Abstract
Purpose
To evaluate the rate of peripapillary choroidal thinning in glaucoma patients and healthy controls using spectral domain optical coherence tomography (SD-OCT).
Design
Cohort study
Methods
Participants from the multicenter African Descent and Glaucoma Evaluation Study (ADAGES) and Diagnostic Innovations in Glaucoma Study (DIGS) were included. The San Diego Automated Segmentation Algorithm (SALSA) was used to automatically segment and measure peripapillary choroidal thickness (PCT) from circle scans centered on the optic nerve head. The rate of PCT thinning was calculated using mixed effects models.
Results
297 eyes with a median follow-up of 2.6 years were included. At baseline, the global mean PCT was significantly thinner in glaucoma patients than healthy controls (141.7±66.3 μm vs 155.7 ±64.8 μm, respectively; P<0.001). However, when age was included in the model, this difference was no longer significant (P = 0.38). Both healthy controls and glaucoma patients showed a significant decrease in mean (95% CI) PCT change over time −2.18 (−2.97, −1.40 μm/year) and −1.88 (−3.08, −0.67 μm/year), respectively and mean PCT percent change over time −3.32% (−4.36, −2.27 μm/year) and −2.85% (−4.64, −0.99 μm/year), respectively. No significant difference was found between healthy controls and glaucoma patients in the mean rate of PCT change (P=0.28) or PCT percentage change over time (P=0.23).
Conclusion
The rate of peripapillary choroidal thinning was not significantly different between healthy and glaucoma eyes during this relatively short follow-up period. Longer follow-up is needed to determine whether monitoring the rate of PCT change has a role in glaucoma management.
Introduction
Glaucoma is a neurodegenerative disease characterized by the loss of optic nerve fibers and associated visual field defects.1 Although it is the leading cause of irreversible blindness worldwide, the exact mechanism of glaucomatous optic neuropathy (GON) remains unclear. It is widely accepted that mechanical factors, such as damage to optic nerve fibers or to the lamina cribrosa via increase in intraocular pressure, play an important role in glaucoma.1 However, vascular factors including decreased blood flow to optic nerve fibers are also thought to be involved in the development and progression of the disease.2–4 The choroid, a vascular layer beneath the retina, is thought to be important in glaucoma pathophysiology due to its significant role in ocular blood flow; it accounts for seventy to eighty percent of blood flow in the eye and has the highest perfusion rate of all vascular beds in the human body.3, 5 The peripapillary region of the choroid is of particular interest to investigators because it’s branches provide essential support to the prelaminar region of the optic nerve head (ONH), a primary site of damage in GON.6
The advent of new imaging techniques, such as Spectral Domain Optical Coherence Tomography (SD-OCT), has allowed researchers and clinicians to obtain unprecedented in-vivo measurements of the choroid. Previous studies using SD-OCT on myopic and healthy eyes have demonstrated that choroidal thickness is negatively associated with age and axial length.7–9 In addition, several groups have utilized SD-OCT to evaluate the relationship between peripapillary choroidal thickness and primary open angle glaucoma, however results are inconsistent.10–16 In contrast to these studies that were largely cross-sectional in design, we used longitudinal SD-OCT data from healthy and glaucoma patients to measure PCT change over time in both groups. The purpose of our study was to evaluate the rate of peripapillary choroidal thinning in primary open angle glaucoma patients and healthy controls using spectral domain optical coherence tomography (SD-OCT).
Methods
This was a cohort study of healthy and glaucoma subjects. The healthy group was comprised of one hundred thirty-two eyes from 68 subjects. The glaucoma group consisted of one hundred sixty-five eyes from 115 subjects. All participants were included from the African Descent and Glaucoma Evaluation Study (ADAGES) and Diagnostic Innovations in Glaucoma Study (DIGS). The patient testing protocols of ADAGES and DIGS are identical and have been described previously.17 All methods adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Ethical approval was obtained from the Institutional Review Boards at the University of California San Diego, New York Eye and Ear Infirmary, and University of Alabama. All participants of the study gave written consent. ADAGES and DIGS were registered at clinicaltrials.gov under NCT00221923 and NCT00221897 respectively. While additional methods and testing were done for ADAGES and DIGS, only the methods relevant to this report will be discussed. In short, subjects underwent a complete ophthalmological examination, including a review of the medical history, determination of best corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, dilated fundus examination, simultaneous stereophotography of the optic disc, SD-OCT imaging and standard automated perimetry (SAP) in both eyes using the 24-2 program with the Swedish Interactive Thresholding Algorithm of the Humphrey Visual Field Analyzer (Carl Zeiss Meditec Inc, Dublin, California, USA).
