Abstract
Aberrant energy metabolism represents a hallmark of cancer and contributes to numerous aggressive behaviors of cancer cells, including cell death and survival. Despite the poor prognosis of mantle cell lymphoma (MCL), due to the inevitable development of drug resistance, metabolic reprograming of MCL cells remains an unexplored area. Post-translational modification of proteins via O-GlcNAcylation is an ideal sensor for nutritional changes mediated by O-GlcNAc transferase (OGT) and is removed by O-GlcNAcase (OGA). Using various small molecule inhibitors of OGT and OGA, we found for the first time that O-GlcNAcylation potentiates MCL response to bortezomib (BTZ). CRISPR interference of MGEA5 (encoding OGA) validated the apoptosis sensitization by O-GlcNAcylation and OGA inhibition. To identify the potential clinical candidates, we tested MCL response to drug-like OGA inhibitor, ketoconazole (KCZ), and verified that it exerts similar sensitizing effect on BTZ-induced apoptosis. Investigations into the underlying molecular mechanisms reveal that BTZ and KCZ act in concert to cause the accumulation of truncated Bid (tBid). Not only does KCZ potentiate tBid induction, but also increases tBid stability through O-GlcNAcylation that interferes with tBid ubiquitination and proteasomal degradation. Remarkably, KCZ strongly enhances BTZ-induced apoptosis in de novo BTZ-resistant MCL cells and in patient-derived primary cells with minimal cytotoxic effect on normal peripheral blood mononuclear cells and hepatocytes, suggesting its potential utility as a safe and effective adjuvant for MCL. Together, our findings provide novel evidence that combination of BTZ and KCZ or other OGA inhibitors may present a promising strategy for the treatment of drug-resistant MCL.
Keywords: O-GlcNAcylation, lymphoma drug resistance, O-GlcNAcase inhibitor, ubiquitination, truncated Bid
Introduction
Metabolic reprogramming is a hallmark of cancer that has emerged as an attractive target in novel therapeutic strategies for cancer treatment (1,2). Mantle cell lymphoma (MCL), an aggressive non-Hodgkin lymphoma (NHL) arising from pregerminal center mature B cells, is typically incurable due to the inevitable development of drug resistance, and thus has the worst prognosis among NHL subtypes (3,4). However, overcoming MCL resistance particularly through metabolic signaling remains largely an unexplored area.
O-GlcNAcylation is an abundant, dynamic, and nutrient-sensitive post-translational modification (PTM) that describes an addition of O-linked β-N-acetylglucosamine (O-GlcNAc) moiety to the serine or threonine residues in proteins in response to changes of the sugar donor UDP-GlcNAc from hexosamine biosynthetic pathway (5,6). As the hexosamine pathway consumes various essential nutrients and metabolic intermediates, e.g. glucose and glutamine, acetyl coenzyme A and nucleotide uridine-5′-triphosphate (UTP), it provides an ideal machinery for cells to sense and respond to a variety of microenvironmental conditions. Alterations in the levels of cycling enzymes O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) that catalyzes and removes O-GlcNAc, and the levels of O-GlcNAcylated proteins themselves, have been linked to many different human malignancies, including breast, lung, colon, prostate, bladder and leukemia (7–11). In chronic lymphocytic leukemia (CLL), a recent study has shown that indolescent and aggressive clinical behaviors of CLL cells correlate well with the higher and lower levels of O-GlcNAc respectively, suggesting the potential involvement of O-GlcNAcylation in CLL pathogenesis (11).
Bortezomib (BTZ) has demonstrated impressive clinical efficacy and is FDA-approved for the treatment of relapse/refractory MCL. However, innate and acquired clinical resistance to BTZ is frequently observed (12,13). Because many nucleocytoplasmic proteins involved in the proliferation and survival of tumor cells are dramatically modified by O-GlcNAcylation, including p53, NF-κB, and c-Myc (7,14,15), targeting O-GlcNAcylation might represent a rational approach to modulate MCL drug response. In this study, we used small molecule inhibitors of OGT and OGA cycling enzymes to modify intracellular O-GlcNAcylation and to investigate its effect on BTZ-induced apoptosis. We found that well known OGA inhibitors, including PugNAc and thiamet G (16,17), sensitize MCL cells to BTZ-induced apoptosis, while OGT inhibitor alloxan (18,19) inhibits it. In an attempt to identify potential drug candidates for clinical utility, we further tested the effect of a drug-like OGA inhibitor, ketoconazole (KCZ) (20), on MCL response. Here, we showed that a conventional antifungal drug KCZ enhances the susceptibility of multiple parental and de novo BTZ-resistant MCL cells as well as patient-derived primary cells to BTZ-induced apoptosis. We also unveil the underlying mechanism of sensitization that is OGA inhibitors induce O-GlcNAcylation of truncated Bid (tBid) and strengthen apoptosis signaling by abrogating tBid ubiquitin-mediated proteasomal degradation, thus stabilizing tBid and prolonging its action. Our findings also suggest the role of hexosamine metabolic pathway in MCL drug response, which could be important in predicting therapeutic response and in developing new treatment strategies for drug-resistant MCL to achieve long-term control of the disease.
Materials and Methods
Reagents
BTZ was obtained from Jannsen-Cilag (Wycombe, UK). Small molecule inhibitors of OGA PugNAc and thiamet G were obtained from Abcam (Cambridge, UK) and Tocris Bioscience (Bristol, UK), while KCZ was obtained from Crosschem Intercontinental Company (Lugano, Switzerland). A small molecule inhibitor of OGT alloxan and a small molecule inhibitor of tBid BI-6C9 (21) were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies for ubiquitin and O-GlcNAc were obtained from Abcam, while antibody for tBid was from Santa Cruz Biotechnology (Dallas, Texas). All other antibodies and reagents including MG132 and cycloheximide were from Cell Signaling Technology (Beverly, MA).
Cell lines and patient-derived primary cells
Human MCL-derived Jeko-1 cells were obtained from ATCC (Manassas, VA), while Granta-519 and SP49 cells were kind gifts of Dr. Siwanon Jirawatnotai (Systems Pharmacology, Faculty of Medicine Siriraj Hospital) (22,23). Mycoplasma contamination was checked every eight weeks using MycoAlertTM mycoplasma detection kit (Lonza, Cologne, Germany) and any cell lines found positive were discarded. Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), supplemented with 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, and maintained in a humidified atmosphere of 5% CO2 environment at 37° C. Patient-derived primary cells were from fresh biopsy-derived lymphoma tissues (lymph nodes) after informed consent and after approval by the Siriraj Institutional Review Board (#733/2557 EC1). Tissues were diced and forced through a fine metal sieve into RPMI culture medium. Mononuclear cells were then isolated by Ficoll-Hypaque gradient centrifugation and cultured in RPMI medium containing 20% FBS and 10% conditioned medium from MCL Granta-519 cells, as previously described (24). Peripheral blood mononuclear cells (PBMC) from healthy donors were used as normal control cells.
Apoptosis assays
Apoptosis was determined by Hoechst 33342 and annexin V/propidium iodide (PI) assays. In the Hoechst assay, cells were incubated with 10 μg/ml Hoechst 33342 (Molecular Probes, Eugene, OR) for 30 min and analyzed for apoptosis by scoring the percentage of cells having condensed chromatin and/or fragmented nuclei by fluorescence microscopy (Eclipse Ti-U with NiS-Elements, Nikon, Tokyo, Japan). The apoptotic index was calculated as the percentage of cells with apoptotic nuclei over total number of cells. For annexin V/PI assay, cells were harvested, washed and stained with annexin V-FITC in binding buffer supplemented with 5 mM calcium chloride for 15 min at room temperature and co-stained with PI (5 μg/ml). Samples were immediately analyzed by BD LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) using a 488-nm excitation beam and 530-nm and 670-nm band-pass filter with CellQuest software.
