Abstract
BACKGROUND
Data are lacking regarding physical functioning, psychological well-being, and quality of life among colorectal cancer survivors more than 10 years post-diagnosis.
OBJECTIVE
To examine self-reported physical functioning, quality of life, and psychological well-being in long-term colorectal cancer survivors compared to age- and gender-matched unaffected controls.
DESIGN
Cross-sectional.
SETTINGS AND PARTICIPANTS
A population-based sample of colorectal cancer survivors (N=296) and their age- and gender-matched unaffected controls (N=255) recruited from a cancer registry. Survivors were on average 15 years post-diagnosis.
STUDY SELECTION
Participants were recruited from the Ontario Familial Colorectal Cancer Registry and completed a cross-sectional survey.
MAIN OUTCOME MEASURES
Quality of life: Functional Assessment of Cancer Therapy-General Scale. Bowel dysfunction: Memorial Sloan-Kettering Cancer Center Scale. Urinary dysfunction: International Consultation on Incontinence Questionnaire Short Form. Fatigue: Functional Assessment of Chronic Illness Therapy-Fatigue scale. Depression: Center for Epidemiologic Studies-Depression Scale.
RESULTS
In linear mixed model analyses adjusting for income, education, race, and comorbid medical conditions, survivors reported good emotional, functional, physical and overall quality of life, comparable to controls. Fatigue and urinary functioning did not differ significantly between survivors and controls. Survivors reported significantly higher social quality of life and lower depression compared to unaffected controls. The only area survivors reported significantly worse deficits was in bowel dysfunction, but the magnitude of differences was relatively small.
LIMITATIONS
Generalizability is limited by moderately low participation rates. Findings are likely biased toward healthy participants. No baseline assessment was available to examine change in outcomes over time.
CONCLUSIONS
Long-term colorectal cancer survivors appear to have comparable quality of life and in some areas, better well-being than their unaffected peers. Bowel dysfunction may continue to be an ongoing issue even 15 years after colorectal cancer diagnosis. Overall quality of life can be expected to be good in this group of older survivors (see video abstract, supplemental digital content 1).
Keywords: colorectal cancer, quality of life, controls, survivors, long-term
Colorectal cancer (CRC) is one of the most common cancers for men and women in both the U.S. and Canada.1 Increased screening, earlier diagnosis, and improved treatments have resulted in declines in mortality by 4.5% annually2 and CRC survivors are living longer than before. Five-year and ten-year relative survival rates are currently 65% and 58%, respectively.2 Yet, data are lacking regarding long-term physical functioning or quality of life (QOL).
Although several studies have examined CRC survivors within the first five years post-diagnosis,3–7 few investigations detail QOL past this time point. Data from the National Surgical Adjuvant Breast and Bowel Project showed that survivors (median 8 years post-surgery) reported better physical and mental QOL, but more fatigue than published norms.8 Studies among survivors more than 5 years post-diagnosis show few significant QOL differences between survivors and normative data.9 However, a lack of comparison groups limits these findings. One study examined 15 year survivors (N=99) and, with the exception of worse diarrhea, found few differences compared to controls.10
The current investigation examined differences in self-reported physical functioning, QOL, and psychological well-being among a population-based sample of long-term CRC survivors and unaffected controls. Directly comparing survivors with an age- and gender-matched control group allowed us to examine the extent to which problems were due to cancer as opposed to comorbidity and/or older age. At 15 years post-diagnosis, we expected CRC survivors (compared to age-and gender-matched unaffected controls) to report worse fatigue and bowel dysfunction, but similar physical and mental QOL after adjusting for race, income, education, and other non-cancer related comorbid health conditions. We also examined QOL within subgroups based on tumor site, disease progression, and type of treatment.
PATIENTS AND METHODS
Participants and Procedures
Participants were recruited from the Ontario Familial Colorectal Cancer Registry (OFCCR), one of six international sites participating in the Consortium of Colon Cancer Familial Registries funded by the U.S. National Cancer Institute in 1997.11 Procedures that established the survivor cohort have been previously published.12 In brief, the population-based Ontario Cancer Registry was used to identify pathology-confirmed, incident CRC patients who were diagnosed between July 1, 1997 and June 30, 2000 and resided in the Ontario province. After the patient was identified from the pathology report, physicians were asked to permit contact, and then patients were mailed study information and consent forms to participate in the OFCCR. Unaffected population controls in the OFCCR were obtained through two methods12: 1) random digit-dialing on Ontario telephone numbers from Bell Canada lists (1999-2000) and 2) random selection through the Ministry of Finance Property Assessment database: a population-based listing of all Ontario residents (2001-2002). Controls were frequency-matched within sex and five to ten-year age groups to the CRC survivors.