All subjects met the following inclusion criteria: 1) Over 18 years of age, 2) Open angles on gonioscopy, and 3) Baseline best corrected visual-acuity (BVCA) of 20/40 or better.
Additional inclusion criteria for healthy subjects were the following: 1) an intraocular pressure (IOP) <22mmHg with no history of elevated IOP 2) a minimum of 2 reliable normal visual fields, defined as a PSD within 95% confidence limits and a GHT result within normal limits.
For inclusion as primary open angle glaucoma patients, in addition to having open angles on gonioscopy, a minimum of 2 consecutive and reliable standard automated perimetry (SAP) examinations with either a pattern standard deviation (PSD) outside 95% confidence limits or a glaucoma hemifield test (GHT) result outside the normal limits was required. Participants with a history of intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), coexisting retinal pathologies, non-glaucomatous optic neuropathy, uveitis, or ocular trauma were excluded from the study. Participants were also excluded if there was a diagnosis of Parkinson’s disease, Alzheimer’s disease, dementia, or a history of stroke.
SD-OCT Imaging
All subjects underwent imaging with Spectralis SD-OCT (software version 5.6.4.0; Heidelberg Engineering). The details of Spectralis SD-OCT have been described elsewhere.18, 19 For this study, high resolution retinal nerve fiber layer (RNFL) circle scans images were acquired as a 3.46mm diameter circle centered on the optic nerve head (ONH), comprising a total of 1536 A-scans. These circle scans were used to obtain PCT measurements. Each eye included in the study had imaging performed on at least 3 separate dates. A total of 297 eyes with a median follow-up of 2.6 years were included.
Segmentation
Raw SD-OCT images were exported using Heidelberg Eye Explorer software (hraviewer version 6.0.9.0). Choroidal thickness was defined as the distance between Bruch’s Membrane (BM) and the choroidal-scleral interface (CSI).20 The San Diego Automated Segmentation Algorithm (SALSA) was used to automatically segment the BM layer and CSI. Details of the SALSA have been described in a prior report.21 In brief, each individual B-scan was assumed to be comprised of multiple inter-retinal layers, such as the BM layer and the retinal nerve fiber layer. To segment the different layers, it is sufficient to estimate their skeletons and the hyper-parameters of the filters. In this study, we were only interested in the segmentation of the BM layer and the CSI. After using SALSA, each scan underwent manual qualitative assessment to ensure the segmentation was completed accurately without any errors. Only good quality scans with appropriate segmentation were included and no manual adjustments were made.
Statistical Methods
Statistical analyses for participant demographics and ocular characteristics were conducted using Stata 13.0 (Stata Corporation, College Station, TX). Categorical variables were compared using the Chi2 test and continuous variables were compared using the 2-tailed, unpaired t test or the Wilcoxon ranksum test (depending on normality).
Mixed-effects models were used to calculate the mean PCT change over time and the mean PCT percentage change over time (calculated as a percentage of baseline PCT). Differences in the intercept and slope were modeled as fixed effects, and the individual distribution of intercept and slope between subjects were modeled as random effects. The model was adjusted for age and axial length. The correlation between eyes was also accounted for in the model.
Results
The study included 132 eyes from 68 healthy subjects and 165 eyes from 115 glaucomatous subjects. Participant demographics and baseline ocular characteristics are presented in Table 1. The participants in the healthy group (were significantly younger than in the glaucoma group (mean ±SD, 56±14 years and 68±11 years, respectively, P<0.001). Compared to the healthy group, the glaucoma group had a smaller percentage of females (57% vs. 75%, respectively, P=0.038), longer mean axial length (24.0±1.0 vs 23.7±0.9, respectively; P=0.02), worse mean baseline MD (−0.31±1.2 dB vs −5.3±7.2, respectively; P<0.001), more SD-OCT visits (median[IQR], 7 visits[5–8] vs 5 visits[4–7], respectively; P<0.001), and longer follow up periods (median 3.0 years [IQR 2.6–3.4] vs 1.6 years [1.2–2.5], respectively; P<0.001). The participants in the glaucoma group had significantly lower IOP compared to the healthy group (14.1±4 vs 15.0±4, respectively; P=0.041), which is likely attributed to the glaucoma group receiving ocular hypotensive treatment for their glaucoma. There were no statistically significant differences found between the glaucoma and healthy group with regards to race and disc area (mm2) [P=0.572 and P=0.094, respectively].
