Abstract
Mounting evidence suggests that the Hippo co-activator Yes-associated protein 1 (YAP1) is a major mediator of cancer stem cell (CSC) properties, tumor progression, and therapy resistance as well as often a terminal node of many oncogenic pathways. Thus, targeting YAP1 may be a novel therapeutic strategy for many types of tumors with high YAP1 expression, including esophageal adenocarcinoma (EAC). However, effective YAP1 inhibitors are currently lacking. Here, we identify a small molecule (CA3) that not only has remarkable inhibitory activity on YAP1/Tead transcriptional activity but also demonstrates strong inhibitory effects on EAC cell growth especially on YAP1 high expressing EAC cells both in vitro and in vivo. Remarkably, radiation resistant cells acquire strong CSC properties and aggressive phenotype, while CA3 can effectively suppresses these phenotypes by inhibiting proliferation, inducing apoptosis, reducing tumor sphere formation, and reducing the fraction of ALDH1+ cells. Further, CA3 combined with 5-FU, synergistically inhibits EAC cell growth especially in YAP1 high EAC cells. Taken together, these findings demonstrated that CA3 represents a new inhibitor of YAP1 and primarily targets YAP1 high and therapy resistant EC cells endowed with CSCs properties
Keywords: Hippo signaling, YAP1, YAP1 inhibitor, radiation resistance, EAC
Introduction
Evolving understanding of tumor biology has led to the hypothesis that tumors may possess a subpopulation of stem cell-like cells known as cancer stem cells (CSCs) that are involved in driving tumor propagation and pathogenesis. Current evidence suggests that CSCs may be responsible for many important characteristics of tumors, including tumor initiation, heterogeneity, therapy resistance, recurrence and metastasis (1). Most cancer therapies (cytotoxics) target mature, proliferating tumor cells without affecting CSCs. Many cancer patients initially experience regression of tumors but eventually experience progressive disease, including therapy resistance. Therefore, new approaches to targeting CSCs are critical to eventually eliminate tumors.
The Hippo pathway and its transcriptional co-activator Yes-associated protein (YAP) have emerged as major regulators of organ size, proliferation, and stem cell properties (2,3). Mounting evidence suggests that YAP1 plays a role in maintaining the stemness of embryonic stem cells and contributes to the functions of CSCs, which are the seeds of therapy resistance, relapse, and metastasis (4–6). Gregorieff et al (5) reported that YAP1 reprograms Lgr5+ intestinal stem cells by inhibiting the Wnt homeostatic program, activates epidermal growth factor receptor (EGFR), and drives cancer initiation. We and others demonstrated that YAP1 confers CSC properties to nontransformed epithelial cells and cancer cells by regulating the expression of CSC genes SOX9, OCT4, and SOX2 (4). Recently, Cebola et al (7) found that YAP1 and its partner TEAD activate key pancreatic signaling and transcription factors, regulate the expansion of pancreatic progenitors, and play major roles in pancreatic cancer development. In addition to their roles in normal and CSCs, deregulation of Hippo signaling and YAP1 have emerged as major players in cancer initiation and development (8). YAP1 overexpression and nuclear localization correlate with poor outcome of several cancers (9–10). Also, overexpression of YAP1 in cancer cell lines can promote epithelial-mesenchymal transition (EMT) and enhance invasion in vitro (11).In transgenic mice, tissue-specific expression of YAP1 in the liver has resulted in tissue overgrowth and tumor formation (12). Recently, we demonstrated that YAP1 regulates SOX9, endows non tumorigenic cells and cancer cells with CSC properties, and drives tumorigenesis in EAC cells, suggesting that the YAP1/SOX9 axis is a new therapeutic target (4).
Therapy resistance of cancer, including chemotherapy, radiation therapy, and targeted therapy resistance, is the major obstacle and challenge in the clinic. Therapy resistance can be inherent or acquired. It has been reported that YAP1 is a major mediator of chemotherapy and targeted therapy resistance (13–15). We found that YAP1 mediated tumor chemo-resistance by activating EGFR signaling (13). A recent study demonstrated that YAP1 mediates RAF- and mitogen-activated protein kinase kinase-targeted therapy resistance (14). YAP1 also cross-talks with and activates many oncogenic signaling such as KRAS (16,17), RhoA (18,19)and Wnt/β-catenin (20,21) to mediate tumor growth and therapy resistance (15,20,22,23). Therefore, targeting YAP1 will provide novel therapeutic strategies by targeting CSCs as well as bulk tumor cells.
In the view of the central role of deregulation of Hippo and activation of YAP1 in regulation of CSCs and many important properties of tumors, targeting YAP1 will be effective novel strategy to target CSCs and inhibit tumor growth. Several small molecule inhibitors identified, however, they are either not potent or less selective. Thus, a novel YAP inhibitor CA3 was recently selected and identified through chemical library screening. We have demonstrated that CA3 has potent inhibitory effects on YAP1/Tead transcriptional activity. As a result, CA3 strongly inhibit EAC cell growth in vitro and exert strong anti-tumor activity in xenograft model with no apparent toxicity. Remarkably, radiation resistant cells acquire strong CSCs properties and aggressive phenotype, while CA3 can effectively suppress tumor cell proliferation, induce apoptosis, reduce tumor sphere formation and the population of ALDH1+ cells. Further, CA3 synergistically inhibits EAC cell growth with 5-FU especially in YAP1 high and resistant EAC cells.
Materials and Methods
Cells and reagents
The human EAC cell lines SKGT-4, JHESO, OACP, YES-6, and Flo-1 have been described previously (24–26). 293T cells generated using published methods (27) were obtained from Dr. Randy L. Johnson of The University of Texas MD Anderson Cancer Center). All cell lines were authenticated at the Characterized Cell Line Core at MD Anderson every 6 months. Verteporfin (VP) was obtained from U.S. Pharmacopeia. Doxycycline (Dox) was obtained from Sigma-Aldrich. An antibody against YAP1 was purchased from Cell Signaling Technology. Anti-CTGF and -SOX9 antibodies were obtained from Chemicon. BRD4 plasmid (pcDNA2-BRD4) was obtained from Addgene Doxycycline inducible YAP1 lentiviral plasmid (PIN20YAP1) was constructed by inserting flag-tagged YAP1S127A cDNA amplified from CMV-S127A-YAP into pINDUCER20 (provided by Thomas Westbrook, Baylor College of Medicine). CA3 and several other novel YAP1 inhibitors were synthesized and provided by Dr. Sheng Ding from University of California, San Francisco.
Establishment of Radiation resistant(XTR) EAC cells
The radiation resistant XTR EAC cell lines Flo-1 XTR and SKGT-4 XTR were generated by continuously irradiating their parental cell lines at 2 Gy four times and repeat several cycles in a stepwise procedure over 2–3 months. Resistant cell lines (XTR) were maintained in normal Dulbecco’s modified Eagle’s medium before analysis.
Cell proliferation assay
The EAC cells and their resistant counterparts were treated with 0.1% dimethyl sulfoxide (control), CA3 at different doses For combination treatment experiments, treatment of the cells with CA3, 5-FU, or a combination at different concentrations was administered for 6 days as indicated, and the cell viability was assessed using an MTS assay as described previously(28). All assays were performed in triplicate and repeated at least three times.