For the current investigation, both survivors and unaffected controls met the following criteria: 1) Provided consent to participate in the OFCCR during 1997-2000, 2) 2014 OFCCR records showed individual was alive with a current address; and 3) consented to participate in the current research project focused on QOL. Exclusion criteria: 1) CRC survivor records did not have abstracted clinical data or 2) unaffected control reported any type of cancer diagnosis since the initial recruitment.
Of the 1200 CRC survivors originally recruited by the OFCCR, 462 were reported through the registry as alive with current contact information and abstracted clinical data at the time of this study. Each survivor who returned a completed QOL questionnaire was matched to an unaffected control on sex and within five years of age. For survivors with multiple possible matches, one match was chosen at random. If the control did not return a completed questionnaire within 6 weeks of the initial mailing and had not responded to reminder phone calls, a new control match was assigned. Of the unaffected controls, 912 (of the 1953 originally recruited) were reported through the registry to be alive and had current contact information, and 571 were able to be matched to survivors based on sex and age. Figure 1 displays the flowchart showing survey process and responses.
Figure 1. Flowchart of Survey Processes and Responses among CRC Survivors and Unaffected Controls.
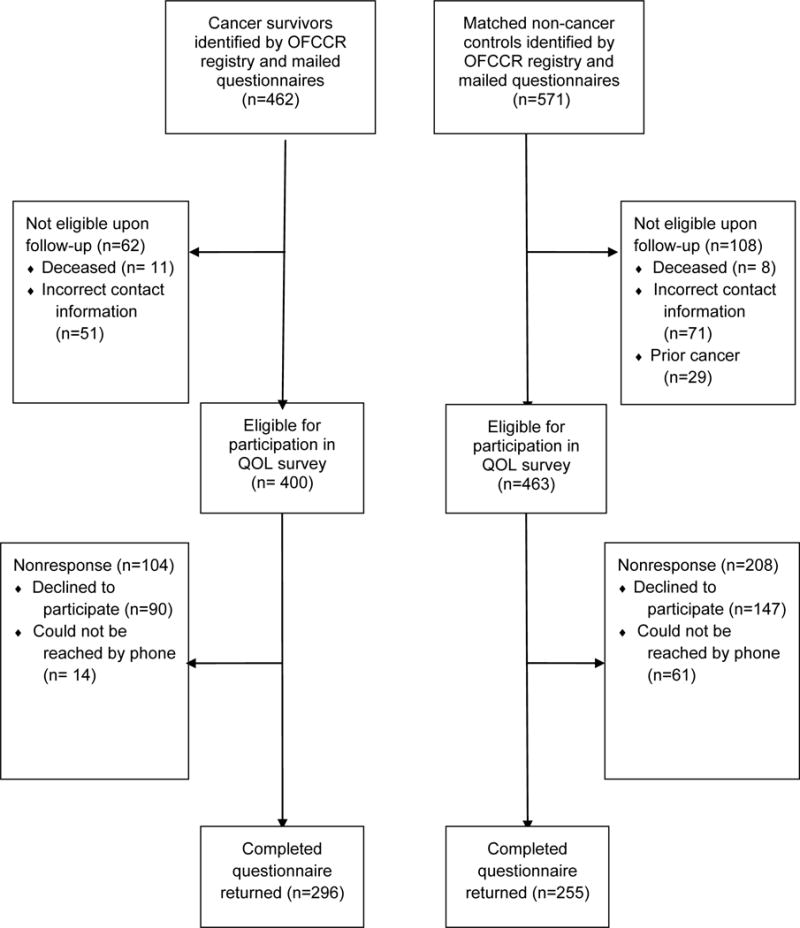
Legend: OFCCR=Ontario Familial Colorectal Cancer Registry. QOL=Quality of Life.
Both CRC survivors and unaffected controls were mailed an invitation letter and consent form, along with a packet of questionnaires and a pre-addressed and stamped return envelopes. Upon completion, participants were mailed a $30 gift card. Within 4 weeks of the initial questionnaire mailing, reminder calls were made to all non-respondents. All study procedures were approved by Mount Sinai Hospital’s research ethics board.
Response Rates
As shown in Figure 1, 462 CRC survivors were eligible through the registry to receive QOL surveys. Of these, 62 questionnaires were returned because the participant was deceased or had incorrect contact information. Of the remaining 400 participants, 296 returned completed questionnaires (response rate=74%). For the unaffected controls, 571 individuals were identified as potential matches who were eligible to receive surveys. After the initial mailing, 108 were found to be deceased, reported prior or current cancer, or had incorrect contact information. Of the 463 eligible, 255 returned completed questionnaires (response rate=55.1%), providing age-and gender-matches for 86.1% of the survivors.
