87:713–8. doi: 10.1016/j.ijrobp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 33.van der Ree TC, Dippel DW, Avezaat CJ, Sillevis Smitt PA, Vecht CJ, van den Bent MJ. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66:225–7. doi: 10.1136/jnnp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang AJ, Huang KE, Page BR, Ayala-Peacock DN, Lucas JT, Jr, Lesser GJ, et al. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120:163–9. doi: 10.1007/s11060-014-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64:664–74. doi: 10.1227/01.NEU.0000341535.53720.3E. discussion 74–6. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MD, Avkshtol V, Baschnagel AM, Meyer K, Ye H, Grills IS, et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94:537–43. doi: 10.1016/j.ijrobp.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–60. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asher AL, Burri SH, Wiggins WF, Kelly RP, Boltes MO, Mehrlich M, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. doi: 10.1016/j.ijrobp.2013.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.