Combination index analysis
Combination index (CI) was analyzed by the fixed-ratio model using CompuSyn software (ComboSyn Inc., Paramus, NJ), which is a computerized analytical simulation using the median-effect principle of the mass-action law and its combination index theorem (25). Cells were treated with 12 concentrations of BTZ each in combination with 12 concentrations of KCZ at the ratio of 1 to 10000 or 1 to 5000, and Fa (fraction affected) on cell apoptosis was determined by Hoechst 33342 assay. Each drug was also used alone and each data point was performed in triplicates.
CRISPR interference design and vector construction
CRISPR interference system containing catalytically inactive version of Cas9 (deactivated Cas9; dCas9) and a single guided RNA (sgRNA) was used to induce transcriptional repression of MGEA5 (encoded OGA). Briefly, sgRNA targeting MGEA5 (CGCAAGCGCAGTGCGGATAAAC) was designed using CRISPR Design tool (http://crispr.mit.edu/) and cloned into human sgRNA expression vector containing a mouse U6 promoter and a constitutive CMV promoter driving an mCherry gene (Addgene, #44248) (26), as described previously (27). The constructed sgRNA plasmid was then transfected into Jeko-1 cells stably expressing human dCas9 vector (Addgene, #44246) (26) by nucleofection using 4D-NucleofectorTM (Lonza, Cologne, Germany) with EW113 device program. Two weeks after nucleofection, mCherry-positive cells were sorted using flow cytometry-based cell sorter (FACS; BD FACSAria, BD Biosciences), recovered for at least three passages and analyzed for MGEA5 by RT-PCR and OGA by Western blotting prior to use.
RNA isolation and RT-PCR
Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was prepared using SuperScript III first-strand synthesis system and oligo (dT) primers (Invitrogen). qPCR analysis was carried out on a 7500 Fast real-time PCR using a Power SYBR Green PCR master mix (Applied Biosystems Foster City, CA). Initial enzyme activation was performed at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec and primer annealing/extension at 60°C for 1 min. Relative expression of each gene was normalized against the housekeeping GAPDH gene product.
PPISURV analysis
PPISURV was used to correlate survival rates in cancer patients to the expression level of MGEA5. Samples were separated into low and high expression groups with respect to expression rank of the gene, which reflects relative mRNA expression level, based on the average expression across the data set. The R statistical package was used to perform survival analyses and to draw Kaplan-Meier plots. Unadjusted P value were generated using standard survival analysis package (28).
Caspase-8 activity assay
Caspase 8 activity was determined by detecting the cleavage of specific substrate IETD-AFC using a commercial assay kit (Biovision, Milpitas, CA). After treatments, cell lysates were prepared and incubated with IETD-AFC (50 μM) for 1 h. Free AFC fluorescence was measured using a fluorescence plate reader (Synergy H1, BioTek, Winooski, VT) at the 400-nm and 505-nm excitation and emission wavelengths. Caspase-8 activity was expressed as the ratio of signals from the treated and control samples.
Western blot analysis
After specific treatments, cells were incubated in a commercial lysis buffer (Cell Signaling Technology) and a protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN, USA) at 4 °C for 30 min. Protein content was analyzed using BCA protein assay (Pierce Biotechnology, Rockford, IL) and 50–150 μg of proteins were resolved under denaturing conditions by SDS-PAGE as described previously (29).
Retrovirus production and short hairpin RNA-mediated gene knockdown
Retroviral plasmids carrying short hairpin (sh) RNA sequence against human BID were obtained from Origene (Rockville, MD) and shBID retroviral production was performed using Platinum-A packaging cells (Cell Biolabs, Inc, San Diego, CA). Cells were incubated with shBID viral particles in the presence of hexadimethrine bromide (8 μg/ml) for 48 h and were cultured and selected for puromycin (1 μg/ml) resistance.
Overexpression plasmid and transfection
Cells were transfected with tBID (Addgene, #21149) (30) or GFP (Invitrogen) plasmid by nucleofection using 4D-NucleofectorTM (Lonza) with EW113 device program. The transfected cells were cultured with G418-containing medium (400 μg/ml) and stable transfectants (clone #1 and #2) were selected and identified by Western blotting.
Co-immunoprecipitation, ubiquitination, and O-GlcNAcylation
Cell lysates (200 μg protein) were immunoprecipitated using Dynabeads magnetic beads (Invitrogen). Briefly, the beads were conjugated with anti-tBid (h71) antibody for 10 min at room temperature. The conjugated beads were then resuspended with cell lysates for 30 min at room temperature. The immune complexes were washed four times and resuspended in 2× Laemmli sample buffer. They were then separated by SDS-PAGE and analyzed for ubiquitination or O-GlcNAcylation using anti-ubiquitin or anti-O-GlcNAc (RL2) antibody respectively.
Cycloheximide-chase assay
Cells were treated with cycloheximide (10 μg/ml) to inhibit new protein synthesis for various times (0–16 h) to follow the degradation of protein by Western blot analysis (29). tBid expression profile was plotted and tBid half-life was calculated using GraphPad Prism software (La Jolla, CA, USA).
Generation of de novo BTZ-resistant cells
BTZ-resistant cell lines were generated by stepwise selection method as described previously with slight modifications (31). Parental MCL-derived Jeko-1 (Jeko/Parent) and Granta-519 (Granta/Parent) cells were continuously exposed to increasing concentrations of BTZ to the maximum concentration of 500 nM in Jeko/Parent cells and 150 nM in Granta/Parent cells, and resistant cells were selected using Dead Cell Removal Kit (Miltenyi Biotec, Auburn, CA) and designated as Jeko/BTZ500R and Granta/BTZ150R.
Statistical analysis
The data represent means ± s.d. from three or more independent experiments as indicated. Statistical analysis was performed by Student’s t-test at a significance level of P < 0.05. An ANOVA followed by Mann-Whitney U test was used for a multiple pairwise comparison.