Measures
Bowel Dysfunction
Bowel Dysfunction was assessed with the Memorial Sloan-Kettering Cancer Center Scale (18-items), a gold standard of measuring bowel frequency, urgency, and dietary restrictions after CRC surgery,13–15 with items rated from “1=always” to “5=never.” The scale possesses good test-retest reliabilities for the scales (r = .62–.87), good internal consistency (>.70), and good discriminant and construct validity.14,15
Urinary Dysfunction
Urinary Dysfunction was assessed with the 4-item International Consultation on Incontinence Questionnaire Short Form (ICQQ) to measure urinary incontinence (e.g., frequency, severity, and interference with daily life).16 The ICIQ has been used with bladder cancer17 and prostate cancer18 research samples as well as healthy older adults, and shows excellent construct, convergent, and divergent validity, sensitivity, and internal consistency (>.95).
Fatigue
Fatigue was measured with the Functional Assessment of Chronic Illness Therapy-Fatigue scale (13 items), commonly-used in research with cancer patients, people with medical problems, and appropriate for use among healthy older adults.19, 20 The intensity of fatigue and related symptoms are rated on a scale from 0=not at all to 4=very much. The scale has good psychometric properties with internal consistency >.90 (19), good sensitivity (>.90) and specificity (>.69).19
Depression
Depression was measured with the Center for Epidemiologic Studies-Depression Scale,21 a 20-item well-known, validated, and measure of clinical depression with both cancer samples22 and the older adults in the general population.23 Each item is measured from “0=not at all” to “3=very often,” where higher scores indicate more severe depressive symptoms. A cutoff score of 16 or greater can also be used as an indication of a possible depressive disorder.
Quality of Life
Quality of Life was measured with the Functional Assessment of Cancer Therapy-General (28 items),24 which has four scales: physical, social, emotional, and functional well-being and a combined total score (known as the FACT-G). Scores range from 0-108, with higher scores representing better QOL. Internal consistency and test-retest reliability is high (>.89) and the scale shows good predictive, convergent, and divergent validity. Although survivors were well-past the treatment phase, the FACT-G captures other relevant issues related to the cancer experience. These include cancer worry, coping, and lack of energy.25–26 Controls completed the FACT-General Population (FACT-GP), a 21-item version of the FACT-G designed for use with non-cancer populations.27 Scores from the 21 identical items are prorated to be comparable with the 28-item FACT-G.27
Clinical and Demographic Data
The OFCCR registry abstracted data on date of CRC diagnosis and clinical treatment including tumor site, surgery, chemotherapy, radiotherapy, cancer stage, and disease progression (i.e., new primaries, recurrences). Age, sex, education, income, race, and marital status were also obtained from the OFCCR database for both CRC survivors and unaffected controls.
Comorbidities
All participants completed the Comorbidity Questionnaire,28 a 12-item list of common medical conditions with good test-retest reliability (>.90), that correlates well with the medical record-based Charlson index (r=0.63). Up to three points can be scored for each of 12 medical conditions (i.e., presence, receipt of treatment for the condition, condition causes functional limitation), for a possible total of 36 points.
Statistical Analysis
Paired t-tests, McNemar’s tests, and tests of marginal homogeneity compared demographic and medical characteristics between matched pairs of CRC survivors and controls using SPSS Version 22.29 Linear mixed-effect models (using matched set as a random effect and CRC history as a fixed effect) compared the means between CRC survivors and controls on physical functioning, QOL, and well-being after adjusting for comorbidity, race, education, and income using all participants available. All variables included in the linear mixed-effect models were continuous. A conditional multivariate logistic regression model examined differences between matched sets in cutoff scores for depression on the CES-D, adjusting for comorbidity, race, income, and education. We further divided our survivors into subgroups based on treatment status (surgery; chemotherapy; chemotherapy plus radiation); disease progression (diagnosis of a new primary), and tumor site (colon; rectal) and compared them with their corresponding matched controls for all outcomes.
RESULTS
Characteristics of the Sample
Sample characteristics are presented in Table 1. The only statistically significant difference between survivors and controls was on race, with 96.6% of survivors versus 92.5% of unaffected controls identifying as Caucasian (McNemar’s test; p=0.019). The average age of CRC survivors (mean = 73.2) and unaffected controls (mean = 72.8) was similar, and both groups were equally comprised of women and men. For both groups, more than 80% were married, over 50% had at least some college education, and just under 30% had a yearly household income of less than $40,000. Comorbidity Index mean was 4.53 and 4.14 for survivors and controls, respectively. Among the CRC survivors, 77% were diagnosed with colon cancer. Approximately 72% of patients had either Stage 1 or Stage 2 cancer. All patients had undergone surgical resection, 53.4% had received chemotherapy and 19.9% had received radiotherapy, 21.3% had received a diagnosis of a new primary cancer (CRC or another site) since initial diagnosis, and 12.5% had a permanent stoma. Less than 5% had a recurrence of CRC. Survivors were 15.5 years post-diagnosis (range 13.7-17.3 years).
