Abstract
Contemporary data on complications and resource utilization after atrial fibrillation (AF) ablation are limited. We evaluated rates and risk factors for procedural complication, rehospitalization and emergency department visits after AF ablation. We identified all adult patients who underwent isolated AF ablation between 2010 and June 2014 in two large integrated healthcare delivery systems and evaluated rates of acute inpatient complication, 30-day and 1-year readmission and emergency evaluation. We used multivariable logistic regression to identify predictors of procedural complications, 30-day readmission or 30-day emergency department evaluation. Among 811 AF ablation patients, procedural complications occurred in 2.5% of patients, 9.7% of patients were rehospitalized within 30 days, and 19.1% of patients had an emergency visit within 30 days. At one year after AF ablation, 28.9% of patients were readmitted, with 18% of patients readmitted for AF or atrial flutter. At one year, 44.5% of patients were seen in an emergency department, with 37.1% related to AF or atrial flutter. Vascular complications and perforation/tamponade were the most common complications, and Hispanic ethnicity, mitral or aortic valvular disease and diabetes mellitus were the strongest risk factors for adverse outcomes at 30 days after AF ablation. Contemporary rates of acute complication and one-year readmission after AF ablation have markedly decreased compared with previous community-based studies.
Keywords: atrial fibrillation, catheter ablation, complication, readmission
Introduction
Catheter ablation for atrial fibrillation (AF) is increasingly performed for AF rhythm control. A wide range of risks of procedural complications after catheter ablation for AF have been reported (1-10%),1,2 with estimates in studies of large, community-based populations converging on ~5%.3 Similarly, studies in community practice have shown rates of hospitalization after AF ablation of 9.4% at 30 days and 39% at one year (22% for arrhythmia recurrence).3 However, these studies relied on data only up to 2009, before widespread adoption of several important advances in AF ablation, including the use of uninterrupted anticoagulation, cryoballoon catheters, open-irrigated and contact force sensing for radiofrequency energy delivery catheters and improved 3-dimensional geometric mapping. In addition, increased operator experience has been shown to be associated with lower rates of complication.3 Furthermore, data have not been reported on rates of emergency department evaluation after AF ablation. To address these knowledge gaps, we examined contemporary rates of procedural complication, hospitalization and emergency department visits in patients undergoing AF ablation between 2010 and 2014 in two large, multicenter community practice settings.
Methods
The source population included members from Kaiser Permanente Northern California and Southern California—two health systems with >8 million persons who are highly representative of the statewide and national populations (Table 1).4 Each health care delivery system also has implemented a Virtual Data Warehouse.5 The Virtual Data Warehouse is comprised of electronic datasets at each participating site that are populated with linked demographic, administrative, ambulatory pharmacy, outpatient laboratory test results, and health care utilization data for members receiving care at participating sites.6 Institutional review boards of Kaiser Permanente Northern California, Kaiser Permanente Southern California and Yale University approved the study. A waiver of informed consent was obtained due to the nature of the study.
Table 1.
Baseline characteristics of patients who underwent atrial fibrillation ablation procedure between January 1, 2010 and June 30, 2014 stratified by the presence or absence of an adverse outcome within 30 days of index procedure (procedural complication, death, hospitalization for any cause, and emergency visit for any cause)