Flow cytometry and apoptotic analysis
Analysis of EAC cell apoptosis using flow cytometry was performed as described previously (29). In brief, SKGT-4 and JHESO cells were seeded onto six-well plates (1 × 105 per well) in Dulbecco’s modified Eagle’s medium and cultured for 24 hours to allow for cell attachment. The cells were then treated with 0.1% dimethyl sulfoxide(control) or CA3 at different doses as indicated for 48 hours. Next, the cells were harvested, fixed with methanol, washed, treated with RNase A, and stained for DNA with propidium iodide (Sigma), and their DNA histograms and cell-cycle phase distributions were analyzed using flow cytometry with a FACS Calibur instrument (Becton, Dickinson).
Protein extraction and Western blot analysis
Proteins were isolated from EAC cells treated with CA3 as indicated and analyzed using Western blotting as described previously (30).
Transient transfection and luciferase reporter assays
The SOX9 luciferase reporter was described previously (28). The 5×-UAS-luciferase reporter and Gal4-TEAD4 constructs, also described previously (31), were obtained from Dr. Johnson of MD Anderson. Transient co-transfection EAC cells with the SOX9 luciferase reporter and a Renilla vector or 5×-UAS-luciferase reporter and Gal4-TEAD4 with a CMV-β-gal construct was performed as described previously (28).
Tumor sphere formation assay
Tumor sphere culture was performed as described previously (28). Briefly, a single-cell suspension of Flo-1 cells and radiation resistant Flo-1 XTRas well as fluorescence-activated cell sorting (FACS)-isolated ALDH1+ or ALDH1− JHESO cells was seeded in triplicate onto a six-well ultralow attachment plate (1000–2500 cells/well) in serum-free Dulbecco’s modified Eagle’s medium/F-12 medium supplemented with 20 ng/mL epidermal growth factor, 5 µg/mL insulin, 0.5 µg/mL hydrocortisone, 2% B27 supplement without vitamin A, and 1% N2 supplement (Invitrogen). After 10–20 days of culture, the tumor spheres that formed (diameter >100 µm) were counted under a microscope.
Immunohistochemistry
Immunohistochemical staining for YAP1, SOX9 and Ki67 were performed on xenograft tumor tissues of SKGT-4 and JHESO xenograft tumors using antibodies against SOX9 (1:2000) and YAP1 (1:100) and KI67 (1:100) as described previously (29).
Indirect immunofluorescence
Indirect immunofluorescent staining for nuclear expression of YAP1 and SOX9 in EAC cells was performed as described previously (4).
Flow cytometry labeling and fluorescence-activated cell sorting
Flo-1 parental and resistant Flo-1XTR cells that were untreated or treated with CA3 were analyzed and collected for fluorescence-activated cell sorting (FACS) using an ALDEFLUOR detection kit (STEMCELL Technologies) as described previously(32).
In vivo xenograft mouse model
In vivo experiments have been conducted in accordance with an Institutional Animal Care and Use Committee (IACUC). SKGT-4 (PIN20YAP1) cells (1 × 106) without (Dox−) or with (Dox+) YAP1 induction by Doxycycline were inoculated into nude mice (n = 5/group). The mice in the Dox+ group were fed drinking water containing 2.5% sucrose and 2.5% Doxycycline, whereas those in the Dox− group were fed water containing only 2.5% sucrose. After 10 days, CA3 was introperitoneally injected into the animals in the Dox+ group at 1 mg/kg/mouse three times a week for total 3 weeks.
In a JHESO xenograft model of EAC, 2 × 106 JHESO cells were subcutaneously injected into nude mice (n = 5/group). After about 10 days, the mice underwent intraperitoneal injection of CA3 at 1 mg/kg/mouse, 5-FU at 30 mg/kg/mouse, or a combination of them three times a week for total 3 weeks. A control group was given phosphate-buffered saline at 100 µl/mouse. The mice’s tumor volumes, tumor weights, and body weights were measured as described previously (4). All measurements were compared using an unpaired Student t-test.
Statistical analysis
Data were analyzed using the Student t-test and Fisher exact test (for immunohistochemistry). P values less than 0.05 were considered statistically significant, and all tests were two-sided. All tests were performed using the SPSS software program (version 10.1; IBM Corporation).
Results
Identification of Novel YAP1 inhibitor CA3 and determination of its effects on YAP1 high EAC cells
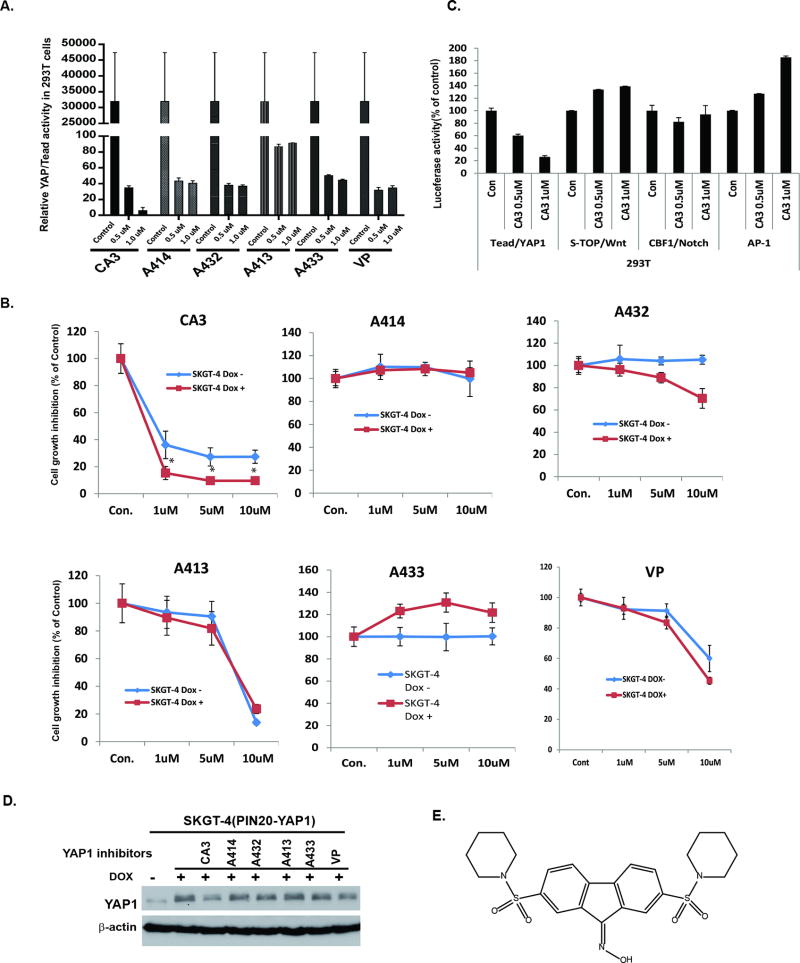
We used the Tead/YAP luciferase reporter system (Gal4-Tead and 5×-UAS-luciferase plasmids, which represent YAP1 activity) as a readout to screen a series of novel compounds synthesized in Dr. Ding’s laboratory from Department of Pharmaceutical Chemistry, University of California, San Francisco based on inhibition of YAP1 activity. For this, 293T cells transfected with Tead/YAP luciferase reporter construct were incubated with different dosage of novel YAP1 inhibitors (CA3, A414, A432, A413 and A433) and compared with commercial available YAP1 inhibitor Verteporfin (VP) (33), as indicated in Figure 1A and Supplemental Figure 1&2, CA3 and other novel YAP inhibitors had similar inhibitory effects on YAP/Tead activity at 0.5 µM, but CA3 was more effective than the other inhibitors and VP at 1 µM. We further validated their antitumor cell activity, especially in high YAP1-expressing SKGT-4 cells, which had stably integrated Dox-inducible YAP1 cDNA. CA3 dramatically reduced SKGT-4 cell growth and was more potent inhibition in YAP1 induced Dox+ SKGT-4 cells than no YAP1 induced (Dox−) SKGT-4 cells. In comparison, the other YAP inhibitors and the commercially available YAP1 inhibitor VP were either not selective for high YAP1-expressing cell inhibition or much less potent than CA3 (Fig. 1B). Further, CA3 specially inhibited Tead/YAP1 transcriptional activity at the concentration indicated but showed no inhibitory activity on other transcriptional factors-Super-TOP/Wnt, CBF1/Notch and AP-1 after co-transfection of their respective individual promoter luciferases in 293T cells (Fig. 1C). We also observed that CA3 was stronger than the other inhibitors in inhibition of YAP expression and EGFR signaling (Fig. 1D). The structure of CA3 is shown in Fig. 1E. Thus, we identified CA3 as a potential novel specific YAP1 inhibitor for further examination.