Table 1.
Demographic and Medical Characteristics of Cancer Survivors and Matched Controls
| Characteristic | Cancer Survivors (N=296) |
Matched Controls (N=255) |
|---|---|---|
|
| ||
| Age, years at time of survey | M=73.2, SD=8.6 | M=72.8, SD=8.2 |
|
| ||
| Sex | ||
| Female | 149 (49.5%) | 129 (50.6%) |
| Male | 146 (50.5%) | 126 (49.4%) |
|
| ||
| Race | ||
| White | 286 (96.6%) | 236 (92.5%) |
| Nonwhite | 10 (3.4%) | 19 (7.5%) |
|
| ||
| Marital Status | ||
| Married | 253 (85.5%) | 208 (82.2) |
| Single, widowed, divorced | 43 (14.5) | 45 (17.8) |
|
| ||
| Education | ||
| Less than 8th grade | 4 (1.4%) | 9 (3.5%) |
| 8th-11th grade | 55 (18.8%) | 37(14.5%) |
| High school graduate | 53 (18.1%) | 34 (13.3%) |
| Vocational school | 30 (10.2%) | 26 (10.1%) |
| Some college | 63 (21.5%) | 69 (27.1%) |
| Bachelor’s degree | 48 (16.4%) | 38 (14.9%) |
| Graduate school | 40 (13.7%) | 40 (15.7%) |
|
| ||
| Income | ||
| <6,000K | 2 (0.9%) | 0 (0%) |
| 6,000–19,999 | 12 (4.0%) | 18 (7.1%) |
| 20,000–29,000 | 26 (8.8%) | 21 (8.2%) |
| 30,000–39,999 | 40 (13.5%) | 34 (13.3%) |
| 40,000–49,999 | 32 (10.8%) | 28 (11%) |
| 50,000–59,999 | 32 (10.8%) | 31 (12.2%) |
| 60,000–69,999 | 28 (9.5%) | 27 (10.6%) |
| 70,000–79,999 | 24 (8.1) | 17 (6.7%) |
| 80,000–89,999 | 17 (5.7%) | 14 (5.5%) |
| unknown | 83 (28%) | 65 (25.5%) |
|
| ||
| Comorbidity Index | M=4.53, SD=3.26 | M=4.14, SD=3.26 |
|
| ||
| Cancer Stage | ||
| 1 | 82 (27.7%) | |
| 2 | 132 (44.6%) | |
| 3 | 74 (25%) | |
| 4 | 7 (2.4%) | |
| unknown | 1 (0.3%) | |
|
| ||
| Cancer Location | ||
| Colon | 228 (77%) | |
| Rectum | 68 (23%) | |
|
| ||
| Cancer Treatment | ||
| Surgery | 296 (100%) | |
| Chemotherapy | 158 (53.4%) | |
| Radiation | 59 (19.9%) | |
|
| ||
| Stoma Status | ||
| Permanent Stoma | 37 (12.5%) | |
|
| ||
| Time Since Cancer Diagnosis | M=15.5, SD=0.9 | |
|
| ||
| New Primary Cancer | ||
| Diagnosis | 63 (21.3%) | |
|
| ||
| Cancer Recurrence | 13 (4.4%) | |
Physical functioning, QOL, and psychological well-being
Means and standard errors calculated from the linear mixed models for survivors and controls are presented in Table 2. Analyses revealed that survivors reported significantly greater dietary restrictions and more bowel urgency. Table 2 shows that higher scores indicate the likelihood of having fewer problems, and that the magnitude of difference was small. Survivors were similar to their matched controls in reported bowel frequency, urinary dysfunction, and fatigue severity. Survivors were more likely to report significantly higher total QOL and higher QOL-social well being than controls. They also reported fewer depression symptoms relative to their matched controls. Descriptive analyses revealed no differences between the percentage of controls (19%) and survivors (19.3%) who met or exceeded the cut-off depression score of 16, (McNemar’s test, p = 0.82), a finding that remained when using a conditional multivariate logistic regression to adjust for covariates, OR = 1.08, 95%CI = 0.57 - 1.52, p = 0.77. No significant differences emerged between survivors and controls on QOL emotional well-being, functional well-being or physical well-being.
Table 2.