| Characteristic | Adverse outcome within 30 days | |||
|---|---|---|---|---|
| Overall (N = 811) |
Yes (N = 227) |
No (N = 584) |
P-value | |
| Age (years) mean (SD) | 59.9 +/− 9.3 | 60.9 +/− 8.7 | 59.5 +/− 9.5 | 0.06 |
| Age Group (years) | 0.50 | |||
| 18-49 | 124 (15.3%) | 27 (11.9%) | 97 (16.6%) | |
| 50-59 | 243 (30.0%) | 72 (31.7%) | 171 (29.3%) | |
| 60-69 | 340 (41.9%) | 97 (42.7%) | 243 (41.6%) | |
| 70-79 | 103 (12.7%) | 31 (13.7%) | 72 (12.3%) | |
| ≥ 80 | 1 (0.1%) | 0 (0.0%) | 1 (0.2%) | |
| Women | 258 (31.8%) | 92 (40.5%) | 166 (28.4%) | |
| Men | 553 (68.2%) | 135 (59.5%) | 418 (71.6%) | |
| White | 689 (85.0%) | 194 (85.5%) | 495 (84.8%) | |
| Black | 13 (1.6%) | 0 (0.0%) | 13 (2.2%) | |
| Asian | 73 (9.0%) | 20 (8.8%) | 53 (9.1%) | |
| Other | 36 (4.4%) | 13 (5.7%) | 23 (3.9%) | |
| Hispanic ethnicity, n (%) | 63 (7.8%) | 25 (11.0%) | 38 (6.5%) | 0.03 |
| Smoker (current or former) | 371 (45.7%) | 116 (51.1%) | 255 (43.7%) | 0.06 |
| Prior coronary artery disease | 47 (5.8%) | 15 (6.6%) | 32 (5.5%) | 0.56 |
| Ischemic stroke or transient ischemic attack | 46 (5.7%) | 9 (4.0%) | 37 (6.3%) | 0.19 |
| Peripheral arterial disease | 12 (1.5%) | 5 (2.2%) | 7 (1.2%) | 0.29 |
| Mitral and/or aortic valvular disease | 88 (10.9%) | 34 (15.0%) | 54 (9.2%) | 0.02 |
| Ventricular tachycardia or fibrillation | 31 (3.8%) | 10 (4.4%) | 21 (3.6%) | 0.59 |
| Implantable cardioverter defibrillator | 6 (0.7%) | 3 (1.3%) | 3 (0.5%) | 0.23 |
| Pacemaker | 42 (5.2%) | 11 (4.8%) | 31 (5.3%) | 0.79 |
| Cardiac resynchronization therapy | 7 (0.9%) | 1 (0.4%) | 6 (1.0%) | 0.42 |
| Hypertension | 429 (52.9%) | 147 (64.8%) | 282 (48.3%) | <0.001 |
| Dyslipidemia | 522 (64.4%) | 160 (70.5%) | 362 (62.0%) | 0.02 |
| Diabetes mellitus | 103 (12.7%) | 47 (20.7%) | 56 (9.6%) | <0.001 |
| Hospitalized bleeding | 10 (1.2%) | 4 (1.8%) | 6 (1.0%) | 0.40 |
| Hyperthyroidism | 48 (5.9%) | 18 (7.9%) | 30 (5.1%) | 0.13 |
| Hypothyroidism | 137 (16.9%) | 42 (18.5%) | 95 (16.3%) | 0.45 |
| Chronic lung disease | 219 (27.0%) | 74 (32.6%) | 145 (24.8%) | 0.03 |
| Chronic liver disease | 45 (5.5%) | 12 (5.3%) | 33 (5.7%) | 0.84 |
| Diagnosed dementia | 20 (2.5%) | 5 (2.2%) | 15 (2.6%) | 0.76 |
| Diagnosed depression | 156 (19.2%) | 61 (26.9%) | 95 (16.3%) | <0.001 |
| Body Mass Index (kg/m2) | 0.06 | |||
| <18.5 | 2 (0.2%) | 0 (0.0%) | 2 (0.3%) | |
| 18.5-25 | 174 (21.5%) | 45 (19.8%) | 129 (22.1%) | |
| 25-30 | 323 (39.8%) | 79 (34.8%) | 244 (41.8%) | |
| 30-40 | 258 (31.8%) | 88 (38.8%) | 170 (29.1%) | |
| >=40 | 48 (5.9%) | 15 (6.6%) | 33 (5.7%) | |
| Missing | 6 (0.7%) | 0 (0.0%) | 6 (1.0%) | |
| Systolic blood pressure (mmHg) mean (SD) | 119.7 +/− 14.3 | 121.4 +/− 14.7 | 119.0 +/− 14.1% | 0.03 |
| Diastolic blood pressure (mmHg) mean (SD) | 70.3 +/− 9.8) | 70.1 +/− 10.0 | 70.3 +/− 9.7 | 0.81 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) mean (SD) | 79.1 +/− 15.9 | 79.2 +/ 15.9 | 79 +/− 15.9 | 0.85 |
| Dipstick proteinuria | 0.05 | |||
| None | 649 (80.0%) | 171 (75.3%) | 478 (81.8%) | |
| Trace | 137 (16.9%) | 44 (19.4%) | 93 (15.9%) | |
| 1+ | 18 (2.2%) | 10 (4.4%) | 8 (1.4%) | |
| 2+ | 5 (0.6%) | 1 (0.4%) | 4 (0.7%) | |
| 3+ | 2 (0.2%) | 1 (0.4%) | 1 (0.2%) | |
| High-density lipoprotein cholesterol (mg/dL) mean (SD) | 49.5 +/− 13.5 | 49.8 +/− 14.5 | 49.4 +/−13.0 | 0.69 |
| Low-density lipoprotein cholesterol (mg/dL) mean (SD) | 100.4 +/− 33 | 98.3 +/− 32.3 | 101.2 +/−33 | 0.34 |
| Hospital days, mean (SD) | 5.3 +/− 4.5 | 5.9 +/− 5.0 | 5.0 +/− 4.2 | 0.08 |
| Outpatient visits, mean (SD) | 16.6 +/− 10.7 | 19.4 +/− 12.6 | 15.6 +/− 9.6 | <0.001 |
| Medications | ||||
| Alpha-adrenergic receptor antagonists | 39 (4.8%) | 13 (5.7%) | 26 (4.5%) | 0.45 |
| Angiotensin converting enzyme inhibitors / angiotensin receptor blockers | 240 (29.6%) | 87 (38.3%) | 153 (26.2%) | <0.001 |
| Anti-arrhythmic medications | 708 (87.3%) | 198 (87.2%) | 510 (87.3%) | 0.97 |
| Diuretics (loop and thiazide) | 162 (20.0%) | 60 (26.4%) | 102 (17.5%) | 0.004 |