Results
O-GlcNAcase inhibitors increase BTZ sensitivity in MCL cells
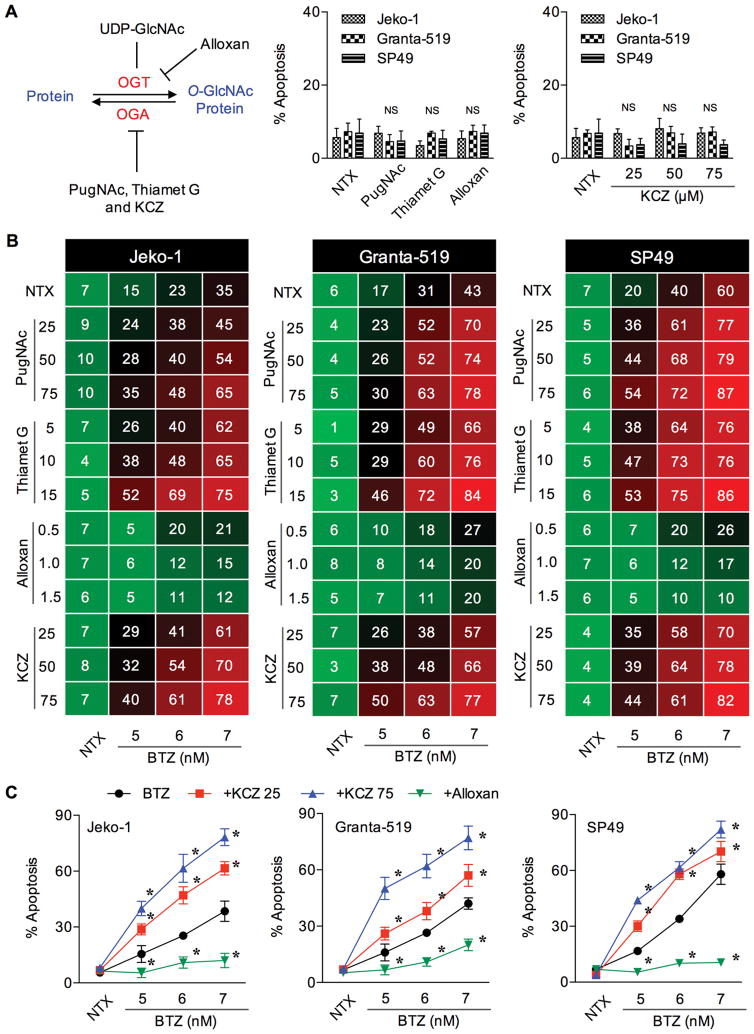
To investigate the potential role of O-GlcNAcylation in BTZ sensitivity, we modulated intracellular O-GlcNAcylation level using various known small molecule inhibitors of specific enzymes in the hexosamine pathway, as schematically depicted in Figure 1A (left). First, we determined the sub-toxic concentrations of these inhibitors in the tested cell systems to ensure that the observed effects are not due to the cytoxicity of the inhibitors. MCL Jeko-1, Granta-519 and SP49 cells were treated with well known inhibitors of OGA (PugNAc and thiamet G), drug-like OGA inhibitor (KCZ) and OGT inhibitor (alloxan), and their effect on cell apoptosis was determined by Hoechst 33342 assay. We identified the appropriate non-cytotoxic concentrations of these inhibitors, i.e. up to 50 μM for PugNAc, 15 μM for thiamet G, 1.5 mM for alloxan, and 75 μM for KCZ (Figure 1A, right; see also Supplementary Figure S1 and S2). To evaluate their effect on BTZ sensitivity, cells were pretreated with the inhibitors for 1 h, followed by BTZ treatment and apoptosis was determined after 24 h. Figure 1B and Supplementary Table S1 show that the OGA inhibitors PugNAc and thiamet G significantly enhanced the sensitivity of MCL cells to BTZ (5–7 nM). By contrast, the OGT inhibitor alloxan abrogated BTZ-induced apoptosis, suggesting the role of O-GlcNAcylation in MCL apoptotic response.
Figure 1.
O-GlcNAcase inhibitors increase the sensitivity of MCL cells to BTZ. (A) (left) Schematic diagram for the O-GlcNAcylation, showing cycling enzymes OGT and OGA, and small molecule inhibitors that modulate OGA, including PugNAc, thiamet G and KCZ, and that modulate OGT, including alloxan. (right) Effect of OGT and OGA inhibitors on cell apoptosis to ensure their sub-cytotoxic concentrations. Data are mean ± s.d. (n=3). NS, not significance vs. non-treated cells. NTX, non-treatment. (B) Effect of small molecule OGA or OGT inhibitors on BTZ-induced MCL cell apoptosis. Cells were treated with BTZ (0–7 nM) in the presence or absence of PugNAc (25–75 μM), thiamet G (5–15 μM), alloxan (0.5–1.5 mM) or KCZ (25–75 μM) and apoptosis was determined after 24 h by Hoechst 33342 assay. Mean percentage of apoptosis from four independent experiments in Jeko-1, Grant-519 and SP49 cells are shown in the drug dose matrix data in 3-color scale, where green, black and red indicate lowest, midpoint and highest apoptosis, respectively (see also Supplementary Table S1 for raw data and statistical analysis). (C) Percentage of apoptosis in response to BTZ and drug-like OGA or OGT inhibitor co-treatment is plotted. Data are mean ± s.d. (n=4). *P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test.
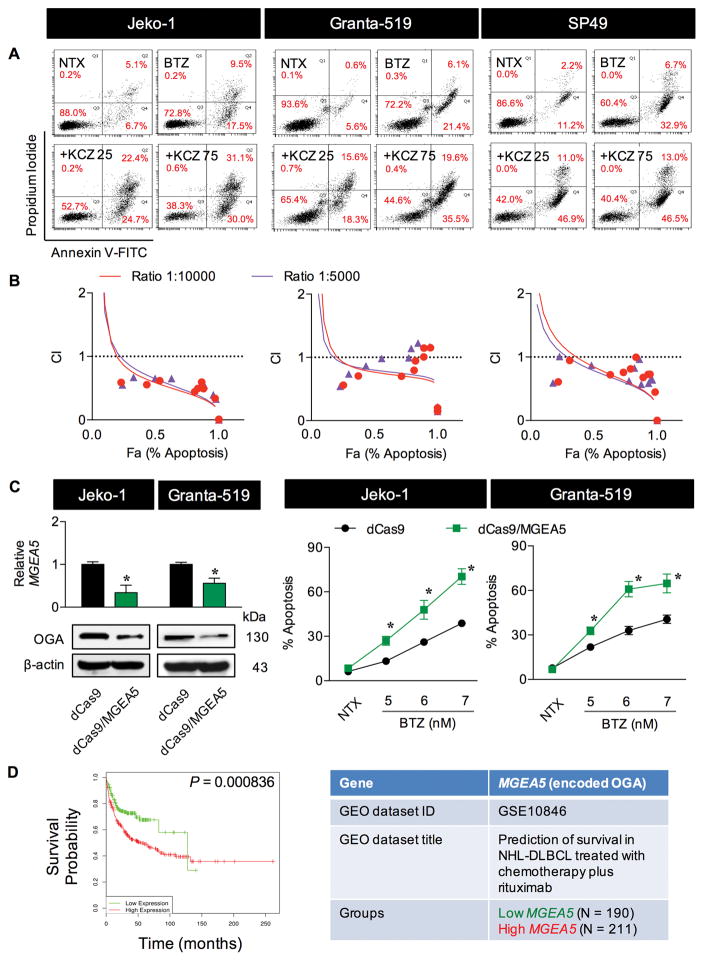
We also investigated the effect of drug-like OGA inhibitor KCZ on BTZ sensitivity due to its potential clinical application. Similar to the well known small molecule OGA inhibitors, KCZ (25–75 μM) dose-dependently promoted the apoptotic effect of BTZ in all MCL cell lines tested (Figure 1C and Supplementary Table S1). Flow cytometric analysis using annexin V-FITC and PI double staining further confirmed the observed sensitizing effect of KCZ on BTZ apoptosis, as indicated by an increase in the proportion of annexin V-positive cells (Figure 2A). To strengthen that the combination of BTZ and KCZ would have added benefits, CI values were calculated based on the fixed-ratio model using CompuSyn software to define whether the two drugs are synergistic (CI < 1), additive (CI = 1) or antagonistic (CI > 1). Strong synergy was observed along the magnitude of apoptosis in all cell tested (Figure 2B), indicating the potential application of KCZ.
Figure 2.