Adjusted means (M) and standard errors (SE) for self-reported quality of life, physical symptoms, and depression for cancer survivors and age- and gender-matched controls from linear mixed models.
| Outcome | Overall | Treatment | Disease Progression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
N=551 |
Surgery only N=274 |
Chemotherapy/Chemotherapy +RT N=318 |
New Primary Cancer N=126 |
|||||||||
| Survivors N=296 |
Controls N=255 |
p | Survivors N=137 |
Controls N=137 |
p | Survivors N=159 |
Controls N=159 |
p | Survivors N=63 |
Controls N=63 |
p | |
| MSKCC Bowel Dysfunction Frequency* | 23.05 (0.28) |
23.18 (0.26) |
.742 | 23.43 (0.48) |
23.05 (0.46) |
.574 | 22.72 (0.29) |
23.26 (0.27) |
.175 | 23.41 (0.47) |
22.50 (0.44) |
.168 |
| MSKCC Bowel Dysfunction Dietary* | 14.61 (0.22) |
15.84 (0.21) |
.001 | 14.35 (0.33) |
15.67 (0.32) |
.004 | 14.86 (0.29) |
16.01 (0.28) |
.005 | 14.81 (0.53) |
16.44 (0.49) |
.032 |
| MSKCC Bowel Dysfunction Urgency* | 17.07 (0.19) |
18.23 (0.18) |
.001 | 16.9 (0.29) |
17.96 (0.28) |
.011 | 17.20 (0.24) |
18.51 (0.22) |
.001 | 16.98 (0.52) |
18.04 (0.48) |
.142 |
| International Consultation on Incontinence Questionnaire | 3.25 (0.25) |
3.23 (0.26) |
.943 | 3.76 (0.37) |
3.40 (0.38) |
.464 | 2.82 (0.34) |
3.03 (0.34) |
.653 | 3.56 (0.51) |
2.88 (0.50) |
.251 |
| FACIT-Fatigue Scale | 42.21 (0.51) |
42.12 (0.52) |
.904 | 40.85 (0.72) |
41.98 (0.72) |
.238 | 43.31 (0.72) |
42.44 (0.72) |
.395 | 42.47 (1.06) |
44.36 (1.05) |
.200 |
| FACT-G Total | 92.17 (0.85) |
88.70 (0.88) |
.004 | 91.11 (1.24) |
87.91 (1.24) |
.063 | 93.00 (1.16) |
89.65 (1.23) |
.036 | 91.97 (1.95) |
90.52 (2.18) |
.627 |
| FACT-G Emotional Well-Being | 20.57 (0.23) |
20.73 (0.23) |
.615 | 20.62 (0.35) |
20.37 (0.35) |
.594 | 20.49 (0.30) |
21.10 (0.30) |
.140 | 20.36 (0.47) |
21.18 (0.47) |
.210 |
| FACT-G Functional Well-Being | 22.70 (0.34) |
21.94 (0.35) |
.119 | 22.37 (0.47) |
21.70 (0.48) |
.331 | 22.95 (0.50) |
22.20 (0.51) |
.276 | 23.06 (0.77) |
22.47 (0.80) |
.571 |
| FACT-G Physical Well-Being | 25.24 (0.21) |
24.89 (0.21) |
.217 | 24.86 (0.31) |
24.92 (0.31) |
.885 | 25.55 (0.27) |
24.91 (0.28) |
.101 | 24.42 (0.58) |
25.64 (0.59) |
.087 |
| FACT-G Social Well-Being | 23.7 (0.34) |
21.33 (0.34) |
.001 | 23.01 (0.51) |
21.32 (0.50) |
.020 | 24.20 (0.45) |
21.40 (0.47) |
.001 | 23.91 (0.82) |
21.67 (0.87) |
.059 |
| Center for Epidemiological Studies Depression Scale | 8.50 (0.47) |
10.61 (0.47) |
.002 | 9.04 (0.68) |
11.05 (0.68) |
.039 | 8.08 (0.67) |
10.12 (0.66) |
.032 | 9.39 (1.13) |
8.66 (1.11) |
.649 |
Abbreviation: SE, standard error. FACT-G, Functional Assessment of Cancer Therapy-General. MSKCC, Memorial Sloan-Kettering Cancer Center. FACIT, Functional Assessment of Chronic Illness Therapy. Means and Standard Errors were calculated after adjusting for comorbidity race, income and education.
Lower scores indicate worse bowel functioning.
Subgroup analyses
Survivors were further stratified based on treatment status, disease progression, and tumor site and were compared to controls. As shown on Table 2, the pattern of significant findings for all outcomes was similar within all subgroups. Because findings were identical in the chemotherapy only and the chemotherapy+RT group, the two groups were combined. Across treatment type, survivors reported significantly more dietary restrictions and bowel urgency than controls. Survivors also reported significantly higher social QOL in both treatment subgroups and total QOL, although the difference was not statistically significant in the surgery only subgroup. Similar to the overall sample, survivors in both treatment subgroups reported significantly fewer depression symptoms than controls. Among survivors who had new primary cancer diagnosis over time, only dietary restrictions were significantly worse compared to controls. With regard to tumor site, the pattern of findings for survivors compared to controls was identical to that for the full sample within the both the colon or rectal cancer subgroups (data not shown). Finally, to explore potential gender differences, models stratified by gender (men versus women) revealed results identical to the non-stratified analyses for all outcomes (data not shown).