Figure 1. Identification of Novel YAP1 inhibitor CA3 and determination of its effects on YAP1 high EAC cells.
A. YAP1/TEAD activity was determined by co-transfection of Gal4-Tead and 5×UAS-luciferase and YAP1 cDNA in 293T cells and then treated with novel YAP1 inhibitors-CA3, A414, A432, A413, A433 and VP; Luciferase reporter activity was measured after 48 h of transfection. B. Cell growth of SKGT-4 (PINYAP120) cells with (DOX+) or without (DOX−) YAP1 induction treated with CA3, A414, A432, A413, A433 and VP as indicated dosage was determined by using the CellTiter Aqueous One Solution Cell Proliferation Assay kit as described in Materials and Methods. C. Luciferase activities of YAP1/Tead, Super-TOP/Wnt, CBF1/Notch and AP-1 were determined by co-transfection of their respective promoter plasmids and Renilla into 293T cells and then treated with CA3 at 0.5µM and 1 µM; Luciferase reporter activities were measured after 48 h of transfection. For all experiments, values shown represent the mean and SD of at least triplicate assays, Experiments were repeated at least three times. D. Expression of YAP1 in SKGT-4 DOX+ treated with CA3, A414, A432, A413, A433 and VP was determined by immunobloting. E. Chemical Structure of CA3 was denoted.
CA3 strongly inhibits EAC cell growth and strongly induces tumor Cell death
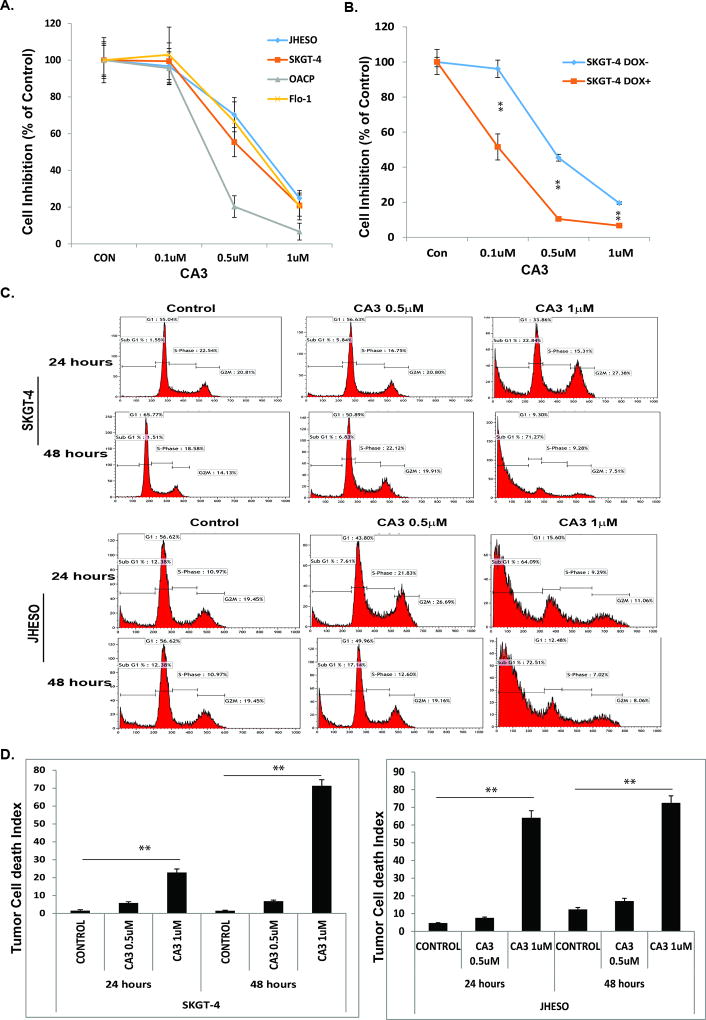
We treated four EAC cell lines (JHESO, SKGT-4, OACP, and Flo-1) with CA3 at different concentrations. As indicated in Fig. 2A, CA3 inhibited the growth of four EAC cell lines in a concentration-dependent manner. Specifically, CA3 inhibited growth at relatively low concentrations. In particular, at 1 µM, treatment with CA3 killed more than 80% of the EAC cells over 3 days. Moreover, CA3 was more selective in inhibiting the growth of cells with inducible YAP1 high SKGT-4 EAC cells (Fig. 1B).
Figure 2. CA3 potently inhibits EAC cell growth and induces tumor cell death.
Four EAC cell lines A.SKGT-4, Flo-1,JHESO and OACP EAC cell lines were treated with 0.1% DMSO (as control), CA3 (left) at dosage of 0.1, 0.5 and 1µM, the cells proliferation and viability were measured using MTS assay and calculated as percent of control. B. SKGT-4 (PINYAP120) cells with (DOX+) or without (DOX−) YAP1 induction were treated with 0.1% DMSO (as control), CA3 (left) at dosage of 0.1, 0.5 and 1µM, the cells proliferation and viability were measured using MTS assay and calculated as percent of control. For all experiments, values shown represent the mean and SD of at least triplicate assays.**P<0.01.C&D. SKGT-4 and JHESO EAC cells were seeded onto 6-well plates and treated with 0.1% DMSO (as control) or CA3 at 0.5 and 1µM for 24 hours and 48 hours and then fixed and stained for DNA with propidium iodide and then analyzed for DNA histograms and cell cycle phase distribution by flow-cytometry (C) and Tumor cell death index were determined by flow cytometry (D). **P<0.01.