Drug-like OGA inhibitor KCZ sensitizes MCL cells to BTZ-induced apoptosis. (A) MCL cells were treated with BTZ (6 nM) in the presence of increasing concentrations of KCZ (0–75 μM) for 24 h and analyzed for apoptosis and necrosis by flow cytometry using annexin V and propidium iodide (PI) as probes. Representative dot plots of annexin V (x-axis) and PI (y-axis) are shown. Early and late apoptotic cells are in the lower and upper right quadrants with annexin V positive. NTX, non-treatment. (B) Combination index (CI) analysis by the fixed-ratio model. MCL cells were treated with BTZ and KCZ at the ratio of 1 to 10 and 1 to 5 and CI values were calculated and predicted (trendlines) using CompuSyn software. CI = 1, additivity; CI>1, antagonism; CI<1, synergy. (C) Transcriptional repression of MGEA5 (encoding OGA) was performed using CRISPR interference. (left) Quantitative real-time PCR of MGEA5 mRNA expression and Western blot analysis of OGA protein level in control (dCas9) and OGA-knockdown (dCas9/MGEA5) Jeko-1 and Granta-519 cells. (right) Effect of OGA inhibition on BTZ-induced apoptosis. Cells were treated with BTZ (0–7 nM) for 24 h and apoptosis was determined by Hoechst 33342 assay. Data are mean ± s.d. (n=3). *P < 0.05 vs. BTZ-treated dCas9 control cells; two-sided Student’s t-test. (D) Kaplan-Meier survival curve of patients with DLBCL, segregated according to high (red) or low (green) expression of MGEA5 (encoding OGA), obtained from public database through a bioinformatics analysis using PPISURV (www.bioprofiling.de).
Next, CRISPR interference (CRISPR/dCas9) targeting MGEA5 (encoding OGA) was used to repress MGEA5 expression. The OGA-knockdown (dCas9/MGEA5) cells were established and their apoptotic response to BTZ was examined in comparison with control (dCas9) cells. Figure 2C shows that BTZ induced more apoptosis in the knockdown cells than control cells, thus confirming the apoptosis sensitization by O-GlcNAcylation and OGA inhibition. Using PPISUV bioinformatics tool, we analyzed the association between MGEA5 (encoding OGA) expression and clinical outcomes of patients with NHL, diffuse large B-cell lymphoma (DLBCL) subtype (due to the availability of data) and found that high expression of MGEA5 correlates well with low survival rates of patients (Figure 2D). These clinical data support the involvement of O-GlcNAcylation in controlling of aggressive lymphoma.
Cleavage of Bid contributes to KCZ-mediated sensitization to BTZ
To characterize the apoptotic response to BTZ and KCZ, MCL Jeko-1, Granta-519 and SP49 cells were treated with BTZ (0–6 nM) alone or in combination with KCZ (75 μM) in the presence or absence of caspase inhibitor z-DEVD-fmk (10–15 μM). z-DEVD-fmk significantly but not completely suppressed BTZ-induced apoptosis, suggesting that BTZ induces both caspase-dependent and -independent apoptotic process. On the other hand, the inhibition of apoptosis by z-DEVD-fmk was more prominent in BTZ and KCZ co-treatment, indicating that apoptosis sensitization by KCZ is associated with caspase activation (Supplementary Figure S3A and B). Western blot analysis shows a dose-dependent increase in caspase-3 activation and PARP cleavage by BTZ, which was further increased by the addition of KCZ, in concomitant with the observed changes in caspase-3 activity (Supplementary Figure S3C). Caspase-9 serves as the apical caspase of the intrinsic apoptosis pathway, while caspase-8 represents the apical caspase of the extrinsic pathway (32,33). Caspase-8 inhibitor (z-LEHD-fmk) and to a lesser extent caspase-9 inhibitor (z-IETD-fmk) significantly inhibited caspase-3 activation induced by the co-treatment, suggesting that the sensitizing effect of KCZ involves both intrinsic and extrinsic pathways.
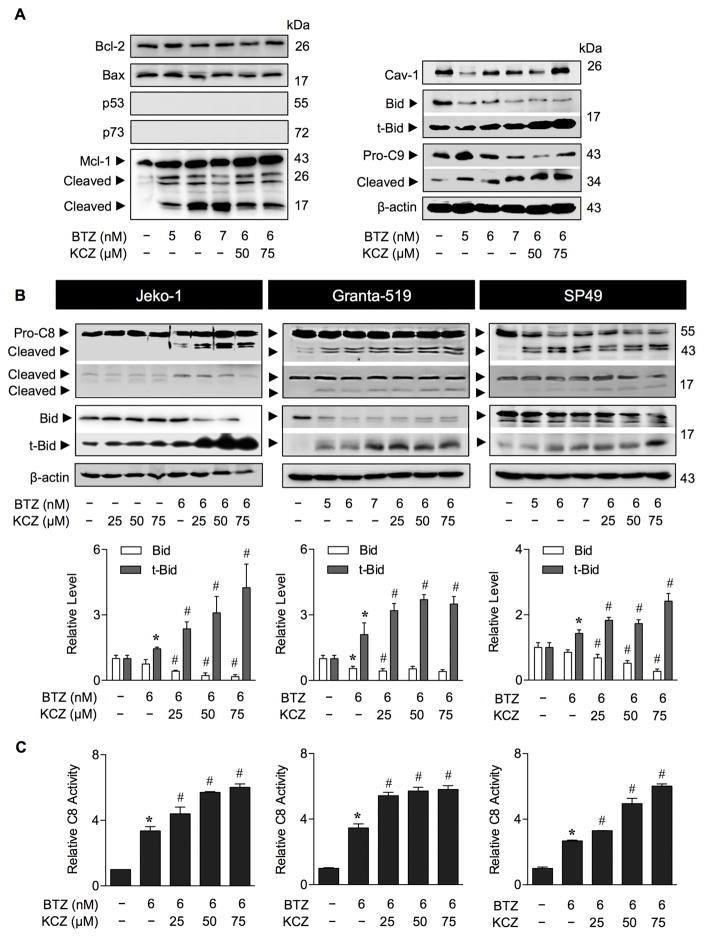
To elucidate the underlying mechanisms of KCZ-mediated sensitization, we monitored the expression levels of various apoptosis regulatory proteins, including Bcl-2, Bax, Bid, Mcl-1, p53, p73, and Cav-1 following the KCZ and BTZ treatment. The results show that Bcl-2/Bax ratio was relatively unchanged, while p53 and p73 were undetectable, and Cav-1 level was fluctuated after the treatment with BTZ alone or with KCZ (Figure 3A), suggesting their non-dominant role in the apoptotic process. While BTZ alone caused a marked increase in the apoptotic fragments of Mcl-1, addition of KCZ did not potentiate this effect, suggesting its unlikely involvement in the KCZ sensitization process. Remarkably, we observed a downregulation of Bid and a striking upregulation of transcated Bid (tBid; Bid cleavage) in the BTZ and KCZ co-treated cells along with an increase in caspase-9 activation. These results suggest the potential involvement of tBid in the sensitization process.
Figure 3.
Drug-like OGA inhibitor KCZ cleaves Bid to its truncated active form tBid. (A) Analysis of apoptosis signaling proteins in response to BTZ and KCZ co-treatment. MCL Jeko-1 cells were treated with various concentrations of BTZ (0–7 nM) and KCZ (25–75 μM) for 24 h and analyzed for key apoptosis regulatory proteins, including Bcl-2, Bax, p53, p73, Mcl-1, caveolin-1 (Cav-1), Bid and caspase-9 (C9) using Western blotting. Blots were reprobed with anti-β-actin antibody to confirm equal loading of the samples. (B) KCZ potentiates caspase-8 activation and Bid cleavage in BTZ-treated cells. Cells were treated with various concentrations of BTZ and KCZ for 24 h and the levels of pro- and activated caspase-8 (C8) as well as Bid and tBid were determined by Western blotting. Quantitative analysis of Bid and tBid by densitometry is shown. Data are mean ± s.d. (n=4). *P < 0.05 vs. non-treated cells; two-sided Student’s t-test. #P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test. (C) C8 activity was evaluated in the cells treated with BTZ (6 nM) and increasing concentrations of KCZ (0–75 μM) for 16 h using the fluorometric substrate IETD-AFC. Data are mean ± s.d. (n=3). Data are mean ± s.d. (n=3). *P < 0.05 vs. non-treated cells; two-sided Student’s t-test. #P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test.