| Beta-blockers | 579 (71.4%) | 174 (76.7%) | 405 (69.3%) | 0.04 |
| Aldosterone receptor antagonists | 9 (1.1%) | 3 (1.3%) | 6 (1.0%) | 0.72 |
| Calcium channel blockers | 254 (31.3%) | 88 (38.8%) | 166 (28.4%) | 0.004 |
| Nitrates | 33 (4.1%) | 11 (4.8%) | 22 (3.8%) | 0.49 |
| Hydralazine | 15 (1.8%) | 7 (3.1%) | 8 (1.4%) | 0.10 |
| Statins | 347 (42.8%) | 111 (48.9%) | 236 (40.4%) | 0.03 |
| Other lipid lowering agents | 38 (4.7%) | 13 (5.7%) | 25 (4.3%) | 0.38 |
| Antiplatelet agents | 26 (3.2%) | 10 (4.4%) | 16 (2.7%) | 0.23 |
| Diabetes therapy | 67 (8.3%) | 31 (13.7%) | 36 (6.2%) | <0.001 |
SD= standard deviation
We identified adults age ≥21 years diagnosed with AF who underwent an isolated ablation for AF. AF was defined as meeting the following criteria between January 1, 2010 through June 30, 2014: one or more inpatient admissions with a primary discharge diagnosis of AF (International Classification of Diseases, Ninth Edition [ICD-9] codes 427.3 or 427.31)7; or two or more outpatient, non-emergency department encounters for diagnosed AF. The subset of patients who underwent ablation for AF was identified through manual review of the electrophysiology laboratory procedure logs. For the present analysis, we excluded those with unknown gender, <12 months of continuous membership or drug benefit, or no membership after the procedure date.
Patients were followed through December 31, 2014 for the outcomes of death, hospitalization, and emergency department visits as well as procedural complications. Patients were censored at the time of health plan disenrollment or the end of follow-up. Death from any cause was identified from health plan databases (inpatient deaths, proxy report of deaths, cancer registry), California state death certificate files and Social Security Administration Death Master File.6,8,9 Hospitalization and emergency visits were identified using electronic medical records and billing claims.10
Acute procedural complications included cardiac perforation and/or tamponade, pneumothorax, hemothorax, procedure-related stroke, transient ischemic attack, vascular complication (consisting of hemorrhage/hematoma, vascular complication requiring surgical repair, and accidental puncture), and in-hospital death (see Supplementary Material Table 1 for ICD-9 codes). Procedural complications were identified using secondary diagnoses coded during the index AF ablation admission.2,3
Data on patient age, gender, and self-reported race/ethnicity were obtained from health plan databases. We ascertained relevant medical history documented up to 5 years prior to and including cohort entry using previously validated approaches based on ICD-9 diagnosis and procedure codes, Current Procedure Terminology procedure codes, laboratory records and pharmacy records.11,12 This included cardiovascular diseases (prior coronary heart disease, ischemic stroke or transient ischemic attack, peripheral artery disease, valvular heart disease), prior ventricular tachycardia or fibrillation, prior implantable cardiac device (pacemaker, implantable cardioverter-defibrillator, cardiac resynchronization device), other cardiovascular risk factors (hypertension, dyslipidemia, and diabetes mellitus), and other coexisting medical illnesses (hospitalized bleeding, hyperthyroidism, hypothyroidism, chronic lung disease, chronic liver disease, dementia, depression). We ascertained body mass index and blood pressure up to 365 days prior to and including cohort entry from outpatient clinic visit information in health plan electronic medical records. We also characterized pre-ablation kidney function using the most recent outpatient serum creatinine concentration values and estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) based on the CKD-EPI equation.13 In an effort to assess other metrics of overall patient illness, we also quantified the number of hospital days, the number of outpatient visits and the number of nursing home or skilled nursing home stays during the prior year.