To further determine whether CA3 affects cell-cycle progression and induces programmed cell death in EAC cells, we treated SKGT-4 and JHESO cells with CA3 at 0.5 and 1 µM for 24 hours and 48 hours respectively; We observed a marked increase in the proportion of cells in sub-G1 phase and decrease in the proportion of those in S phase with CA3-based treatment in a dose- and time-dependent manner (Fig. 2C). When we further analyzed cell death by flow cytometry, we found that CA3 significantly induced tumor cell death in both SKGT4 and JHESO cells in a time- and dose-dependent manner (Fig. 2D).
CA3 inhibits YAP1 expression and transcriptional activity in EAC cell lines, especially those with high YAP1
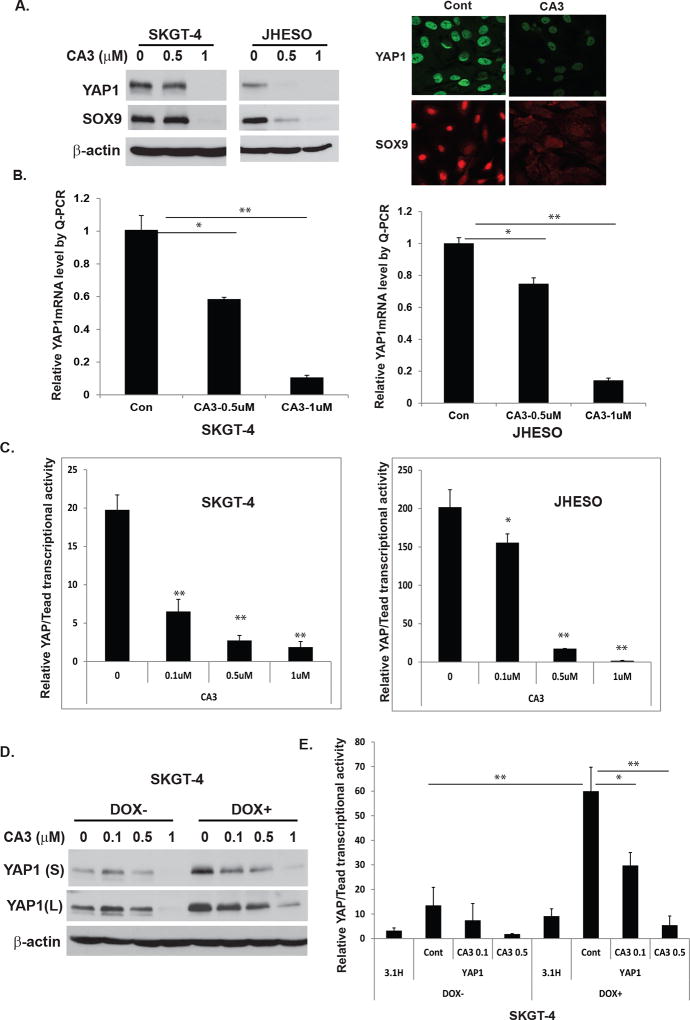
To gain insight into potential mechanisms by which CA3 inhibits growth and induces apoptosis of EAC cells, we first determined its effect on YAP1 expression and transcriptional activity. As shown in Fig. 3A, treatment with CA3 strongly inhibited expression of YAP1 and its target SOX9 in EAC cells in a dose-dependent manner, which correlated with its inhibition of growth (Fig. 2A) and induction of apoptosis (Fig. 2C) in these cells. Also, immunofluorescence analysis demonstrated markedly reduced nuclear expression of YAP1 and SOX9 in EAC cells treated with CA3 (Fig. 3A, right panel). Furthermore, CA3 greatly reduced transcription levels ofYAP1 detected by quantitative real-time polymerase chain reaction (Q-PCR) in both SKGT-4 and JHESO cells (Fig. 3B). To further determine if YAP1’s transcriptional activity is affected by treatment with CA3 in EAC cell lines, we co-transfected Gal4-Tead and 5×-UAS-luciferase plasmids, which represent YAP1 activity (31), into SKGT-4 and JHESO EAC cells and treated them with CA3 at the doses indicated. The transcriptional activity of YAP1 in SKGT-4 and JHESO EAC cells was suppressed dramatically and dose-dependently (Fig. 3C). More importantly, CA3 suppressed YAP1 expression (Fig. 3D) and transcriptional activity in SKGT-4 cells with induced high YAP1 in a dose-dependent manner (Fig. 3D and 3E). These data suggested that CA3 is a strong novel YAP1 inhibitor, suppressing not only YAP1 expression but also its transcriptional activity.
Figure 3. CA3 inhibits YAP1 expression and transcriptional activity in EAC cell lines, especially those with high YAP1.
A. Cell lysate from SKGT-4 and JHESO EAC cells treated with CA3 at indicated dosage were selected for immunoblotting analysis for YAP1 and SOX9 (left). Immunofluorescent staining of YAP1 and SOX9 in JHESO cells were observed by confocal microscopy (right). B. YAP1 mRNA levels were determined by quantitative real-time PCR in SKGT-4 and JHESO EAC cells treated with CA3 at indicated dosage. C. YAP1/Tead transcriptional activity was determined by co-transfection of Gal4-Tead and 5×UAS-luciferase and YAP1 cDNA in SKGT-4 and JHESO EAC cells and then treated with CA3 at indicated dosage. Luciferase reporter activity was measured after 48 h of transfection. For all experiments, values shown represent the mean and SD of at least triplicate assays (*P<0.05; **P<0.01). D&E. YAP1 expression (D) and transcriptional activity (E) were determined by immunoblotting or co-transfection of Gal4-Tead and 5×UAS-luciferase and YAP1 cDNA in SKGT-4 DOX− and DOX+ cells treated with CA3 at dosage indicated.