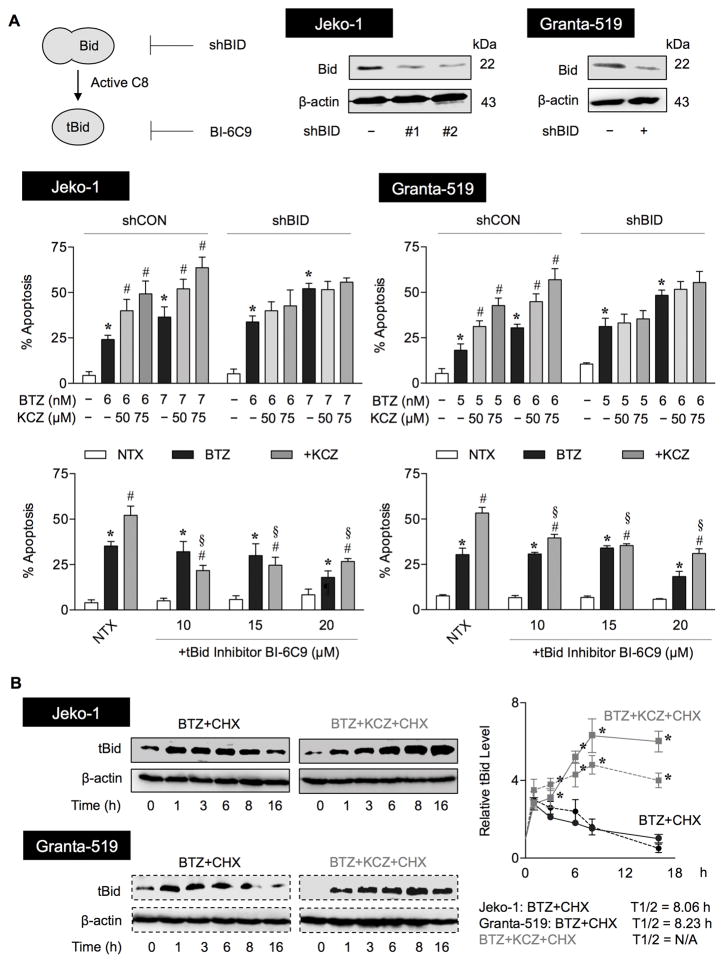
Because tBid links the extrinsic to intrinsic apoptosis pathway (34,35), we evaluated and found an increase in caspase-8 activation in parallel with tBid induction in all MCL cell lines tested following their treatment with BTZ and KCZ (Figure 3B). We confirmed by qPCR analysis that BCL2, BAX, TP53, MCL1, CAV1 and BID showed no appreciable changes in their expression in a way that would promote apoptosis (Supplementary Figure S4), except for TP53 in response to KCZ at 75 μM. We postulate that although p53 was not a likely target of O-GlcNAcylation in the present setting, due to its undetectable protein level, KCZ at higher doses might induce apoptosis in part through an upregulation of TP53. To validate the role of tBid in the KCZ sensitizing effect, tBid was inhibited directly and indirectly by small molecule inhibitor of tBid (BI-6C9) and RNA interference using shRNA against BID. Consistent with a recent report on the anti-apoptotic function of full-length Bid in mouse embryonic fibroblasts (36), we observed that knockdown of Bid slightly promoted BTZ-induced apoptosis, but abolished the sensitizing effect of KCZ (Figure 4A, middle), suggesting the role of Bid/tBid in the sensitizing process. Specific inhibition of tBid by BI-6C9 effectively reversed the KCZ sensitizing effect (Figure 4A, lower), indicating that tBid is a key mediator in this process. Notably, the inhibition of BI-6C9 on BTZ-induced apoptosis was observed only at a high dose (20 μM), suggesting that other regulators are likely to be involved in the apoptosis induced by BTZ alone.
Figure 4.
tBid is a key mediator of KCZ sensitization of BTZ-induced apoptosis. (A) (upper) A schematic diagram for the inhibition of truncated Bid (tBid) using a small molecule inhibitor of tBid (BI-6C9) and RNA interference of Bid (shBid) in MCL Jeko-1 and Granta-519 cells. (middle) Inhibition of Bid by shBid diminishes the sensitizing effect of KCZ on BTZ-induced apoptosis. shCON and shBID cells were similarly treated with BTZ (0–7 nM) in the presence or absence of KCZ (50–75 μM) for 24 h and apoptosis was determined by Hoechst 33342 assay. Data are mean ± s.d. (n=3) *P < 0.05 vs. non-treated cells; two-sided Student’s t-test. #P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test. (lower) Inhibition of tBid reverses the sensitizing effect of KCZ on BTZ-induced apoptosis. Cells were co-treated with BTZ (6 nM) and KCZ (75 μM) in the presence or absence of the tBid inhibitor BI-6C9 (10–20 μM) and analyzed for apoptosis by Hoechst 33342 assay. Data are mean ± s.d. (n=3). *P < 0.05 vs. non-treated cells; two-sided Student’s t-test. #P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test. §P < 0.05 vs. BTZ-treated cells in the presence of KCZ; two-sided Student’s t-test. NTX, non-treatment. (B) KCZ increases tBid stability in BTZ-treated cells. Jeko-1 and Granta-519 cells were treated with BTZ and KCZ in the presence or absence of the protein translation inhibitor cycloheximide (CHX; 10 μg/ml) for various times (0–16 h) to follow the degradation of tBid protein. Cell lysates were prepared and tBid expression was determined by Western blotting. Representative immunoblots of tBid and loading control β-actin are shown. Expression-time profiles of tBid in Jeko-1 (solid lines) and Granta-519 (dashed lines) cells treated with CHX and BTZ alone (circle) or with KCZ (square) were plotted and tBid half-lifves were calculated and shown. Data are mean ± s.d. (n=3). *P < 0.05 vs. BTZ and CHX co-treated cells; two-sided Student’s t-test.
tBid degradation is mediated by ubiquitin-mediated proteasomal degradation
The stability of tBid is an important factor in determining its apoptotic activity (37,38). We tested whether tBid stability is controlled by proteasomal degradation in MCL cells. MCL Jeko-1 cells were treated with the proteasome inhibitor MG132 and tBid expression was determined by Western blotting using specific tBid antibody (h71). The result shows that the MG132 treatment increases the level of tBid, suggesting its regulation by proteasomal degradation (Supplementary Figure 5A). Ubiquitination is a PTM process that triggers proteasomal degradation (39,40). With a low basal level of tBid, cells were first induced to overexpress tBid by gene transfection, and its ubiquitination was examined by Western blotting. Supplementary Figure 5C shows that treatment of the cells with the proteasome inhibitor MG132 strongly induced polyubiquitination of tBid, supporting ubiquitin-mediated proteasomal degradation of the protein.
Based on the observed sensitizing effect of KCZ and the role of tBid in the process, we hypothesized that KCZ might cause tBid accumulation by regulating of tBid stability. To test this possibility, we evaluated the expression-time profile of tBid in MCL cells treated with BTZ with or without KCZ. The results show that the combination treatment dramatically shortened tBid induction from 16 h to 6 h compared to BTZ treatment alone (Supplementary Figure S6). tBid protein half-life was further evaluated in both treatment groups by a cycloheximide-chase assay. In this assay, the tBid half-life following inhibition of new protein synthesis by cycloheximide was calculated, revealing a gradual decrease in tBid expression over time when the cells were treated with BTZ alone (Figure 4B). In contrast, the tBid expression continued to increase when the cells were treated with both BTZ and KCZ even in the absence of new protein synthesis, indicating the stabilization of tBid following the combination treatment.