Table 1.
Baseline Demographic and Ocular Characteristics of Healthy and Glaucoma Subjects in the Longitudinal Analysis of Peripapillary Choroidal Thinning
| By Subject | Healthy N=68 |
Glaucoma N=115 |
P Value |
|---|---|---|---|
| Mean age at baseline (years) | 56±14 | 68±11 | <0.001 |
| Gender, Female (%) | 51 (75%) | 66 (57%) | 0.038 |
| Race: European Descent | 34 (50%) | 53 (54%) | 0.572 |
| African Descent | 34 (50%) | 62(46%) | 0.572 |
| By Eye | N=132 | N=165 | |
| Mean IOP during follow-up (mmHg) | 15.0±4 | 14.1±4 | 0.041 |
| Disc area (mm2) | 1.99±0.50 | 2.09±0.47 | 0.094 |
| Mean Axial Length (mm) | 23.7±0.9 | 24.0±1.0 | 0.020 |
| Mean Baseline MD (dB) | −0.23±1.2 | −5.3±7.2 | <0.001 |
| Median No. of OCT visits, (IQR) | 5 (4–7) | 7 (5–8) | <0.001 |
| Median Follow up (years) (IQR) | 1.6 (1.2–2.5) | 3.0 (2.6–3.4) | <0.001 |
The sectoral differences in baseline average PCT between healthy and glaucoma patients are shown in Table 2. For both glaucomatous and healthy eyes, mean baseline PCT was thinnest in the inferior region (mean (95% CI), 137.6(127.5, 149.4μm) and 124.7(114.0, 133.5 μm), respectively). At baseline, the global mean PCT was significantly thinner in glaucoma patients compared to healthy controls (141.7μm vs 155.7μm, respectively; P<0.001). However, when we adjusted the model for age and axial length, the difference in global mean PCT at baseline between groups was no longer significant (P = 0.38; Table 2).
Table 2.
Comparison Of Baseline Mean (95% Confidence Interval) Peripapillary Choroidal Thickness In Healthy And Glaucoma Subjects By Sector (adjusted for age and axial length) (um)
| Sector | Healthy N=132 |
Glaucoma N=165 |
P Value |
|---|---|---|---|
| Global | 155.66 (145.5, 167.01) | 141.74 (130.9, 151.2) | 0.38 |
| Inferior | 137.64 (127.5, 149.38) | 124.66 (114.0, 133.5) | 0.29 |
| Superior | 162.02 (153.7, 179.5) | 147.59 (136.2, 158.9) | 0.31 |
| Nasal | 157.20 (147.3, 168.1) | 146.21 (134.7–156.6) | 0.44 |
| Temporal | 165.76 (151.6, 173.0) | 148.47 (136.5–157.9) | 0.36 |
Table 3 presents the rate of PCT change in healthy subjects and glaucoma patients after adjusting for both age and axial length. In healthy eyes, there was a significant correlation between age and PCT (−1.74 um/year p-value<0.001) even after adjusting for axial length (−1.98 um/year p-value <0.001). For this reason, we adjusted for age and axial length in the multivariable model. Both healthy and glaucoma groups showed a significant decrease in mean rate of global PCT change over time when measured as μm/year (p<0.001 and p=0.003, respectively) and as percentage change of PCT over time (p<0.001 and p<0.001, respectively). PCT in the temporal region showed the largest rate of change in both healthy and glaucoma groups, decreasing on average 2.56 μm/year and 2.02 μm/year respectively. We found no significant differences in the mean rate of global PCT change over time between healthy and glaucoma eyes when measured as μm/year (−2.18 μm/year vs −1.88 μm/year, respectively; P=0.28; Table 3), and as a percentage change of PCT over time (−3.32%/year vs −2.85%/year, respectively; P=0.23; Table 4). No differences were found in any sectors when comparing rate of PCT change or percentage change of PCT over time between healthy and glaucoma subjects.
Table 3.