Discussion
To our knowledge, this is the largest population-based study examining physical functioning, QOL, and well-being in CRC survivors at least 15 years post-diagnosis. Findings reveal equivalent physical, emotional, and functional QOL and similar fatigue and urinary dysfunction for CRC survivors compared to their age and gender-matched unaffected controls.
Prior studies also show that CRC survivors are similar to those unaffected by cancer on physical and psychosocial QOL measures. For example, one study of female-only CRC patients found that QOL was comparable to published age-specific norms on all scales of the SF-36.30 Moreover, QOL scores among long-term male and female CRC survivors (80% of whom were less than 15 years post-diagnosis) were comparable or higher to published norms.31 These findings differ from studies of survivors within the first five years of diagnosis, which show significant problems in QOL (pain, physical functioning).3, 32 However, problems appear to largely resolve over the long-term,33–34 with the exception of bowel dysfunction.4
Our findings suggest that QOL is good for CRC survivors, even after considering the effect of tumor site, disease progression, treatment, or gender. This corresponds to findings from a small (N=99) population based study in France10 of CRC survivors 15 years post-diagnosis, who significantly differed from controls only on diarrhea but not on overall QOL, fatigue or anxiety. A German population-based study,35 in contrast, found that CRC patients over the age of 70 reported worse QOL than controls on role, social, cognitive and emotional functioning, in addition to worse bowel functioning. However, this study used historical controls who reported their QOL 10 years prior to survivors. Moreover, survivor QOL was comparable to population-based published norms. The current study is the largest in a North American sample to demonstrate equivalent QOL in a tightly controlled population-based cohort of long-term survivors and controls, assessed within the same time frame.
The current study also provides novel information regarding survivors’ significantly higher social QOL compared to controls, which meets the minimally important difference criteria of 2-3 points on this scale.36 Our study is the first to demonstrate this in CRC patients. Little research has examined social QOL or depression in long-term survivors. Higher social QOL for survivors may be related to increased social support garnered during the treatment and survivorship phase of CRC. In other studies, cancer patients have also reported greater social support compared to healthy controls.37, 38 Regarding depression, a study examining the National Comorbidity Survey-Replication sample found those at least 5 years post-cancer diagnosis had no increased risk of depression compared to people without cancer.39 Our sample found that survivors reported significantly lower mean CES-D scores than controls, however, the scores for both groups indicate little depression. Taken together, the findings suggest social QOL and depression are not significant problems for long-term CRC survivors.
The areas where survivors reported comparative deficits were in bowel urgency and dietary restrictions, but these differences were relatively small in magnitude. Worse bowel functioning in the short- and long-term after CRC diagnosis has been consistently reported.5, 8, 10, 31, 35 Clinicians need to inform patients that bowel dysfunction is an expected long-lasting problem, and symptom self-management interventions may help patients cope these problems.40–41
Strengths of the current study are the use of age- and gender-matched population-based unaffected controls; analyses that adjusted for income, education, race, and comorbidities; excellent response rates on the QOL survey for survivors; well-validated cancer-specific measures; and survivors and controls recruited from a population-based registry. This study also compared differences by tumor site, type of treatment, disease progression, and gender as possible contributors to QOL; specificity has been lacking in prior research. Some limitations, however, should be noted. No baseline assessment was available to examine change in outcomes over time. The few published longitudinal studies show that although patients report worse QOL one year post-surgery compared to controls,32 QOL tends to improve over time.10 It is also possible that response-shift may account for good QOL in the long-term, as research shows positive self-evaluations often follow a cancer diagnosis.33 Nonrandom drop-out of survivors by 15 years post-diagnosis is also a limitation. It is possible that participating survivors had low comorbidity levels, as prior data show comorbidity is related to all-cause death in cancer patients.42 Moreover, those who survive for 15 years are the healthiest, reflected by earlier stage disease in this sample. Prior data demonstrate that Stage IV patients typically have the worst QOL and depression scores,32 so our findings likely underestimate difficulties for the entire sample during these earlier years. In addition, a lower response rate for unaffected controls compared to survivors suggests that healthier controls may have been more likely to participate, which also limits the generalizability of the findings. Furthermore, as the participation rate for CRC survivors was moderate (61% for the first year of the OFCCR registry),12 it is possible that healthier individuals participated in the registry and thus our survivor sample is skewed towards persons with better QOL and well-being. However, both survivors and controls were similar to participants in the larger OFCCR registry with regard to demographic variables.43
However, none of these limitations detract meaningfully from the validity and importance of the findings. Health care providers should be aware that bowel dysfunction may be an ongoing issue for CRC survivors even 15 years after diagnosis, although a small difference from their age and gender-matched controls. Moreover, the overall message is encouraging: Health care providers can reassure CRC survivors that, with the exception of bowel symptoms, QOL is similar to those who have not had cancer.