We also characterized baseline exposure to cardiovascular medications within 120 days before cohort entry based on estimated day supply information per dispensed prescription and refill patterns found in health plan outpatient pharmacy databases using previously validated methods.11 For this study, we included the following medications: α- adrenergic receptor antagonists, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, anti-arrhythmic medications, diuretics, beta-blockers, aldosterone receptor antagonists, calcium channel blockers, nitrates, hydralazine, statins, other lipid lowering agents, anti-platelet agents, and diabetic medications.14
All analyses were conducted using SAS, version 9.3 (Cary, N.C.). We compared baseline characteristics for patients with and without an adverse outcome (procedural complication, death, hospitalization, emergency visit) within 30 days of the index procedure using ANOVA or relevant non-parametric test for continuous variables and χ2 test for categorical variables. Outcomes were presented as frequencies and percentages of all index AF ablation recipients. We used Kaplan-Meier analyses to estimate rates of all-cause rehospitalization, rehospitalization for AF and/or atrial flutter, all-cause emergency visits, and emergency visits for AF and/or atrial flutter within 1 year after the index procedure date. We sought to identify predictors of inpatient complication and/or 30-day adverse events, with candidate factors including demographic, comorbidity, and laboratory test covariates listed previously. We performed multivariable logistic regression with generalized estimating equations to account for clustering of patients by site to evaluate factors independently associated with the occurrence of acute procedural complications and/or 30-day all-cause readmissions. We included in the final models all variables previously reported to be associated with death or hospitalization for cardiovascular causes as well as any variables with significant differences at baseline between those with and those without an adverse outcome within 30 days of the index procedure using a cutoff of P<0.05. We also performed a secondary analysis to evaluate predictors of complications and/or 1-year all-cause readmissions.
Results
We identified 811 eligible adults who received an isolated AF ablation procedure between January 1, 2010 and June 30, 2014. The mean age was 59.9 years old, with dyslipidemia (64.4%) and hypertension (52.9%) being the most common comorbidities (Table 1). Patients who experienced an emergency visit, hospitalization or death within 30 days after AF ablation were more likely to be women and have a history of aortic or mitral valvular disease, hypertension, dyslipidemia, diabetes mellitus, chronic lung disease, and known depression. They also had a higher mean number of outpatient visits in the prior year and were more likely to be receiving angiotensin receptor blockers, diuretics, beta blockers, calcium channel blockers, statins, and diabetes medications (Table 1). Cryoballoon ablation was performed in 256 (31.6%) of the AF ablations and radiofrequency energy catheters were used in the remainder of the procedures.
Acute procedural complications occurred during the index hospitalization in 2.5% of AF ablations (n=20). Vascular complications (1.1%), accidental puncture (0.5%, most vascular access) and cardiac perforation/ tamponade (0.7%) accounted for the vast majority of all inpatient complications. There were no documented ischemic or hemorrhagic strokes during the index hospitalization for the procedure (Table 2).
Table 2.
Procedural complications, rehospitalizations, and emergency department visits after atrial fibrillation ablation.