Comparison Of The Mean (95% Confidence Interval) Rate Of Peripapillary Choroidal Thickness Change Over Time In Healthy Subjects, Glaucoma Subjects Measured From The First 5 Visits, And Glaucoma Subjects Measured From All Visits By Sector (adjusted for age and axial length) (um/year)
| Sector | Healthy N=132 |
Glaucoma (all visits) N=165 |
Glaucoma (5 visits) N=165 |
P Value* | P Value** |
|---|---|---|---|---|---|
| Global | −2.18 (−2.97, −1.40) p<0.001 |
−1.88 (−3.08, −0.67) p=0.003 |
−1.72 (−3.11, −0.47) p=0.001 |
0.45 | 0.28 |
| Inferior | −2.23 (−3.10, −1.36) p<0.001 |
−1.70 (−2.86, −0.54) p=0.004 |
−1.59 (−3.01, −0.67) p=0.002 |
0.25 | 0.31 |
| Superior | −2.13 (−2.88, −1.38) p<0.001 |
−1.79 (−3.08, −0.51) p=0.007 |
−1.85 (−2.87, −0.23) p=0.001 |
0.54 | 0.43 |
| Nasal | −1.81 (−2.70, −0.93) p<0.001 |
−1.99 (−3.21, −0.78) p=0.002 |
−2.13 (−3.41, −0.54) p=0.005 |
0.41 | 0.28 |
| Temporal | −2.56 (−3.39, −1.74) p<0.001 |
−2.02 (−3.35, −0.69) p=0.003 |
−1.88 (−3.45, −0.37) p=0.001 |
0.32 | 0.23 |
p-value of the difference between long and short follow-up in glaucoma eyes,
p-value of the difference between healthy and glaucoma eyes (all visits)
Table 4.
Comparison Of The Mean (95% Confidence Interval) Rate Of Percent Peripapillary Choroidal Thickness Change Over Time In Healthy And Glaucoma Subjects By Sector (adjusted for age and axial Length) (%/Year)
| Sector | Healthy N=132 |
Glaucoma N=165 |
P Value* |
|---|---|---|---|
| Global | −3.32% (−4.36, −2.27) p<0.001 |
−2.85% (−4.64, −0.99) p=0.003 |
0.37 |
| Inferior | −3.44% (−4.78,−2.08) p<0.001 |
−2.57% (−4.35,−0.78) p=0.005 |
0.33 |
| Superior | −3.15% (−4.25,−2.03) p<0.001 |
−2.63% (−4.53,−0.72) p=0.007 |
0.49 |
| Nasal | −2.69% (−4.00,−1.36) p<0.001 |
−2.29% (−4.73,−1.11) p=0.002 |
0.34 |
| Temporal | −3.36% (−4.44,−2.19) p<0.001 |
−2.29% (−4.34,−0.85) p=0.004 |
0.41 |
p-value of the difference between healthy and glaucoma eyes
As previously mentioned, the length of follow up was greater for glaucoma subjects compared to healthy subjects (median 3.0 years [IQR 2.6–3.4] vs 1.6 years [1.2–2.5], respectively; P<0.001). In order to determine if this increased length of follow-up influenced the PCT rate of change in glaucoma patients, we reduced the number of visits of the glaucoma eyes to 5 visits (follow-up median (IQR) 1.73 years (1.24–2.6) years) to match the follow-up of the healthy eyes (p-value = 0.27) and ran additional analysis on this subset of glaucoma patients. The PCT rate of change in glaucoma eyes analyzed with shorter follow-up was similar to the PCT rate of change with the longer follow-up, with differences ranging from .06 um/year in the inferior region to 0.16 um/year for global PCT. Similarly, we did not find significant differences in PCT rate of change between glaucoma eyes with the shorter follow-up and healthy eyes.
Discussion
In this longitudinal study, RNFL circle scans centered on the optic disc were used to measure baseline PCT, rate of PCT change, and percentage change of PCT over time. We found significant mean rates of PCT change in both healthy and glaucoma eyes. However, we found that the rate of peripapillary choroidal thinning, measured by the rate of PCT change and percentage change of PCT over time did not significantly differ between healthy and glaucoma groups during this follow-up period. While we initially found an association between glaucoma and thinner choroids at baseline, this association was no longer present when age and axial length were accounted for in our multivariable model.
Our findings are consistent with several reports utilizing SD-OCT and SS-OCT, suggesting that peripapillary choroidal thickness is not significantly different in glaucoma patients compared to healthy controls.10, 11, 14, 22–26 However, our study is unique in that we also evaluated change in peripapillary choroidal thickness over time in healthy and glaucoma subjects, as opposed to previous cross-sectional studies that examined PCT at one point in time.