O-GlcNAcylation of tBid reduces its ubiquitination
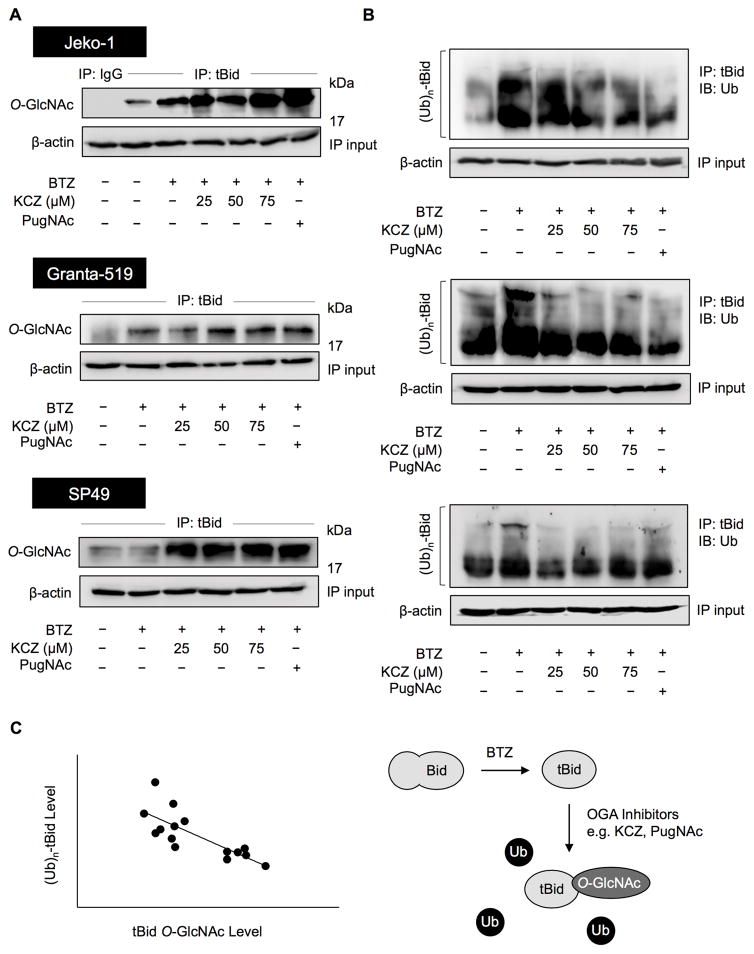
PTMs like phosphorylation are known to influence protein stability through ubiquitin-mediated proteasomal degradation (41,42). As O-GlcNAcylation occurs on serine and/or threonine residues of protein similarly to phosphorylation (5,6), it is conceivable that KCZ might induce O-GlcNAcylation of tBid which interferes with its ubiquitination. MCL Jeko-1, Granta-519 and SP49 cells were treated with BTZ and KCZ, and tBid was immunoprecipitated and subjected to Western blot analysis using an O-GlcNAc-specific antibody (RL2). The level of tBid O-GlcNAcylation was found to increase markedly in the presence of KCZ in all tested cells (Figure 5A). tBid ubiquitination was further analyzed after the BTZ and KCZ co-treatment. Figure 5B shows that KCZ indeed reduced tBid ubiquitination, possibly through its induction of tBid O-GlcNAcylation. A similar result was observed when the cells were treated with another OGA inhibitor, PugNAc. Further analysis of the correlation between tBid O-GlcNAcylation and ubiquitination in the tested cells reveals the inverse relationship of the two events with a correlation coefficient (r) of −0.7760 (Figure 5C), thus supporting the interfering effect of O-GlcNAcylation on tBid ubiquitination.
Figure 5.
Drug-like OGA inhibitor KCZ increases the O-GlcNAcylation but decreases the ubiquitination of tBid. (A) Analysis of O-GlcNAcylation of tBid in response to bortezomib (BTZ) and ketoconazole (KCZ) co-treatment. MCL cells were co-treated with BTZ (6 nM) and increasing concentrations of KCZ (25–75 μM) or PugNAc (50 μM) for 3 h. Cell lysates were prepared and immunoprecipitated using control IgG or anti-tBid (h71) antibody and probed with anti-O-GlcNAc antibody. Immunoblots were performed on cell lysates used as IP input using anti-β-actin antibody to confirm equal loading of the samples. (B) Analysis of tBid ubiquitination in response to BTZ and KCZ co-treatment. Cells were co-treated with BTZ (6 nM) and KCZ (25–75 μM) or PugNAc (50 μM) in the presence of MG132 (50 μM) for 3 h. Cell lysates were immunoprecipitated using anti-tBid (h71) antibody and analyzed for ubiquitin by Western blotting. (C) Correlation analysis of tBid O-GlcNAcylation and ubiquitination in BTZ-treated cells in the presence or absence of KCZ and PugNAc (r = −0.7760) (left). A schematic diagram showing the interfering effect of O-GlcNAc on tBid ubiquitination (right).
KCZ enhances BTZ sensitivity in patient-derived primary cells and de novo BTZ-resistant MCL cells
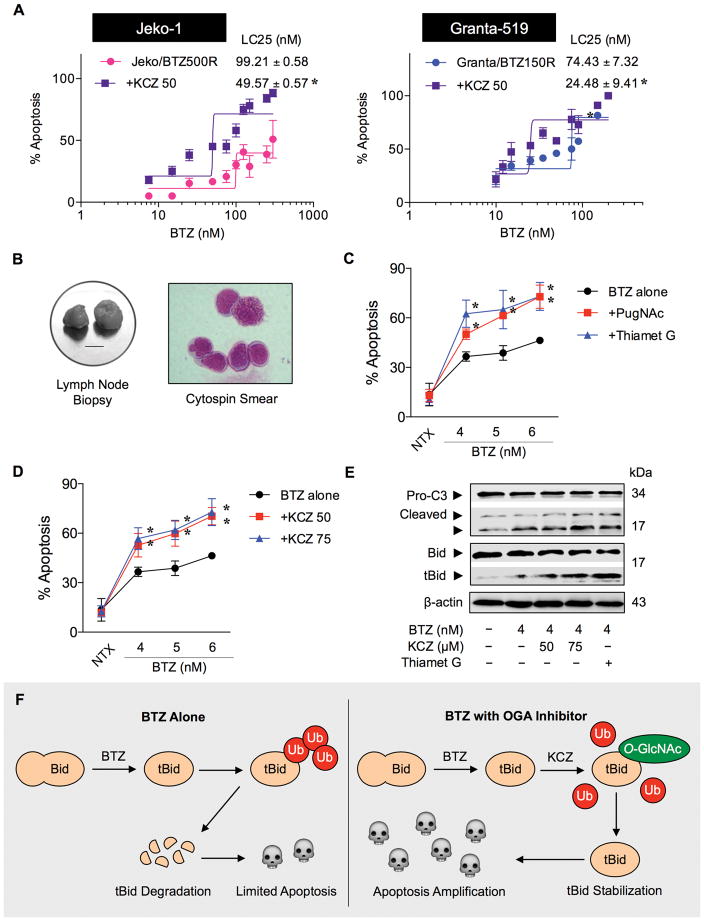
To investigate the potential clinical application of BTZ and KCZ combination therapy for MCL, we further tested the sensitizing effect of KCZ in acquired BTZ-resistant MCL cells and in patient-derived primary cells. Drug resistance remains a major clinical challenge for MCL treatment, we thus established de novo BTZ-resistant cells by continuous exposure of MCL Jeko-1 and Granta-519 cells to increasing concentrations of BTZ over time. Dose-response curve of the parental and BTZ-resistant cells was generated and LC25 was calculated from the plot. BTZ-resistant Jeko/BTZ500R and Granta/BTZ150R cells exhibited an LC25 approximately 20-fold and 10-fold higher than that of their parental cells, respectively (Supplementary Figure S7). Treatment of BTZ-resistant Jeko/BTZ500R and Granta/BTZ150R cells with KCZ reduced their LC25 by approximately 50–65% (Figure 6A).