| Outcome | N (%) (N = 811) |
|---|---|
|
Procedural complications during index hospitalization
| |
| Total persons with at least one complication | 20 (2.5%) |
| Death | 0 |
| Cardiac perforation / Tamponade | 6 (0.7%) |
| Stroke – ischemic | 0 |
| Stroke – hemorrhagic | 0 |
| Pneumothorax | 0 |
| Hemothorax | 0 |
| Vascular complications | 9 (1.1%) |
| Accidental puncture | 4 (0.5%) |
| Major bleeding | 0 |
| Minor bleeding | 1 (0.1%) |
|
30-day rehospitalizations | |
| Rehospitalization for any cause | 79 (9.7%) |
| Rehospitalization for atrial fibrillation or fluttera | 33 (4.1%) |
| Rehospitalization for atrial fibrillation | 27 (3.3%) |
| Rehospitalization for atrial flutter | 6 (0.7%) |
| Rehospitalization for heart failure | 5 (0.6%) |
| Rehospitalization for procedural complicationsb | 17 (2.1%) |
| Death – all cause | 1 (0.1%) |
| Cardiac perforation / Tamponade | 0 |
| Stroke – ischemic | 0 |
| Stroke – hemorrhagic | 0 |
| Pneumothorax | 0 |
| Hemothorax | 0 |
| Vascular complications | 16 (2.0%) |
| Accidental puncture | 1 (0.1%) |
| Major bleeding | 0 |
| Minor bleeding | 1 (0.1%) |
|
30-day emergency visits | |
| First emergency visit for any cause | 155 (19.1%) |
| Emergency visit only for atrial fibrillation or flutter | 119 (14.7%) |
| First emergency visit only for atrial fibrillation | 110 (13.6%) |
| First emergency visit only for atrial flutter | 35 (4.3%) |
| Emergency visit for heart failure | 13 (1.6%) |
| Emergency visit for procedural complications | 8 (1.0%) |
| Death – all cause | 0 |
| Cardiac perforation / Tamponade | 0 |
| Stroke – ischemic | 0 |
| Stroke – hemorrhagic | 0 |
| Pneumothorax | 0 |
| Hemothorax | 0 |
| Vascular complications | 8 (1.0%) |
| Accidental puncture | 0 |
| Major bleeding | 0 |
| Minor bleeding | 0 |
|
30-day death from all cause | |
| Death from all cause | 1 (0.1%) |
Atrial fibrillation or atrial flutter as the primary discharge/emergency visit diagnosis
Procedural complication as either a primary or secondary discharge/emergency visit diagnosis
Overall, 9.7% of patients were rehospitalized within 30 days (n=79, Table 2). AF or atrial flutter was the primary diagnosis for readmission for 4.1% of patients (n=33). Rehospitalization within 30 days for a procedural complication occurred in 2.1% of patients (n=17). Vascular complications accounted for most of the complication-related readmissions. No patients were readmitted for stroke or cardiac perforation/tamponade. Readmission within 30 days for heart failure occurred in 0.6% of patients (n=5). There was also 1 patient death 29 days after the procedure due to acute respiratory failure in a patient with chronic pulmonary disease in whom atrioesophageal fistula was ruled out (Table 2).
Overall, 19.1% patients were seen in an emergency department but not admitted to the hospital within 30 days post-procedure. Emergency department visits with a diagnosis of AF or atrial flutter occurred in 14.7% of patients (n=119). Emergency department visits with a procedural complication occurred in 1.0% of patients (n=8). Vascular complications accounted for most of the complication-related emergency visits. Emergency evaluation for heart failure occurred in 1.6% of patients (n=13, Table 2).
In multivariable models, we identified several variables independently associated with a risk of procedural complication, 30-day hospitalization, or 30-day emergency department visits. We found that there was an increased risk of these adverse outcomes with Asian/Pacific Islander race compared with white race (adjusted odds ratio [OR] 1.14, 95% confidence interval [CI] 1.11–1.17), Hispanic ethnicity (OR 2.49, 95% CI 2.47-2.51), known mitral or aortic valvular disease (OR 1.49, 95% CI 1.10-2.03), hypertension (OR 1.31, 95% CI 1.17-1.46), diabetes mellitus (OR 1.65, 95% CI 1.18-2.32), and diagnosed depression (OR 1.44, 95% CI 1.22-1.72) (Table 3). Factors that were protective against adverse events included black/African American race compared with white race (OR 0.46, 95% CI 0.46-0.47) and a history of ischemic stroke or transient ischemic attack (OR 0.42, 95% CI 0.41-0.44). A secondary analysis evaluating predictors of procedural complication, 30-day hospitalization, or 30-day emergency department visits at 1 year showed similar results, except Asian race, black race, and diabetes mellitus were no longer statistically significant predictors, while female sex was a statistically significant (Supplementary Material Table 2).
Table 3.