There have been several cross-sectional studies which have reported an association between glaucoma and choroidal thickness utilizing SD-OCT.12, 27–29 For example, studies by Park et al28 and Hirooka et al27 found that PCT was significantly reduced in glaucomatous eyes without a history of increased IOP compared to healthy eyes using Enhanced Depth Imaging (EDI) SD-OCT. In a study looking at specific subtypes of glaucoma, Roberts et al found lower PCT values in glaucoma patients with severe sclerotic optic disc damage compared to healthy controls and subjects with focal and diffuse damage.29 In contrast, several other studies have failed to find an association between PCT and glaucoma.11, 14, 23–26 In a cross-sectional study using Swept Source OCT (SS-OCT) imaging, Zhang and coworkers reported no significant difference in PCT between healthy and glaucoma subjects.26 Further confirming these results, in the largest meta-analysis to date on the subject, Zhang et al. concluded there is no difference in either peripapillary or macular choroidal thickness between glaucoma patients and normal controls.22
In addition, our results also indicate that the peripapillary choroid is thinnest in the inferior location, which is also well documented in the literature.30–33 Our baseline average PCT for healthy subjects of 156μm is consistent with studies completed by Zhang et al. and Li et al. who reported an average PCT of 154 ± 44 μm and 154 ± 61 μm respectively for healthy eyes. Moreover, our baseline PCT values in healthy eyes are also similar to reports by Robert et al. (154±40 μm).29
Most importantly, the current study provides new information on the rate of peripapillary choroidal thinning. While we did not find an association between the rate of peripapillary choroidal thinning and glaucoma, we observed a significant rate of peripapillary choroidal thinning with age. In healthy eyes, we observed a global decrease in PCT of 2.2 μm/year, which would equate to a decrease of 22 μm per decade. Previous studies have estimated the change in PCT over time through extrapolation of cross-sectional data, however these estimates differ across studies. Using multivariate models adjusting for axial length, Zhang and coworkers estimated a 9 um decrease in PCT per decade.26 Similarly, Roberts et al estimated an 11 um decrease per decade using a simple linear regression model.29 More recently, Jiang and colleagues23 estimated a decrease in 20 um/decade, which is more consistent with the current study.
There are several limitations in the current study which should be noted. First, studies have shown there is a linear increase in PCT with increasing distance from the BMO.34 In our study using RNFL circle scans, the measurements were taken at only one location relative to the BMO. Moreover, the RNFL circle scans were not aligned to the Bruch’s Membrane Opening (BMO), which may affect comparability of our PCT measurements and sectoral analyses across groups as the measurements relative to the BMO varied across eyes. Second, this study has a relatively short follow-up period, 1.6 years in our healthy group, and 3.0 years in our glaucoma group. Having longer follow-up periods in both groups will allow for a better assessment of the relationship between glaucoma and change in PCT over time. However, most clinical decisions are made within a relatively short period of time, often within 1.6 years. Third, our healthy group was younger than the glaucoma group. For this reason, we adjusted for age in our statistical analysis.
In conclusion, although we found significant peripapillary choroidal thinning in both healthy and glaucoma eyes during this relatively short follow-up period of 2.6 years, the rate of thinning was not significantly different between healthy and glaucoma groups. As such, our results did not show an association between glaucoma and peripapillary choroidal thinning measured using SD-OCT. Longer follow-up is needed to determine whether monitoring the rate of PCT change has a role in glaucoma management.
Supplementary Material
Figure 1.
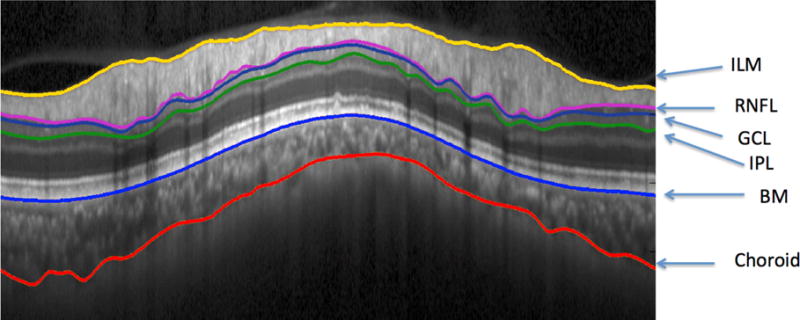
Spectral-Domain Ocular Coherence Tomography (SD-OCT) image with San Diego Automated Segmentation Algorithm (SALSA) segmentation of the Internal Limiting Membrane (ILM), Retinal Nerve Fiber Layer (RNFL), Ganglion Cell Layer (GCL), Inner Plexiform Layer (IPL), Bruch’s Membrane (BM), and Choroidal-Scleral Interface
Figure 2.