Figure 6.
Drug-like OGA inhibitor KCZ enhances BTZ sensitivity in de novo BTZ-resistant cells and patient-derived primary cells. (A) KCZ reduces the LC25 of BTZ-resistant MCL cells. BTZ-resistant Jeko/500R and Granta/150R cells were generated by stepwise selection method (Supplementary Figure S6) and treated with various concentrations of BTZ in the presence or absence of KCZ (50 μM). Dose-response curves were generated and LC25 of BTZ in cells treated with BTZ alone or in combination with KCZ were determined and compared. Data are mean ± s.d. (n=3). *P < 0.05 vs. LC25 of BTZ-treated Jeko/BTZ500R or Granta/BTZ150R in the absence of KCZ; two-sided Student’s t-test. (B) Primary cells were obtained from fresh biopsy-derived lymphoma tissues (lymph nodes; left; scale bar = 1 cm) of MCL patient and were captured for Wright-stained cytospin (right). Clinical characterization of the cells is shown in Supplementary Table S2. (C, D) MCL patient-derived primary cells were treated with increasing concentrations of BTZ (0–6 nM) in the presence or absence of OGA inhibitors, PugNAc (50 μM) and thiamet G (10 μM) (C) or KCZ (50–75 μM) (D) for 24 h and apoptosis was determined by Hoechst 33342 assay. Data are mean ± s.d. (n=3). *P < 0.05 vs. BTZ-treated cells; two-sided Student’s t-test. (E) Western blot analysis of activated caspase-3 (cleaved C3) and truncated Bid (tBid) in patient-derived primary cells in response to BTZ (4 nM) and KCZ (50–75 μM) co-treatment. (F) A schematic diagram for the regulation of tBid by BTZ and KCZ. In the presence of KCZ and other OGA inhibitors, e.g. PugNAc and thiamet G, tBid O-GlcNAcylation occurs to inhibit tBid ubiquitination and proteasomal degradation. tBid stabilization intensifies the apoptotic signal and sensitizes MCL to BTZ-induced apoptosis.
MCL patient-derived primary cells were obtained from fresh patient-derived lymphoma tissues (Figure 6B; see Supplementary Table S2 for their clinical characterization) and treated with BTZ in the presence or absence of two well known small molecule inhibitors, PugNAc and thiamet-G, or drug-like OGA inhibitor KCZ for 24 h. Figure 6C and D shows that the addition of either small molecule inhibitor or drug-like inhibitor KCZ similarly sensitized the cells to BTZ-induced apoptosis. Western blot analysis reveals a marked increase in caspase-3 activation in response to the co-treatment of BTZ and KCZ or thiamet G (Figure 6E). A parallel increase in tBid expression and a reduction in Bid were also observed in the treated cells, thus validating the role of tBid in the apoptosis sensitization in MCL cell lines. Importantly, the combination treatment of BTZ and KCZ had no significant cytotoxic effect on normal PBMC and hepatocytes at a similar dosing condition (Supplementary Figure S8). Taken together, our results support the potential clinical application of BTZ and KCZ combination therapy for MCL.
Discussion
Aberrant hexosamine metabolism and O-GlcNAcylation have been linked to various aggressive behaviors of solid tumors (6,7), as yet relatively little is known about their roles in hematologic malignancies, particularly MCL. Here, we provide compelling evidence that O-GlcNAcylation of tBid promotes apoptosis of MCL cells in response to its frontline therapeutic agent BTZ. This finding is in line with a previous report showing reduced doubling time and enhanced cytogenetic abnormalities in CLL cells with a low level of O-GlcNAcylation (RL2 index < 1) relative to those with a higher index (RL2 > 1) (11) and with the survival analyses of DLBCL patients showing better clinical outcomes with low MGEA5 (encoding OGA) expression (Figure 2D). We further demonstrate that inhibition of OGA and subsequent O-GlcNAcylation by either small molecule inhibitors or drug-like inhibitor KCZ potentiates BTZ sensitivity in MCL. We highlight for the first time the potential clinical application and molecular mechanism of BTZ and KCZ combination therapy for MCL.
KCZ is a conventional antifungal drug that was recently found to be one of the most potent inhibitors of human and C. perfringen OGAs based on high-throughput screening assays of OGA proteins against a commercial library of 880 off-patent small molecules (20). In the present study, we found that KCZ, like well known OGA inhibitors including PugNAc and thiamet G, was able to sensitize MCL cells to BTZ-induced apoptosis, albeit at approximately 5 times higher molar concentration (Figures 1–2). To our knowledge, this is the first demonstration of the sensitizing effect of OGA inhibitors, specifically KCZ, on MCL apoptosis induced by chemotherapeutic agent. KCZ, which sensitizes MCL cells for BTZ-induced apoptosis, markedly induces Bid cleavage (tBid) with no or minimal effects on other major apoptosis-regulatory proteins (Figure 3). We herein validate the role of tBid in KCZ sensitizing effect and demonstrate that KCZ stabilizes tBid, which is generated on stimulation of BTZ itself and greatly potentiated by KCZ co-treatment (Figure 4). This finding suggests that BTZ and KCZ cooperatively cause tBid accumulation, linking the extrinsic and intrinsic apoptosis pathway and intensifying the death signal.
O-GlcNAcylation is a PTM process that regulates the expression and function of several proteins and has a ubiquitous role in many types of cancer (6,7). In pancreatic carcinoma MiaPaCa-2 and PANC-1 cells, a decrease in O-GlcNAcylation triggers the intrinsic apoptosis pathway by reducing Bcl-xL, while its upregulation in BxPC-3 cells promotes anoikis resistance in part through NF-κB signaling (43). On the other hand, an accumulation of O-GlcNAcylation reduces the viability of breast cancer MCF-7 cells likely through the accumulation of p53 (44). Thus, the role of O-GlcNAcylation in cell death and survival remains unclear and appears to be cell type- and context-dependent. In the present study, we found that O-GlcNAcylation of tBid renders MCL cells more susceptible to BTZ-induced apoptosis. Previous studies have shown that tBid undergoes ubiquitination and subsequently degrades by 26s proteasome (36,45). The crosstalk between O-GlcNAcylation and ubiquitination are also reported, for example, the counteraction of O-GlcNAcylation and ubiquitination was earlier found to regulate circadian clock proteins, BMAL1 and CLOCK (46), as yet their precise interplay is unclear as enhanced ubiquitination was observed upon increasing of O-GlcNAcylation in the other report (47). Our data indicate that O-GlcNAcylation of tBid interferes with its ubiquitination, as supported by their inverse correlation in all cell systems tested and under various treatment conditions (Figure 5). Degradation of tBid is known to limit the extent of apoptosis (35,37), thereby tBid accumulation and stabilization would aid in the amplification and maintenance of apoptosis signal. It is worth noting that the reported sensitizing effect of KCZ on BTZ-induced apoptosis was also seen in patient-derived primary cells and in BTZ-resistant MCL cells (Figure 6).