Supplementary Material
Supplemental Digital Content 1. Video abstract. Mp4.
Acknowledgments
Source of Funding:
This work was supported by grant UM1 CA167551 from the National Cancer Institute and through a cooperative agreement with the Ontario Familial Colorectal Cancer Registry (U01/U24 CA074783). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or the Ontario Familial Colorectal Cancer Registry, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CCFR. This work was also supported by an operating grant from the Canadian Institutes of Health Research to Dr. Tae Hart.
Footnotes
Author Contributions: Conceptualization: Hart, Baxter. Methodology: Hart, Baxter, Cotterchio, Gallinger. Formal Analysis: Hart, Charles, Gunaratne. Investigation: Hart, Gunaratne. Resources: Cotterchio, Cohen, Gallinger. Data Curation: Hart, Gunaratne, Cotterchio. Writing: Hart, Charles, Gunaratne, Baxter, Cotterchio, Cohen, Gallinger. Visualization: Hart, Charles, Gunaratne. Supervision: Hart. Project administration: Hart, Gunaratne. Funding acquisition: Hart, Baxter, Cotterchio, Gallinger. Hart is responsible for the overall content as guarantor.
Conflicts of Interest
There are no conflicts of interest to report.
References
- 1.Canadian Cancer Society’s Steering Committee: Canadian Cancer Society. Canadian Cancer Statistics 2016. 2016 [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: 2016. [Google Scholar]
- 3.Domati F, Rossi G, Benatti P, Roncucci L, Cirilli C, Ponz de Leon M. Long-term survey of patients with curable colorectal cancer with specific reference to the quality of life. Intern Emerg Med. 2011;6:529–535. doi: 10.1007/s11739-011-0590-y. [DOI] [PubMed] [Google Scholar]
- 4.Gatta G, Ciccolallo L, Faivre J, Bouvier AM, Berrino F, Gerard JP. Late outcomes of colorectal cancer treatment: a FECS-EUROCARE study. J Cancer Surviv. 2007;1:247–254. doi: 10.1007/s11764-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 5.Downing A, Morris EJA, Richards M, et al. Health-related quality of life after colorectal cancer in England: A patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33:616–624. doi: 10.1200/JCO.2014.56.6539. [DOI] [PubMed] [Google Scholar]
- 6.Clark JC, Fino NF, Liang JH, Hiller D. Depressive symptoms in older long-term colorectal cancer survivors: A population based analysis using the SEER-Medicare healthcare outcomes survey. Support Care Cancer. 2016;24:3907–3914. doi: 10.1007/s00520-016-3227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams SV, Ceballos R, Newcomb PA. Quality of life and mortality of long-term colorectal cancer survivors in the Seattle Colorectal Cancer Family Registry. PLoS ONE. 2016;11:e0156534. doi: 10.1371/journal.pone.0156534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunitake H, Russel MM, Zheng P, et al. Quality of life and symptoms in long-term survivors of colorectal cancer: Results from NSABP protocol LTS-01. J Cancer Surviv. 2016;11:111–118. doi: 10.1007/s11764-016-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey S, Berry K, Moinpour C, Giedzinska A, Andersen MR. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97:1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 10.Caravati-Jouvenceaux J, Launoy G, Klein D, et al. Health-related quality of life among long-term survivors of colorectal cancer: a population-based study. Oncologist. 2011;16:1626–1636. doi: 10.1634/theoncologist.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newcomb P, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resources for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2334–2341. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 12.Cotterchio M, McKeown-Eyssen G, Sutherland H, et al. Ontario familial colon cancer registry: methods and first-year response rates. Chronic Dis Can. 2000;21:81–86. [PubMed] [Google Scholar]
- 13.Temple L, Bacik J, Savatta S, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–1365. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 14.Zotti P, Del Bianco P, Serpentini S, et al. Validity and reliability of the MSKCC Bowel Function instrument in a sample of Italian rectal cancer patients. Eur J Surg Oncol. 2011;37:589–596. doi: 10.1016/j.ejso.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Murata A, Brown C, Raval M, Phang P. Impact of short-course radiotherapy and low anterior resection on quality of life and bowel function in primary rectal cancer. Am J Surg. 2008;195:611–615. doi: 10.1016/j.amjsurg.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Avery K, Donovan J, Peters T, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–330. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 17.Novara G, Ficarra V, Minja A, De Marco V, Artibani W. Functional results following vescica ileale Padovana (VIP) neobladder: midterm follow-up analysis with validated questionnaires. Eur Urol. 2010;57:1045–1051. doi: 10.1016/j.eururo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Salonia A, Gallina A, Briganti A, et al. Postoperative orgasmic function increases over time in patients undergoing nerve-sparing radical prostatectomy. J Sex Med. 2010;7:149–155. doi: 10.1111/j.1743-6109.2009.01518.x. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Lai J, Chang C, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 20.Cella D. FACIT manual: manual of the functional assessment of chronic illness therapy (FACIT) measurement system. Center on Outcomes, Research and Education; 1997. [Google Scholar]