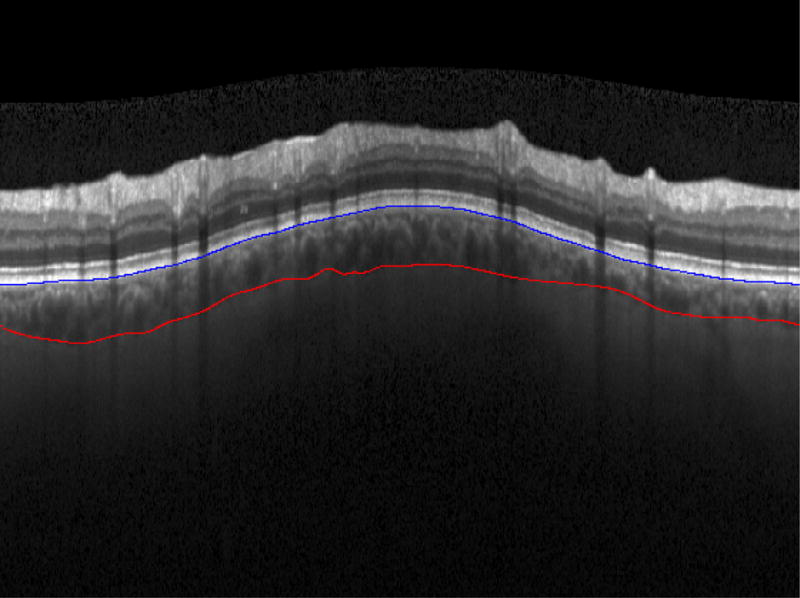
Spectral-Domain Ocular Coherence Tomography (SD-OCT) image with San Diego Automated Segmentation Algorithm (SALSA) segmentation of the choroid, seen between Bruch’s membrane (blue line) and the Choroidal-Scleral Interace (red line)
Figure 3.
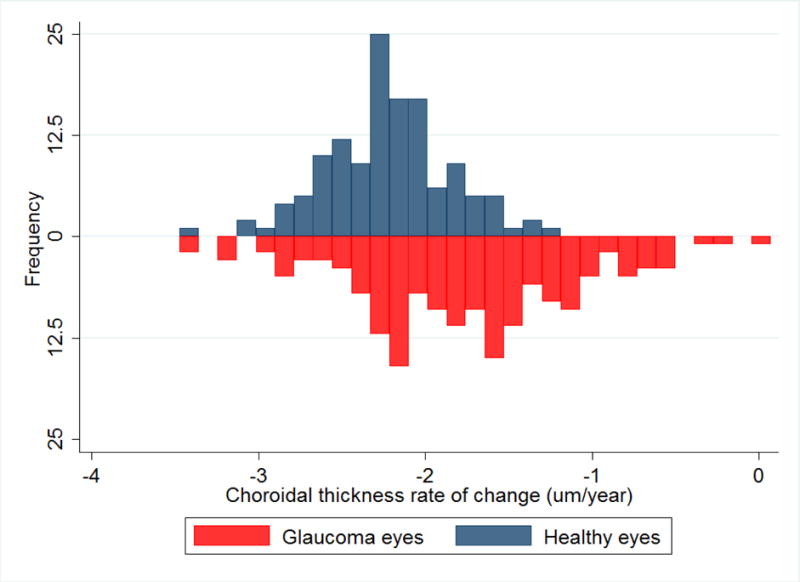
Inverted histogram showing the peripapillary choroidal thickness rate of change (um/year) adjusted for axial length in glaucoma eyes (red) and healthy eyes (blue)
Acknowledgments
a) FUNDING/SUPPORT. This work was supported by NIH Grants P30EY022589, EY11008, EY019869, EY021818, EY025056, EY022039 and grants from Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; and the Edith C. Blum Research Fund of the New York Glaucoma Research Institute, New York, NY; Unrestricted grant from Research to Prevent Blindness, New York, New York.
b) FINANCIAL DISCLOSURES.
Robert N. Weinreb: Alcon Laboratories Inc, Fort Worth, Texas, USA; Allergan Inc, Dublin, Ireland; Bausch+Lomb Inc, Bridgewater, New Jersey, USA; Carl-Zeiss Meditec Inc, Dublin, California, USA; Topcon Medical Systems Inc, Tokyo, Japan; Heidelberg Engineering GmbH, Heidelberg, Germany; Genentech Inc, San Francisco, California, USA; Optovue Inc, Fremont, California, USA.