Taken together, the evidence presented here demonstrates the role of tBid O-GlcNAcylation in the regulation of apoptosis in MCL cells. Such PTM improves the stability of tBid by blocking its ubiquitination and subsequent proteasomal degradation, leading to sustained apoptosis, as schematically summarized in Figure 6F. Our findings not only point to the involvement of hexosamine metabolic pathway and O-GlcNAcylation in MCL response to chemotherapy, but also advise the potential clinical application of drug-like OGA inhibitor KCZ in combination therapy to battle against drug-resistant MCL and other malignancies whose development and apoptosis is dependent on tBid dysregulation.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grants from Thailand Research Fund (RTA 488-0007 to S. Issaragrisil and TRG5980013 to S. Luanpitpong), the Commission on Higher Education (CHE-RES-RG-49 to S. Issaragrisil) and National Institutes of Health (NIH; R01-ES022968 to Y. Rojanasakul).
This work was supported by grants from Thailand Research Fund (RTA 488-0007 to S. Issaragrisil and TRG5980013 to S. Luanpitpong), the Commission on Higher Education (CHE-RES-RG-49 to S. Issaragrisil) and National Institutes of Health (NIH; R01-ES022968 to Y. Rojanasakul). S. Issaragrisil is a Senior Research Scholar of Thailand Research Fund. We would like to acknowledge Dr. S. Jirawatnotai from Systems Pharmacology, Department of Pharmacology, Faculty of Medicine Siriraj Hospital, Mahidol University for his technical assistance and Profs. D. Solter and B. Knowles for their comments on the manuscript.
Footnotes
Supplementary information: Supplementary information accompanies this manuscript includes two Supplementary Tables and eight Supplementary Figures.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:645–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–8. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah BD, Martin P, Sotomayor EM. Mantle cell lymphoma: a clinically heterogeneous disease in need of tailored approaches. Cancer Control. 2012;19:227–235. doi: 10.1177/107327481201900307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208:869–80. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jóźwiak P, Forma E, Bryś M, Krześlak A. O-GlcNAcylation and metabolic reprogramming in cancer. Front Endocrinol. 2014;5:145. doi: 10.3389/fendo.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Queiroz RM, Carvalho E, Dias WB. O-GlcNAcylation: the sweet side of the cancer. Front Oncol. 2014;4:132. doi: 10.3389/fonc.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, et al. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013;13:2088–99. doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514–9. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, et al. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–98. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouroukis CT, Fernandez LA, Crump M, Gascoyne RD, Chua NS, Buckstein R, et al. A phase II study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the National Cancer Institute of Canada Clinical Trials Group (IND 172) Leuk Lymphoma. 2011;52:394–9. doi: 10.3109/10428194.2010.546015. [DOI] [PubMed] [Google Scholar]
- 13.Hegde GV, Nordgren TM, Munger CM, Mittal AK, Bierman PJ, Weisenburger DD, et al. Novel therapy for therapy-resistant mantle cell lymphoma: multipronged approach with targeting of hedgehog signaling. Int J Cancer. 2012;131:2951–60. doi: 10.1002/ijc.27602. [DOI] [PubMed] [Google Scholar]
- 14.Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem. 2014;289:34457–65. doi: 10.1074/jbc.R114.577718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–65. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 16.Hurtado-Guerrero R, Dorfmueller HC, van Aalten DM. Molecular mechanisms of O-GlcNAcylation. Curr Opin Struct Biol. 2008;18:551–7. doi: 10.1016/j.sbi.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–90. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 18.Konrad RJ, Zhang F, Hale JE, Knierman MD, Becker GW, Kudlow JE. Alloxan is an inhibitor of the enzyme O-linked N-acetylglucosamine transferase. Biochem Biophys Res Commun. 2002;293:207–12. doi: 10.1016/S0006-291X(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 19.Dehennaut V, Lefebvre T, Sellier C, Leroy Y, Gross B, Walker S, et al. O-linked N-acetylglucosaminyltransferase inhibition prevents G2/M transition in Xenopus laevis oocytes. J Biol Chem. 2007;282:12527–36. doi: 10.1074/jbc.M700444200. [DOI] [PubMed] [Google Scholar]
- 20.Dorfmueller HC, van Aalten DMF. Screening-based discovery of drug-like O-GlcNAcase inhibitor scaffolds. FEBS Lett. 2010;584:694–700. doi: 10.1016/j.febslet.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC, et al. Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol. 2004;11:1107–17. doi: 10.1016/j.chembiol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Amin HM, McDonnell TJ, Medeiros LJ, Rassidakis GZ, Leventaki V, O’Connor SL, et al. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127:424–31. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- 23.Kridel R, Meissner B, Rogic S, Boyle M, Telenius A, Woolcock B, et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood. 2012;119:1963–71. doi: 10.1182/blood-2011-11-391474. [DOI] [PubMed] [Google Scholar]
- 24.Drexler HG. Establishment and culture of leukemia-lymphoma cell lines. Methods Mol Biol. 2011;731:181–200. doi: 10.1007/978-1-61779-080-5_16. [DOI] [PubMed] [Google Scholar]
- 25.Zhang N, Fu JN, Chou TC. Synergistic combination of microtubule targeting anticancer fludelone with cytoprotective panaxytriol derived from panax ginseng against MX-1 cells in vitro: experimental design and data analysis using the combination index method. Am J Cancer Res. 2016;6:97–104. [PMC free article] [PubMed] [Google Scholar]
- 26.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–83. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8:2180–96. doi: 10.1038/nprot.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonov AV, Krestyaninova M, Knight RA, Rodchenkov I, Melino G, Barlev NA. PPISURV: a novel bioinformatics tool for uncovering the hidden role of specific genes in cancer survival outcome. Oncogene. 2014;33:1621–8. doi: 10.1038/onc.2013.119. [DOI] [PubMed] [Google Scholar]
- 29.Luanpitpong S, Li J, Manke A, Brundage K, Ellis E, McLaughlin SL, et al. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene. 2016;35:2824–33. doi: 10.1038/onc.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Galán P, Mora-Jensen H, Weniger MA, Shaffer AL, 3rd, Rizzatti EG, Chapman CM, et al. Bortezomib resistance in mantle cell lymphoma is associated with plasmacytic differentiation. Blood. 2011;117:542–52. doi: 10.1182/blood-2010-02-269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 33.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unterkircher T, Cristofanon S, Vellanki SH, Nonnenmacher L, Karpel-Massler G, Wirtz CR, et al. Bortezomib primes glioblastoma, including glioblastoma stem cells, for TRAIL by increasing tBid stability and mitochondrial apoptosis. Clin Cancer Res. 2011;17:4019–30. doi: 10.1158/1078-0432.CCR-11-0075. [DOI] [PubMed] [Google Scholar]
- 35.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]
- 36.Luo W, Li J, Zhang D, Cai T, Song L, Min X-M, et al. Bid mediates anti-apoptotic COX-2 induction through the IKKβ/NFκB pathway due to 5-MCDE exposure. Curr Cancer Drug Targets. 2010;10:96–106. doi: 10.2174/156800910790980160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000;275:21648–52. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- 38.Ying S, Seiffert BM, Häcker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–89. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 40.Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature. 2002;416:763–7. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- 41.Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S, et al. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat Methods. 2013;10:676–82. doi: 10.1038/nmeth.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28:730–8. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–30. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 45.Azakir BA, Desrochers G, Angers A. The ubiquitin ligase Itch mediates the antiapoptotic activity of epidermal growth factor by promoting the ubiquitylation and degradation of the truncated C-terminal portion of Bid. FEBS J. 2010;277:1319–30. doi: 10.1111/j.1742-4658.2010.07562.x. [DOI] [PubMed] [Google Scholar]
- 46.Li MD, Ruan HB, Hughes ME, Lee JS, Singh JP, Jones SP, et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17:303–10. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, et al. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22:2901–11. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.