- 21.Radloff L. The CES-D scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Hart S, Charles S. Negative affect and appraisals about cancer: application of strength and vulnerability integration (SAVI) Health Psychol. 2013;32:302–310. doi: 10.1037/a0028523. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn P, Seeley J, Roberts R, Allen N. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Cella D, Tulsky D, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 25.Deimling GT, Brown SP, Albitz C, Burant CJ, Mallick N. The relative importance of cancer-related and general health worries and distress among older adult, long-term cancer survivors. Psychooncology. 2017;26:182–190. doi: 10.1002/pon.4015. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Harden JK. Symptom burden and quality of life in survivorship. Cancer Nurs. 2015;38:E29–54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 27.Brucker P, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 28.Katz J, Chang L, Sangha O, Fossel A, Bates D. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 29.IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp; 2013. Version 22. [Google Scholar]
- 30.Trentham-Dietz A, Remington PL, Moinpour CM, Hampton JM, Sapp AL, Newcomb PA. Health-related quality of life in female long-term colorectal cancer survivors. Oncologist. 2003;8:342–349. doi: 10.1634/theoncologist.8-4-342. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey S, Andersen M, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 32.Quach C, Sanoff HK, Williams GR, Lyons JC, Reeve BB. Impact of colorectal cancer diagnosis and treatment on health-related quality of life among older Americans: A population-based, case-control study. Cancer. 2015;121:943–950. doi: 10.1002/cncr.29125. [DOI] [PubMed] [Google Scholar]
- 33.Chambers SK, Meng X, Youl P, Aiken J, Dunn J, Baade P. A five-year prospective study of quality of life after colorectal cancer. Qual Life Res. 2012;21:1551–1564. doi: 10.1007/s11136-011-0067-5. [DOI] [PubMed] [Google Scholar]
- 34.Husson O, Mols F, Ezendam NPM, Schep G, van de Poll-Franse LV. Health-related quality of life is associated with physical activity levels among colorectal cancer survivors: A longitudinal, 3-year study of the PROFILES registry. J Cancer Surviv. 2015;9:472–480. doi: 10.1007/s11764-014-0423-x. [DOI] [PubMed] [Google Scholar]
- 35.Jansen L, Herrmann A, Stegmaier C, Singer S, Brenner H, Volker A. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: A population-based study. J Clin Oncol. 2011;29:3263–3269. doi: 10.1200/JCO.2010.31.4013. [DOI] [PubMed] [Google Scholar]
- 36.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual Life Outcomes. 2003;16:79–86. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson T, Rodebaugh TL, Perez M, Schootman M, Jeffe DB. Perceived social support change in patients with early stage breast cancer and controls. Health Psychol. 2013;32:886–895. doi: 10.1037/a0031894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Ah DM, Russell KM, Carpenter J, et al. Health-related quality of life of African American breast cancer survivors compared with health African American women. Cancer Nurs. 2012;35:337–346. doi: 10.1097/NCC.0b013e3182393de3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirl WF, Greer J, Temel JS, Yeap BY, Gilman SE. Major depressive disorder in long-term cancer survivors: Analysis of the National Comorbidity Survey Replication. J Clin Oncol. 2009;27:4130–4134. doi: 10.1200/JCO.2008.16.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vij A, Kowalkowski MA, Hart T, et al. Symptom management strategies for men with early-stage prostate cancer: results from the Prostate Cancer Patient Education Program (PCPEP) J Cancer Educ. 2013;28:755–761. doi: 10.1007/s13187-013-0538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borosund E, Cvancarova M, Moore SM, Ekstedt M, Ruland CM. Comparing effects in regular practice of e-communication and Web-based self-management support among breast cancer patients: Preliminary results from a randomized controlled trial. J Med Internet Res. 2014;16:e295. doi: 10.2196/jmir.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braithwaite D, Moore DH, Satariano WA, et al. Prognostic impact of comorbidity among long-term breast cancer survivors: Results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21:1115–1125. doi: 10.1158/1055-9965.EPI-11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: A case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control. 2005;16:865–875. doi: 10.1007/s10552-005-2370-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Video abstract. Mp4.