Felipe A. Medeiros: Carl-Zeiss Meditec Inc, Dublin, California, USA; Heidelberg Engineering GmbH, Heidelberg, Germany; Topcon Medical Systems Inc, Tokyo, Japan; Ametek Inc, Berwyn, Pennsylvania, USA; Bausch+Lomb Inc. Bridgewater, New Jersey, USA; Allergan Inc, Dublin, Ireland; Sensimed AG, Lausanne, Switzerland; Alcon Laboratories Inc, Fort Worth, Texas, USA.
Linda M. Zangwill: Carl Zeiss Meditec Inc, Dublin, California, USA; Heidelberg Engineering GmbH, Heidelberg, Germany; Optovue Inc, Fremont, California, USA; Topcon Medical Systems Inc, Tokyo, Japan; Quark Pharmaceuticals Inc, Fremont, California, USA.
Christopher A. Girkin: National Eye Institute, EyeSight Foundation of Alabama, Research to Prevent Blindness, Carl Zeiss Meditech, Inc.; Heidelberg Engineering, GmbH, SOLX
Jeffrey M. Liebmann: Alcon, Inc. ;Allergan, Inc. ; Bausch & Lomb, Inc. ; Carl Zeiss Meditech, Inc.; Diopysis, inc.; Heidelberg Engineering, GmbH ; Merz Phamaceuticals, Inc. ; National Eye Institute ; New York Glaucoma Research Institute; Optovue, inc; Quark Pharmaceuticals, Inc.; Reichert, Inc. ;Sensimed, Inc.; SOLX, Inc.; Sustained Nano System ; Topcon, Inc.; Valeant Pharmaceutiicals, Inc.
The following authors have no financial disclosures: Rusdeep S. Mundae, Sami Kabbara, Christopher Bowd, Naama Hammel, Akram Belghith
c) OTHER ACKNOWLEDGMENTS:
Funding: P30EY022589 (the core grant); EY11008, U10EY14267, EY019869 (LMZ); EY021818 (FAM); EY022039 (CB); Genentech and participant retention incentive grants in the form of glaucoma medication at no cost from Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; Unrestricted grant from Research to Prevent Blindness, New York, NY
Biography
Biosketch
Rusdeep Mundae was born in Canada and moved to San Diego, California in 2000. He earned his BS in Neuroscience at the University of California, Los Angeles (UCLA) in 2012. He has presented at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting and the Learn Serve Lead Association of American Medical Colleges (AAMC) Annual Meeting. He is currently a fourth year medical student at St. Louis University (SLU).

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311(18):1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54(7):461–72. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Progress in Retinal and Eye Research. 2002;21(4):359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 4.Fuchsjager-Mayrl G, Wally B, Georgopoulos M, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45(3):834–9. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 5.Weigelin E. The blood circulation of the retina and the uvea. Adv Ophthalmol. 1972;25:2–27. [PubMed] [Google Scholar]
- 6.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53(11):721–48. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara T, Imamura Y, Margolis R, et al. Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Highly Myopic Eyes. American Journal of Ophthalmology. 2009;148(3):445–50. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147(5):801–10. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Fedilkuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Investigative Ophthalmology and Visual Science. 2010;51(4):2173–6. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 10.Maul EA, Friedman DS, Chang DS, et al. Choroidal Thickness Measured by Spectral Domain Optical Coherence Tomography: Factors Affecting Thickness in Glaucoma Patients. Ophthalmology. 2011;118(8):1571–9. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini H, Nilforushan N, Moghimi S, et al. Peripapillary and macular choroidal thickness in glaucoma. J Ophthalmic Vis Res. 2014;9(2):154–61. [PMC free article] [PubMed] [Google Scholar]
- 12.Lamparter J, Schulze A, Riedel J, et al. Peripapillary Choroidal Thickness and Choroidal Area in Glaucoma, Ocular Hypertension and Healthy Subjects by SD-OCT. Klin Monbl Augenheilkd. 2015;232(4):390–4. doi: 10.1055/s-0035-1545819. [DOI] [PubMed] [Google Scholar]
- 13.McCourt EA, Cadena BC, Barnett CJ, et al. Measurement of subfoveal choroidal thickness using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S28–S33. doi: 10.3928/15428877-20101031-14. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich JR, Peterson J, Parlitsis G, et al. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Experimental Eye Research. 2011;92(3):189–94. doi: 10.1016/j.exer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Banitt M. The choroid in glaucoma. Curr Opin Ophthalmol. 2013;24(2):125–9. doi: 10.1097/ICU.0b013e32835d9245. [DOI] [PubMed] [Google Scholar]