CA3 preferentially inhibits CSC properties enriched in radiation-resistant EAC cells
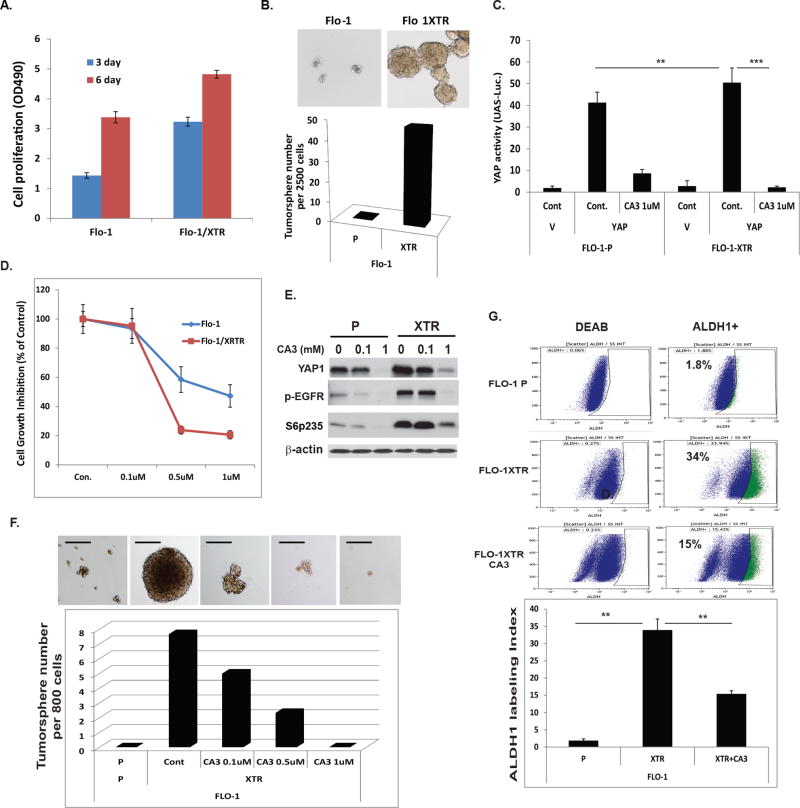
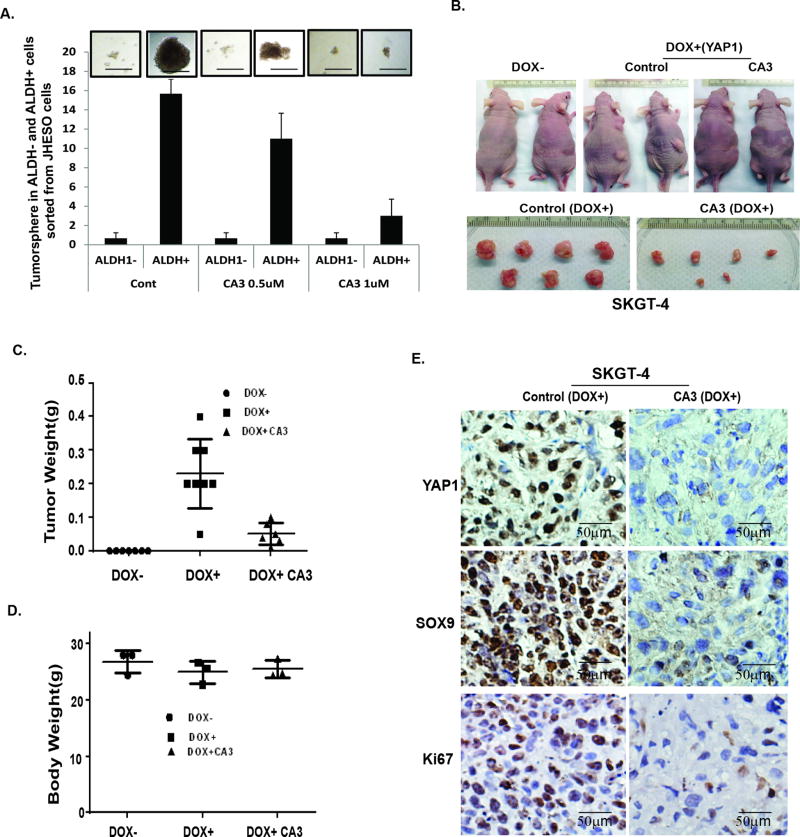
In collaboration with Dr. Heath Skinner (MD Anderson), we developed radiation resistant Flo-1 XTR cells as indicated in Fig. 4. We observed that these radiation resistant XTR cells had greater potential to proliferate and form large tumor spheres and in greater numbers than did their parental cells (Fig. 4A and 4B). Treatment with CA3 preferentially inhibited the transcriptional activity of YAP1/Tead in XTR cells (Fig.4C) and suppressed XTR cell growth compared to that in parental cell (Fig. 4D). Further, treatment with CA3 significantly suppressed YAP1 and other oncogenes (phosphorylated S6 and phosphorylated EGFR) (Fig.4E). CA3 dramatically blocked tumor sphere formation by XTR Flo-1 cells in a dose-dependent manner; while parental Flo-1 cells do not form tumor spheres (Fig. 4F). Most importantly, CA3 also markedly decreased the population of CSCs as determined by ALDH1 labeling (ALDEFLUOR™ Kit) (Fig.4G). To further confirm that treatment with CA3 inhibits CSC properties, ALDH1 positive and ALDH1 negative cells were sorted from JHESO cells and treated with CA3. We found that CA3 significantly decreased ALDH1+ tumor sphere growth in a dose dependent manner, whereas ALDH1− cells hardly form any tumor sphere. (Fig. 5A). This indicated that the novel YAP1 inhibitor CA3 preferentially inhibits the CSC properties of radiation resistant XTR EACs, suggesting that CA3 can target CSC-enriched radiation resistant XTR EAC cells.
Figure 4. CA3 preferentially inhibits CSC properties enriched in radiation-resistant EAC cells.
A. The cells proliferation and viability (OD490) were measured in Flo-1 and Flo-1 XTR, the radiation resistant cells at 3 day and 6 day using MTS assay respectively as described in Materials&Methods. B. Representative images (top) and quantification (low) of tumor sphere formation in Flo-1 and Flo-1 XTR cells were demonstrated. C. YAP transcriptional activity was determined by co-transfection of Gal4-Tead and 5×UAS-luciferase and YAP1 cDNA in Flo-1-P and Flo-1 XTR cells treated with CA3 at dosage indicated, D. Cell growth inhibition in both Flo-1-P and Flo-1 XTR cells treated with CA3 at dosage indicated was determined using MTS assay as described in Materials&Methods; E. Immunoblotting for YAP1 and phospho-EGFR and phosph-S6 in Flo-1-P and Flo-1 XTR cells treated with CA3 at dosage indicated. F. Representative images (top) and quantification (low) of tumor sphere formation in Flo-1 and Flo-1 XTR cells treated with CA3 at dosage indicated. Experiments were repeated three times. G. Flo-1, Flo-1 XTR and Flo-1 XTR cells treated with CA3 at 0.5µM for 48 hours and then labeling with ALDH1 using ALDH1 labeling kit.
Figure 5. CA3 suppresses ALDH1+ cell tumor sphere and exerts strong antitumor effects in inducible high YAP xenograft model.
A. ALDH1 positive or negative cells were sorted from JHESO EC cells and tumor sphere assays were done in the sorted cells and add CA3 at 0.5µM at the beginning of the tumor sphere culture. After 8–10 days of culture, the tumor sphere numbers formed were counted under microscope. Representative fields (top) and the bar graph (low) were demonstrated. B–D. SKGT-4 (PIN20YAP1) cells with (DOX+) or without (DOX−) YAP1 induction were inoculated into nude mice of both sites (n = 5 per group). Representative tumors after 6 weeks are shown (B).Tumor weight (C) and body weight(D) were calculated as described in Materials and Methods after 6 weeks. E. immunohistochemistry for YAP1, SOX9, and Ki67 was performed in mouse tumor tissues derived from xenograft nude mice. Scale bar, 50 µm; 200× magnification.