Multivariable predictors for procedural complications, hospitalizations, or emergency department visits within 30 days after atrial fibrillation ablation between January 1, 2010 and June 30, 2014.a
| Variable | Adjusted Odds Ratio (95% Confidence Interval) |
|---|---|
| Age per year increase | 1.00 (0.98-1.02) |
| Women | 1.32 (0.87-2.00) |
| White | Reference |
| Asian | 1.14 (1.11-1.17) |
| Black | 0.46 (0.46-0.47) |
| Hispanic | 2.49 (2.47-2.51) |
| Coronary heart disease | 0.72 (0.41-1.24) |
| Ischemic stroke or transient ischemic attack | 0.42 (0.41-0.44) |
| Peripheral arterial disease | 1.09 (0.52-2.32) |
| Mitral and/or aortic valvular disease | 1.49 (1.10-2.03) |
| Hypertension | 1.31 (1.17-1.46) |
| Diabetes mellitus | 1.65 (1.18-2.32) |
| Dyslipidemia | 0.99 (0.76-1.30) |
| Chronic lung disease | 1.11 (0.83-1.48) |
| Diagnosed depression | 1.44 (1.22-1.72) |
Model also adjusted for the following covariates: pre-procedural systolic blood pressure, angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, anti- arrhythmic medications, diuretics (loop and thiazide), beta-blockers, calcium channel blockers, statins, diabetes therapy, number of hospital days within 12 months before ablation, and number of outpatient visits within 12 months before ablation.
In longer-term follow up, 28.9% of patients were readmitted within one year after AF ablation, with 18% of patients readmitted for AF or atrial flutter. The probability of surviving free of hospital admission for any cause was 70% (95% CI 66%-73%), and the probability of surviving free of hospital admission for AF or atrial flutter was 81% (95% CI 78%-84%) (Figure 1).
Figure 1.
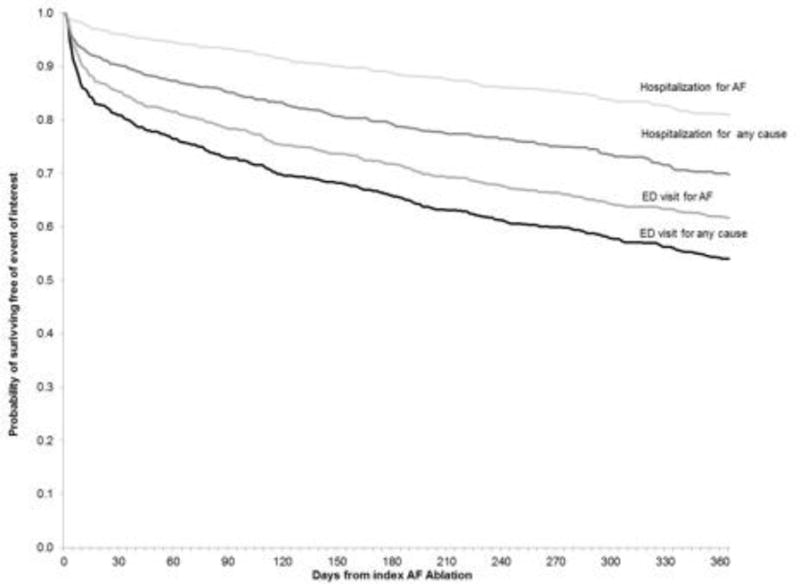
Kaplan-Meier survival plots for survival free from all-cause and atrial arrhythmia (atrial fibrillation or atrial flutter) related hospitalization and emergency visits after atrial fibrillation ablation.
At one year after AF ablation, 44.5% of patients had an emergency visit for any cause and 37.1% of patients had an emergency visit for AF or atrial flutter. At one year, the probability of surviving free of an emergency visit for any cause was 54% (95% CI 50%-57%), and the probability of surviving free of emergency visit for AF or atrial flutter was 62% (95% CI 58%-65%) (Figure 1).
Among 811 recipients of an initial AF ablation, 10.5% of patients received one additional ablation, 0.9% received two additional ablations, and 0.1% received three additional ablations within one year.
Discussion
Our study demonstrates that in a contemporary multicenter cohort of patients undergoing AF ablation between 2010 and 2014, the rate of acute inpatient complication was 2.5%, which was lower than with previous community-based studies.3 The rates of hospitalization and emergency visit within 30 days after ablation were 9.7% and 19.1%, respectively, and 1-year readmission was 28.9%, with only 18% of patients readmitted for AF or atrial flutter. The rate of emergency visit at one year was notably higher at 44.5%, with 37.1% for AF or atrial flutter. Vascular complications and perforation/tamponade were the most common complications, and Hispanic ethnicity, mitral or aortic valvular disease and diabetes mellitus were the strongest risk factors for adverse outcomes at 30 days after AF ablation.