- 16.Mwanza J-C, Hochberg JT, Banitt MR, et al. Lack of Association between Glaucoma and Macular Choroidal Thickness Measured with Enhanced Depth-Imaging Optical Coherence Tomography. Investigative Ophthalmology & Visual Science. 2011;52(6):3430–5. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khachatryan N, Medeiros FA, Sharpsten L, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): Predictors of Visual Field Damage in Glaucoma Suspects. American Journal of Ophthalmology. 2015;159(4):777–87.e1. doi: 10.1016/j.ajo.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, de Boer JF, Chen TC. Reproducibility of Retinal Nerve Fiber Layer Thickness Measurements Using Spectral Domain Optical Coherence Tomography. Journal of glaucoma. 2011;20(8):470–6. doi: 10.1097/IJG.0b013e3181f3eb64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langenegger SJ, Funk J, Töteberg-Harms M. Reproducibility of Retinal Nerve Fiber Layer Thickness Measurements Using the Eye Tracker and the Retest Function of Spectralis SD-OCT in Glaucomatous and Healthy Control Eyes. Investigative Ophthalmology & Visual Science. 2011;52(6):3338–44. doi: 10.1167/iovs.10-6611. [DOI] [PubMed] [Google Scholar]
- 20.Mrejen S, Spaide RF. Optical coherence tomography: Imaging of the choroid and beyond. Survey of Ophthalmology. 2013;58(5):387–429. doi: 10.1016/j.survophthal.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Belghith A, Bowd C, Weinreb RN, Zangwill LM. A hierarchical framework for estimating neuroretinal rim area using 3D spectral domain optical coherence tomography (SD-OCT) optic nerve head (ONH) images of healthy and glaucoma eyes. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:3869–72. doi: 10.1109/EMBC.2014.6944468. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Yu M, Wang F, et al. Choroidal Thickness and Open-Angle Glaucoma: A Meta-Analysis and Systematic Review. J Glaucoma. 2016;25(5):e446–54. doi: 10.1097/IJG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 23.Jiang R, Wang YX, Wei WB, et al. Peripapillary Choroidal Thickness in Adult Chinese: The Beijing Eye Study. Invest Ophthalmol Vis Sci. 2015;56(6):4045–52. doi: 10.1167/iovs.15-16521. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Bian A, Zhou Q, Mao J. Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol. 2013;156(6):1277–84.e1. doi: 10.1016/j.ajo.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Suh W, Cho HK, Kee C. Evaluation of peripapillary choroidal thickness in unilateral normal-tension glaucoma. Jpn J Ophthalmol. 2014;58(1):62–7. doi: 10.1007/s10384-013-0290-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Tatham AJ, Medeiros FA, et al. Assessment of choroidal thickness in healthy and glaucomatous eyes using swept source optical coherence tomography. PLoS One. 2014;9(10):e109683. doi: 10.1371/journal.pone.0109683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirooka K, Tenkumo K, Fujiwara A, et al. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;12:29. doi: 10.1186/1471-2415-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park HY, Lee NY, Shin HY, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23(4):225–31. doi: 10.1097/IJG.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 29.Roberts KF, Artes PH, O’Leary N, et al. PEripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Archives of Ophthalmology. 2012;130(8):980–6. doi: 10.1001/archophthalmol.2012.371. [DOI] [PubMed] [Google Scholar]
- 30.Gupta P, Cheung CY, Saw S-M, et al. Peripapillary Choroidal Thickness in Young Asians With High MyopiaPeripapillary Choroidal Thickness in High Myopes. Investigative Ophthalmology & Visual Science. 2015;56(3):1475–81. doi: 10.1167/iovs.14-15742. [DOI] [PubMed] [Google Scholar]
- 31.Ho J, Branchini L, Regatieri C, et al. Analysis of Normal Peripapillary Choroidal Thickness via Spectral Domain Optical Coherence Tomography. Ophthalmology. 2011;118(10):2001–7. doi: 10.1016/j.ophtha.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X-S, Shen L-J, Chen R-R, Lyu Z. Peripapillary choroidal thickness in Chinese children using enhanced depth imaging optical coherence tomography. International Journal of Ophthalmology. 2016;9(10):1451–6. doi: 10.18240/ijo.2016.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Wang W, Zhou M, et al. Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol. 2013;13:23. doi: 10.1186/1471-2415-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes LA, Huisingh C, Johnstone J, et al. Peripapillary Choroidal Thickness Variation With Age and Race in Normal EyesPeripapillary Choroidal Thickness Varies With Race. Investigative Ophthalmology & Visual Science. 2015;56(3):1872–9. doi: 10.1167/iovs.14-16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