CA3 exerts strong antitumor effects in inducible high YAP xenograft mouse model in vivo
To further confirm the antitumor effects of CA3 result from targeting YAP1 in vivo, we utilized inducible YAP1 high SKGT-4 (Dox+) cells xenograft model. Nude mice were implanted with Dox− and Dox+ SKGT-4 cells at 1 × 106 cells per mouse. At this concentration, we observed that only Dox+ SKGT-4 cells were able to form tumors after 10–14 days of injection (Fig. 5B), indicating that YAP1 is necessary to drive tumor growth in vivo. To determine the effects of treatment with CA3 in inhibition of YAP-inducible tumor growth in vivo, we randomly placed mice bearing Dox+ SKGT-4 EAC xenografts in two groups and then gave them treatment with control phosphate-buffered saline or CA3 at 1 mg/kg. At the end of our 3-week dosing schedule, SKGT-4 xenograft tumor weights and volumes and the mice’s body weights were measured. Results from in vivo SKGT-4 Dox+ xenograft model demonstrated that mice with Dox+ SKGT-4 xenografts treated with CA3 greatly reduced tumor sizes and weights in vivo (Fig.5B&5C), whereas tumors did not form in mice implanted with Dox− SKGT-4 cells over the whole experimental period (Figure 5B). Mice’s body weights did not differ significantly between CA3 treatment and control group (Fig 5D). In addition, immunohistochemistry further confirmed in mice tumor tissues that expression of YAP1, SOX9 and KI67 was greatly reduced in mice with SKGT-4 Dox+ treated with CA3 (Fig. 5E). Thus, CA3 effectively suppress EAC tumor growth in vivo and these effects owe, at least in part, to inhibition of the stemness genes YAP1 and SOX9.
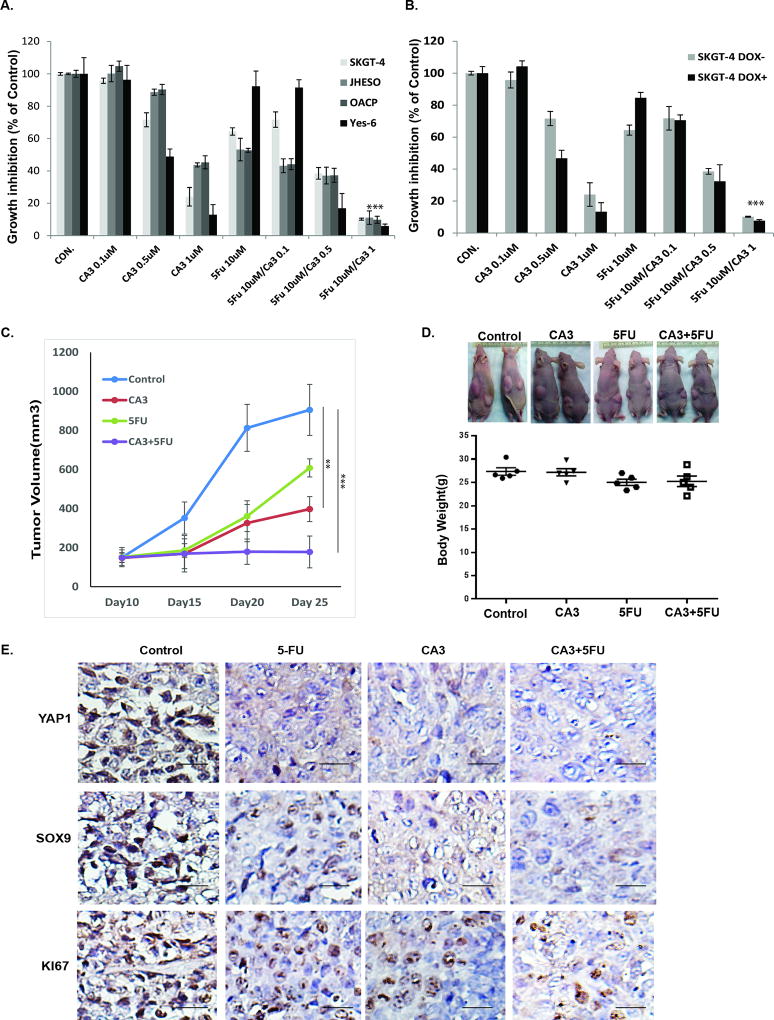
CA3 synergizes with 5-FU in inhibiting growth of EAC cells in vitro and in vivo
To determine the effects of treatment with CA3 alone or in combination with 5-FU on inhibition of the growth of EAC cell lines, we first seeded four EC cell lines with constitutive high YAP1 expression (SKGT-4, JHESO, OACP, and YES-6) in 96-well plates and treated them with CA3 alone, 5-FU alone, or the combination of CA3 and 5-FU at indicated concentrations. The results shown in Fig. 6A demonstrated that although CA3 produced dose-dependent decreases in the growth of these four cell lines, the combination of CA3 and 5-FU significantly synergized to inhibit their growth especially in the combination of CA3 and 5-FU at the maximum extent. To further examine the inhibition of CA3 on EAC cells are YAP1 dependent, we treated Dox+ (YAP1 induced) and Dox− SKGT4 (PIN20YAP1) cells with CA3 alone, 5-FU alone, or the combination, we found that CA3 alone preferentially inhibited the growth of YAP1 high SKGT-4 cells (Dox+) compared to YAP1 low SKGT-4 cells (Dox−) and in a dose dependent manner (Fig. 6B). Moreover, treatment with the combination of CA3 and 5-FU produced the greatest inhibition of growth of both Dox+ and Dox− SKGT-4 cells (Fig.6B). These findings indicated that treatment with CA3 synergize with 5-FU in GAC cell growth inhibition.
Figure 6. CA3 synergizes with 5-FU in inhibiting growth of EAC cells in vitro and in vivo.
A. Four EAC cell lines-SKGT-4, JHESO, OACP and Yes-6 were treated with 5-FU and CA3 either alone or in combination at the concentration indicated for six days, cell growth inhibition was measured using MTS assay. ***p<0.01. B. SKGT-4 (PIN20YAP) cells with (DOX+) or without (DOX−) YAP induction by doxycycline and treated with 5-FU and CA3 either alone or in combination at the concentration indicated for six days, cell growth inhibition was measured using MTS assay. ***p<0.01. C&D. JHESO cells (1.5×106) were injected subcutaneously in nude mice, each mouse have two sites (left, right) injections; 5 mice/group and treated with either CA3 alone, 5-FU alone or in combination as described in Materials&Methords. Tumor Volume and mouse body weight in each group were measured and calculated as described in Materials & Methods. E. Immunohistochemistry for YAP1, SOX9 and Ki67 was performed in mouse tumor tissues derived from JHESO xenograft nude mice. Scale bar, 50 µm; 200× magnification.
To further determine the antitumor effects of treatment with CA3 and in combination with 5-FU in vivo, we tested CA3 alone and or in combination with 5-FU in JHESO xenograft mouse model. We implanted nude mice with JHESO cells with constitutively high YAP1 expression, which easily form large tumors in mice. We then randomly placed the mice bearing the JHESO xenografts in four groups and treated with control phosphate-buffered saline alone, CA3 alone, 5-FU alone, or CA3 and 5-FU. Tumor growth and tumor volumes were observed and measured over 3 weeks of treatment. Xenograft tumor weights and mouse body weights were measured at the end of treatment. The results shown in Fig. 6C demonstrated markedly lower tumor volumes in the combination group than in the 5-FU and CA3 groups but that the mice’s body weights did not differ significantly (Fig. 6D, lower panel). In addition, the expression of YAP1, SOX9, and the proliferation marker KI67 in mouse tumors was dramatically reduced by treatment with the combination of CA3 and 5-FU (Fig. 6E). Thus, treatment with CA3 in combination with 5-FU have synergistic antitumor effects in vivo.