Data from patients treated with AF ablation prior to 2009 in the California Statewide Inpatient Database, showed rates of inpatient complication of 5%, which is roughly twice the rate we report in our analysis.3 That study showed rates of rehospitalization of 9.4% at 30 days and almost 40% at 1 year3, while our study showed substantially lower rates. Increased hospital and physician experience has been previously shown to improve outcomes including complications and readmission after AF ablation and other cardiac procedures3,15,16 In addition, since 2009 there have been several advances in AF ablation including the use of uninterrupted anticoagulation, cryoballoon catheters, open-irrigated and contact force sensing for radiofrequency energy delivery catheters, and improved 3-dimensional geometric mapping which may have contributed to the lower rates of procedural complications we observed.
The stable rate of 30-day readmission after AF ablation suggests that more investigation is needed into the short-term peri-procedural management of patients in the 1-3 month “blanking period” after AF ablation. Nonetheless, these rates of short-term readmission are substantially lower than the readmission rates for Medicare beneficiaries after percutaneous coronary intervention17 and heart failure hospitalization.18
Our study shows that the most common acute inpatient complications after AF ablation were vascular complications and cardiac perforation/tamponade, which is consistent with previous studies.3,19 While the rate of vascular complication we observed was less than half of previous reports,3 the consistent finding of vascular events as the most common complication represents an opportunity for innovation in equipment and medical management.20
Importantly, we observed no ischemic or hemorrhagic strokes occurring acutely or within the first 30 days after AF ablation. Studies of AF ablation have shown that stroke rates have steadily declined over time. Possible explanations include improvements in equipment and periprocedural management, including the use of open irrigated catheters and standardized approaches to peri-procedural anticoagulation and pre-procedure left atrial appendage screening.21 The recent widespread adoption of uninterrupted oral anticoagulation for AF ablations may be particularly important in explaining the very low rates of short-term stroke we observed in our study.
We showed that 19% of patients were seen in an emergency department within 30 days after an AF ablation and 44.5% were seen in an emergency department within one year. Prior studies of AF ablation have not evaluated rates of emergency evaluation after the procedure. The most common causes for emergency evaluation within 30 days after AF ablation were atrial arrhythmias, heart failure and vascular complications. Our data suggest that peri-procedural interventions targeted at arrhythmia recurrence and heart failure such as increased use of short term anti-arrhythmic drugs or diuretics after AF ablation may improve short-term morbidity and resource utilization.22
Prior studies have shown that the elderly, women, and possibly those with coronary disease may be at increased risk for adverse events after AF ablation.2,3,19,23 We identified several new patient characteristics associated with adverse events. Mitral and/or aortic valvular disease, hypertension, and diabetes mellitus are all risk factors that have been associated with both an increased risk of AF24–26 and AF-associated ischemic stroke.27 Our finding that Hispanic ethnicity and depression were associated with adverse events suggests that targeted interventions for these groups may be warranted. Depression has been shown to be associated with increased long-term cardiovascular mortality in patients with AF and heart failure.28 Patients with a history of ischemic stroke or transient ischemic attack were at lower risk of events despite a known increased risk of recurrent stroke,27,29 which may reflect greater vigilance with these patients. Black patients have been shown to have lower rates of AF,30 and our findings suggest they may have lower rates of AF recurrence and adverse events after ablation.
Our study has several limitations. The study was conducted within two large health systems in California, so the results may not be generalizable to all populations and practice settings. However, the patients within Kaiser Permanente Northern California and California have been shown to be broadly generalizable to the statewide and national populations.4 Our study was observational in design and used combined clinical and administrative data for the identification of comorbidities and outcomes, which may have led to underestimation of true event rates due to under-reporting of clinical events and misclassification with administrative codes. However, the algorithms used for the identification of comorbid conditions and outcomes have been well validated in prior studies. Detailed information about procedural technique, intra-procedural medications, and AF chronicity (e.g. paroxysmal versus persistent) were unavailable for our assessment of predictors of adverse events, but to our knowledge, our study evaluated the most complete list of risk factors to date in the literature.
In conclusion, in a contemporary, multicenter cohort of patients undergoing AF ablation between 2010 and 2014, the rate of acute inpatient complication was 2.5%, which was approximately half that in older community-based studies. The rate of one-year readmission was also substantially lower than previously reported, but nearly 1 in 2 were evaluated in the emergency department at one year post-ablation, with most related to AF or atrial flutter. Vascular complications and perforation/tamponade remain the most common complications after AF ablation. Hispanic ethnicity, mitral or aortic valvular disease and diabetes mellitus were the strongest risk factors for adverse outcomes at 30 days in our cohort, suggesting that targeted intervention to improve outcomes in these groups may be warranted.