Discussion
CSCs have received considerable attention because of their ability to initiate tumors and importance as sources of therapy resistance, and progression, establishing the importance of targeting CSCs for treatment. Mounting evidence suggests that deregulation of Hippo signaling and activation of its co-activator YAP1 leads to acquisition of CSC properties in many tumor types, including in EAC. In the present study, we identified a novel, potent small molecule inhibitor CA3 and showed that it has a remarkable inhibitory effect on YAP1/Tead transcriptional activity and potent growth-inhibitory effects on EAC cell lines, especially those with high YAP1 expression. Remarkably, radiation resistant EAC cells had enriched CSC properties and malignant behavior, whereas treatment with CA3 effectively suppressed CSC properties and reduced the fraction of ALDH1+ cells enriched in radiation resistant cells. Moreover, CA3 and 5-FU synergistically inhibited EAC growth, especially that of high YAP1-expressing and resistant cells. Thus, CA3 represents a newly identified inhibitor of Hippo YAP1, and primarily targeting high YAP1-expressing and therapy-resistant EAC with enriched CSC properties.
Dysregulation of stem cell signaling pathways like Hippo/YAP1, Wnt/β-catenin, and Hedgehog have been implicated in the maintenance of CSC populations and confer therapy resistance in cancer cases(5,34).YAP1, the downstream effector of the Hippo signaling pathway, is frequently overexpressed in many types of cancer tissues and associated with poor survival (35,36). Our recent studies demonstrated that YAP1 regulation of SOX9 is a key modulation of the CSC phenotype, the YAP/SOX9 axis is potentially an important new therapeutic target for EAC (4), and YAP mediates constitutive and acquired treatment resistance in EAC cells (37,38). Many oncogenic signaling pathways, such as KRAS/EGFR, G-protein-coupled receptor, and Wnt/β-catenin, use YAP1/TAZ to integrate and enhance their oncogenic signaling (23,39). Thus, YAP1 is often a terminal node of many oncogenic pathways and a critical target for many cancer types.
Verteporfin (VP) is a porphyrinic photosensitizer clinically used for the photodynamic treatment of age-related macular degeneration. Investigators recently identified VP as a small molecule inhibitor of YAP1/TEAD association and a selective means of inhibiting YAP1’s oncogenic activity (33). Since the identification of VP as a YAP/TEAD inhibitor, several studies have revealed the potential for this molecule in inhibition of different cancers in which YAP is overexpressed and also as a research tool to study Hippo/YAP1 functions in their systems. Recently, Basu D et al identified C19, a candidate small molecule inhibitor of Hippo, transforming growth factor-β, and Wnt signaling as a multi-epithelial-mesenchymal transition pathway inhibitor that not only inhibits Hippo YAP/TAZ signaling but also suppresses TGF-β and Wnt signaling (40). We observed that although VP suppresses YAP/Tead activity and tumor sphere formation induced by YAP1(4), the effects of VP on high YAP1-expressing EAC cells are less potent than CA3 and has been administered at high concentration levels (50–100 mg/kg) in vivo animal experiments(33). In addition, C19, another reported Hippo/YAP1 inhibitor; is not a pure YAP inhibitor owing to its multiple mechanisms against various pathways, such as transforming growth factor-β and Wnt (40). To search for more specific and potent YAP1 inhibitors, we screened a large chemical library in collaboration with Dr. Ding based on inhibition of YAP1/Tead transcriptional activity using co-transfection of Gal4-Tead and 5×-UAS-luciferase plasmids, which represent YAP1 activity. We identified several potent YAP1 inhibitors, including CA3, A414, A432, A413, and A433 (Fig. 1A). To determine the specific YAP-inhibitory effects of these inhibitors in EAC cells, we used our EAC cell line with Dox-inducible YAP1 expression with PIN20YAP1 SKGT-4 (SKGT-4 DOX− vs SKGT-4 DOX+). Although all YAP1 inhibitors including VP inhibited YAP/Tead transcriptional activity similar as shown in Fig. 1A, we found that CA3 had the most potent and specific inhibitory effects on SKGT-4 DOX+ cell growth. The rest of the inhibitors either had minimal specificity or were far less potent. The mechanism of CA3 in tumor cells inhibition primarily though attenuation of YAP1 protein expression in EAC cells. How CA3 affects YAP1 protein levels are not clear at present. CA3 (CAS Registry Number 300802-28-2 2,7-bis(piperidinosulfonyl)-9H-fluoren-9-one oxime) is also known as CIL56. CIL56 has been reported to affect iron-dependent ROS production and fatty acid biosynthesis and to trigger cell death dependent upon the rate-limiting de novo lipid synthetic enzyme ACC1(41). However, there is currently no report linking CA3/CIL56 to cancer therapy and to YAP1 inhibition. It will be of interest to explore whether there is a link between the ability of CA3/CIL56 to trigger tumor cell death through YAP1 inhibition and cell death induced by ROS production and modulation of fatty acid production. Further, our in vivo study demonstrated CA3 has strong antitumor effect when administrating in relative low concentration at 1mg/kg compared to VP in 50–100mg/kg (33) which is more practical to apply to human subjects. Thus, we identified the novel small molecule YAP1 inhibitor CA3 as a more selective and potent inhibitor of the growth of high YAP1-expressing EAC tumor cells.
Resistance to chemo-, radiation-, and targeted therapy is the major obstacle and challenge in the clinic. The strength of present study is that we demonstrated that radiation resistant EAC cells are enriched with CSC properties and treatment with CA3 preferentially suppresses radiation resistant cell growth and tumor sphere formation. More strikingly, treatment with CA3 significantly reduced CSC populations ALDH1+ in resistant EAC cells and preferentially inhibited ALDH1+ EAC cell tumor sphere formation, and decreased the proportion of ALDH1+ EAC cells. We also demonstrated that CA3 can sensitize resistant EAC cells to radiation therapy. Our previous study reported that ALDH1 and YAP1/SOX9, which are reliable CSC markers were either associated with or mediated chemo-resistance in EAC (13,32). Our current study demonstrated that treatment with CA3 in combination with 5-FU strongly inhibits the growth of many types of EAC cells with constitutively high YAP1 expression (Fig 6A) and that these two agents synergistically inhibit the growth of EAC cells with inducible high YAP1 (Fig. 6B). Most importantly, studies in vivo indicated that CA3 potently suppresses doxycycline induced YAP1 high (Dox+) EAC cell growth (Fig. 5). Furthermore, in another JHESO xenograft mouse model, CA3 synergistically with 5-FU, suppresses constitutive high YAP1 JHESO xenograft growth (Fig. 6C&D). This was accompanied by decreased expression of Ki67 as well as YAP1 and SOX9 in mouse tumor tissues (Fig. 6E), while CA3 did not have any apparent signs of toxicity as judged by mice body weights among groups suggesting that this compound may represent an effective and potentially safe agent. Further testing of its efficacy against many other tumor types in vitro and in vivo is warranted and identification of the mechanism of its action is under our further investigation.
In conclusion, our studies identified the novel small molecule YAP1 inhibitor CA3 as having potent and selective activity in targeting CSCs in radiation resistant EAC cells with high YAP1 expression. CA3 has remarkable inhibitory activity against YAP/Tead transcription, strongly inhibits the growth of these cells in vitro and in vivo, and greatly reduces CSCs populations enriched in therapy resistant EAC cells. The findings of this study shed light on targeting of CSCs, the seeds of tumor progression and treatment resistance. The combination of CA3 that target CSCs and cytotoxic agents that target proliferating cells synergistically inhibit tumor growth that could be the best strategy for treating therapy-resistant EACs with activation of YAP1.
Supplementary Material
Acknowledgments
The authors thank the Department of Scientific Publications at MD Anderson for their wonderful revision of the manuscript. Authors thank Lianchun Xiao in the Department of Biostatistics of MDACC for statistical analysis of some in vitro and in vivo data. This work was supported by American Gastroenterological Association Research Scholar Award (Song S), and Public Health Service Grant DF56338 which supports the Texas Medical Center Digestive Diseases Center (Song S); UTMDACC IRG (3-0026317, Song S); CA160433 (Song S); NIH, CA129906, CA138671, CA172741(JAA) and P30CA016672 from NIH/NCI.
Financial Supports: S. Song, UTMDACC IRG (3-0026317); S Song, DOD (CA160433); J.A. Ajani, NIH/NCI (CA138671, CA172741); J.A. Ajani, DOD (CA150334, CA160445).
References
- 1.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer Stem Cells: The Promise and the Potential. Semin Oncol. 2015;42(Suppl 1):S3–S17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Tumaneng K, Russell RC, Guan KL. Organ size control by Hippo and TOR pathways. Curr Biol. 2012;22(9):R368–79. doi: 10.1016/j.cub.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14(12):1322–9. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, et al. Hippo coactivator YAP1 upregulates SOX9 and endows stem-like properties to esophageal cancer cells. Cancer Res. 2014;74:4170–82. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526(7575):715–8. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 6.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, et al. YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells. Stem cells. 2015;33(6):1705–18. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cebola I, Rodriguez-Segui SA, Cho CH, Bessa J, Rovira M, Luengo M, et al. TEAD and YAP regulate the enhancer network of human embryonic pancreatic progenitors. Nat Cell Biol. 2015;17(5):615–26. doi: 10.1038/ncb3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nature reviews Cancer. 2015;15(2):73–9. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115(19):4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130–9. doi: 10.1158/1078-0432.CCR-10-2467. [DOI] [PubMed] [Google Scholar]
- 11.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin Cancer Res. 2015;21(11):2580–90. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47(3):250–6. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keren-Paz A, Emmanuel R, Samuels Y. YAP and the drug resistance highway. Nat Genet. 2015;47(3):193–4. doi: 10.1038/ng.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greten FR. YAP1 takes over when oncogenic K-Ras slumbers. Cell. 2014;158(1):11–2. doi: 10.1016/j.cell.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25(6):822–30. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Xia H, Ge X, Chen Q, Yuan D, Chen Q, et al. CD44 acts through RhoA to regulate YAP signaling. Cellular signalling. 2014;26(11):2504–13. doi: 10.1016/j.cellsig.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Regue L, Mou F, Avruch J. G protein-coupled receptors engage the mammalian Hippo pathway through F-actin: F-Actin, assembled in response to Galpha12/13 induced RhoA-GTP, promotes dephosphorylation and activation of the YAP oncogene. BioEssays : news and reviews in molecular, cellular and developmental biology. 2013;35(5):430–5. doi: 10.1002/bies.201200163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780–94. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Jeong S. Wnt activated beta-catenin and YAP proteins enhance the expression of non-coding RNA component of RNase MRP in colon cancer cells. Oncotarget. 2015;6(33):34658–68. doi: 10.18632/oncotarget.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158(1):171–84. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158(1):185–97. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raju U, Ariga H, Koto M, Lu X, Pickett J, Valdecanas D, et al. Improvement of esophageal adenocarcinoma cell and xenograft responses to radiation by targeting cyclin-dependent kinases. Radiother Oncol. 2006;80(2):185–91. doi: 10.1016/j.radonc.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Soldes OS, Kuick RD, Thompson IA, 2nd, Hughes SJ, Orringer MB, Iannettoni MD, et al. Differential expression of Hsp27 in normal oesophagus, Barrett's metaplasia and oesophageal adenocarcinomas. Br J Cancer. 1999;79(3–4):595–603. doi: 10.1038/sj.bjc.6690094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21(3):374–87. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strick-Marchand H, Weiss MC. Inducible differentiation and morphogenesis of bipotential liver cell lines from wild-type mouse embryos. Hepatology. 2002;36(4 Pt 1):794–804. doi: 10.1053/jhep.2002.36123. [DOI] [PubMed] [Google Scholar]
- 28.Song S, Maru DM, Ajani JA, Chan CH, Honjo S, Lin HK, et al. Loss of TGF-beta adaptor beta2SP activates notch signaling and SOX9 expression in esophageal adenocarcinoma. Cancer Res. 2013;73(7):2159–69. doi: 10.1158/0008-5472.CAN-12-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song S, Krishnan K, Liu K, Bresalier RS. Polyphenon E inhibits the growth of human Barrett's and aerodigestive adenocarcinoma cells by suppressing cyclin D1 expression. Clin Cancer Res. 2009;15(2):622–31. doi: 10.1158/1078-0432.CCR-08-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69(4):1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69(3):1089–98. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 32.Ajani JA, Wang X, Song S, Suzuki A, Taketa T, Sudo K, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 2014;8(1):142–9. doi: 10.1016/j.molonc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144(7):1530–42. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, Xu R, Li X, Ren W, Ou C, Wang Q, et al. Prognostic Value of Yes-Associated Protein 1 (YAP1) in Various Cancers: A Meta-Analysis. PLoS One. 2015;10(8):e0135119. doi: 10.1371/journal.pone.0135119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenzetto E, Brenca M, Boeri M, Verri C, Piccinin E, Gasparini P, et al. YAP1 acts as oncogenic target of 11q22 amplification in multiple cancer subtypes. Oncotarget. 2014;5(9):2608–21. doi: 10.18632/oncotarget.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa K, Noguchi K, Nakano Y, Yamamura M, Takaoka K, Hashimoto-Tamaoki T, et al. The Hippo pathway transcriptional co-activator, YAP, confers resistance to cisplatin in human oral squamous cell carcinoma. Int J Oncol. 2015;46(6):2364–70. doi: 10.3892/ijo.2015.2948. [DOI] [PubMed] [Google Scholar]
- 38.Huo X, Zhang Q, Liu AM, Tang C, Gong Y, Bian J, et al. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncology reports. 2013;29(2):840–6. doi: 10.3892/or.2012.2176. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123–35. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu D, Lettan R, Damodaran K, Strellec S, Reyes-Mugica M, Rebbaa A. Identification, mechanism of action, and antitumor activity of a small molecule inhibitor of hippo, TGF-beta, and Wnt signaling pathways. Molecular cancer therapeutics. 2014;13(6):1457–67. doi: 10.1158/1535-7163.MCT-13-0918. [DOI] [PubMed] [Google Scholar]
- 41.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10(7):1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.