Supplementary Material
Acknowledgments
This study was supported by funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants K23 HL118147-01, 1RC2 HL101589 and U19 HL91179). Dr. Freeman also receives salary support from the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) and modest consulting fees from Janssen Pharmaceuticals. Dr. Go has received a research grant through his institution from iRhythm Technologies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have nothing to disclose.
References
- 1.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 2.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. doi: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC) 2014;2:1049. doi: 10.13063/2327-9214.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Magid DJ, Wells B, Sung SH, Cassidy-Bushrow AE, Greenlee RT, Langer RD, Lieu TA, Margolis KL, Masoudi FA, McNeal CJ, Murata GH, Newton KM, Novotny R, Reynolds K, Roblin DW, Smith DH, Vupputuri S, White RE, Olson J, Rumsfeld JS, Gurwitz JH. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1:138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 7.Prescription Cost Analysis England 2008: Prescription items dispensed in the community in England and listed alphabetically within chemical entity by therapeutic class. NHS Information Centre for Health and Social Care. 2009;1 [Google Scholar]
- 8.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4:233–237. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arellano MG, Petersen GR, Petitti DB, Smith RE. The California Automated Mortality Linkage System (CAMLIS) Am J Public Health. 1984;74:1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 12.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155– 2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 15.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 16.McGrath PD, Wennberg DE, Dickens JD, Jr, Siewers AE, Lucas FL, Malenka DJ, Kellett MA, Jr, Ryan TJ., Jr Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA. 2000;284:3139–3144. doi: 10.1001/jama.284.24.3139. [DOI] [PubMed] [Google Scholar]
- 17.Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, Brindis RG, Krumholz HM. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of medicare patients. J Am Coll Cardiol. 2009;54:903– 907. doi: 10.1016/j.jacc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 18.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baman TS, Jongnarangsin K, Chugh A, Suwanagool A, Guiot A, Madenci A, Walsh S, Ilg KJ, Gupta SK, Latchamsetty R, Bagwe S, Myles JD, Crawford T, Good E, Bogun F, Pelosi F, Jr, Morady F, Oral H. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:626–631. doi: 10.1111/j.1540-8167.2010.01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shurrab M, Di Biase L, Briceno DF, Kaoutskaia A, Haj-Yahia S, Newman D, Lashevsky I, Nakagawa H, Crystal E. Impact of Contact Force Technology on Atrial Fibrillation Ablation: A Meta-Analysis. J Am Heart Assoc. 2015;4:e002476. doi: 10.1161/JAHA.115.002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oza SR, Hunter TD, Biviano AB, Dandamudi G, Herweg B, Patel AM, Pollak SJ, Wang H, Fishel RS. Acute safety of an open-irrigated ablation catheter with 56-hole porous tip for radiofrequency ablation of paroxysmal atrial fibrillation: analysis from 2 observational registry studies. J Cardiovasc Electrophysiol. 2014;25:852–858. doi: 10.1111/jce.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, Bala R, Dixit S, Riley M, Russo AM, Hutchinson MD, Cooper J, Verdino R, Patel V, Joy PS, Gerstenfeld EP. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study) Circulation. 2009;120:1036– 1040. doi: 10.1161/CIRCULATIONAHA.108.839639. [DOI] [PubMed] [Google Scholar]
- 23.Spragg DD, Dalal D, Cheema A, Scherr D, Chilukuri K, Cheng A, Henrikson CA, Marine JE, Berger RD, Dong J, Calkins H. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627–631. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 24.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 25.Grigioni F, Avierinos JF, Ling LH, Scott CG, Bailey KR, Tajik AJ, Frye RL, Enriquez- Sarano M. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40:84–92. doi: 10.1016/s0735-1097(02)01922-8. [DOI] [PubMed] [Google Scholar]
- 26.Diker E, Aydogdu S, Ozdemir M, Kural T, Polat K, Cehreli S, Erdogan A, Goksel S. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am J Cardiol. 1996;77:96–98. doi: 10.1016/s0002-9149(97)89145-x. [DOI] [PubMed] [Google Scholar]
- 27.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 28.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D, Atrial F, Congestive Heart Failure I Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140. doi: 10.1161/CIRCULATIONAHA.109.851675. 133p following 140. [DOI] [PubMed] [Google Scholar]
- 29.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 30.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–441. doi: 10.1001/jamacardio.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


