Abstract
The devastating consequences of alcohol-use disorder (AUD) on the individual and the society are well established. Current treatments of AUD encompass various strategies, all of which have only modest effectiveness. Hence, there is a critical need to develop more efficacious therapies. Recently, specific glutamatergic receptors have been identified as potential novel targets for intervention in AUD. Thus, the current study was designed to evaluate the effects of acute administration of sub-anesthetic doses of ketamine, an NMDA receptor antagonist, as well as NBQX, an AMPA/kainate receptor antagonist on alcohol intake and its possible behavioural consequences. Adult male Wistar rats were trained in drinking in dark paradigm (3 weeks), and following stable alcohol intake, ketamine, NBQX as well as their combination were injected prior to a 90 min drinking session. In addition to alcohol intake, sucrose preference (overnight), and locomotor activity and forced swim test (FST) were also evaluated before and following alcohol intake. Both doses of ketamine (5 and 10 mg/kg) and NBQX (5 and 10 mg/kg) significantly attenuated percent alcohol intake. The combination of the higher dose of ketamine and NBQX, however, did not significantly affect percent alcohol intake. Moreover, animals exposed to alcohol showed decreased sucrose intake (reflective of anhedonia), decreased locomotor activity and swimming in the FST (reflective of helplessness), that were not affected by ketamine and/or NBQX. These results suggest that selective antagonism of the NMDA or AMPA/kainate receptors may be of therapeutic potential in AUD.
Keywords: Alcoholism, Alcohol use disorder, Glutamatergic receptors, NMDA receptor, AMPA receptor, Kainate receptor
Graphical abstract
Introduction
Although various drugs, e.g. disulfiram, naltrexone, and acamprosate that may act as a functional NMDA (N-Methyl-D-Aspartate) receptor antagonist [1,2], or calcium modulator [3] are available in combating alcohol-use disorders (AUD), none has the desired efficacy and/or optimal safety. Thus, there is a critical need to develop efficient AUD medications. Recent findings have highlighted specific central glutamatergic receptors as a potential innovative target.
Glutamate, a major excitatory central neurotransmitter, has a number of functions that are primarily mediated through its interactions with two distinct classes of receptors. The ligand-gated ionotropic receptors that consist of NMDA (N-methyl-D-aspartate), AMPA (alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid) and kainate [4,5], and the metabotropic receptors (mGLUR) that are G-protein coupled and consists of at least 8 different subtypes [6]. The ionotropic receptors, considered “fast” mediators of the glutamate transmission, may play an important role in AUD. Indeed, it is well established that chronic consumption of high alcohol can lead to alcohol-use disorder (AUD), which is associated with hyper-activation of ionotropic glutamatergic receptors [7–10]. Moreover, normalization of the activity of these receptors, including use of the receptor antagonists, can be an effective intervention in AUD and reduction of alcohol intake [7–10].
To further elucidate the involvement of specific glutamatergic ionotropic receptors in acute alcohol intake and on possible behavioural effects of long term alcohol exposure, we investigated the effects of sub-anesthetic doses of ketamine, an NMDA receptor antagonist, and NBQX (2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(F)quinoxaline), an AMPA/kainate receptor antagonist, alone and in combination on alcohol intake and depressive-like behaviour. Thus, we examined whether alcohol intake could be modified by specific ionotropic glutamatergic receptor manipulation and whether our alcohol intake paradigm might also result in depressive like behaviour, as such behavioural effects of alcohol exposure have been reported [11]. Moreover, since ketamine has been shown to possess rapid and lasting antidepressant effects [12–14] we were curious to find out whether our drug treatments would also affect any behavioural effects of alcohol.
Based on previous reports that the antidepressant effects of ketamine could be blocked by NBQX, thus implicating AMPA/kainate receptors in antidepressant effects of ketamine [14], we hypothesized that in our paradigm (i.e., alcohol drinking) the effects of ketamine would also be blocked by NBQX and that NBQX by itself will not have any effect. Our findings, however, did not support these expectations.
Materials and Methods
Animals
Sixty male albino Wistar rats (8 weeks old, with weight range of 250 to 300 g at the beginning of the experiment) were purchased from the Masaryk University breeding facility (Brno, Czech Republic). The rats were housed individually in standard rodent polycarbonate cages. Environmental conditions during the whole study were constant: relative humidity 50–60 %, room temperature 23 ºC ± 1 ºC, inverted 12-hour light-dark cycle (6 a.m. to 6 p.m. darkness). Food and water were available ad libitum with the exception of daily 90 min alcohol drinking sessions when no food was available and the water was substituted for alcohol solution. All procedures were performed in accordance with EU Directive no. 2010/63/EU and approved by the Animal Care Committee of the Faculty of Medicine, Masaryk University, Czech Republic and Czech Governmental Animal Care Committee, in compliance with Czech Animal Protection Act No. 246/1992.
Drugs and Treatments
Ethanol was purchased from a local pharmacy and dissolved by distilled water to desired concentration (10 to 20%). Ketamine (KET) solution was prepared by diluting a ready-made preparation Calypsol® inj. sol. (50 mg in 1 ml, Gedeon Richter, Hungary) with saline to obtain concentration of 5 or 10 mg in 1 ml. NBQX was purchased from Alomone Labs (Jerusalem, Israel) and dissolved in saline to obtain a concentration of 5 or 10 mg in 1 ml. Alcohol was delivered in a standard drinking bottle, ketamine and NBQX were administered intraperitoneally (ip) in a volume of 1 ml/kg (bw).
Alcohol drinking paradigm
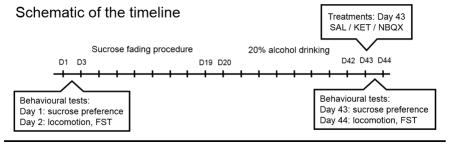
The drinking in dark paradigm with sucrose fading was used as described previously [15]. The daily drinking session started in animal’s home cage (3 hours after lights went off) and lasted 90 minutes. During this time the animal had access to alcohol/sucrose solution only. The sucrose fading procedure consisted of gradual increase of alcohol concentration followed by a decrease of sugar content. Specifically: 10% alcohol and 5% sucrose solution was presented for 3 days, followed by 15% alcohol and 5% sucrose for another 3 days, 20% alcohol and 5% sucrose (4 days), 20% alcohol and 2% sucrose (3 days) and lastly 20% alcohol and 1% sucrose (4 days). This training period lasted for a total of 17 days.
From day 18 onward, for 23 consecutive days the animals were given 20% alcohol only during the 90 min period. On the test day (day 24 of being on 20% alcohol), rats were randomly divided into 6 treatment groups (6–8 animals/group) as follows: saline 1 ml/kg (SAL), ketamine 5 or 10 mg/kg (KET-5 or KET-10), NBQX 5 or 10 mg/kg (NBQX-5 or NBQX-10), and ketamine 10 mg/kg + NBQX 10 mg/kg (KET-NBQX). The injections were made 20 min prior to alcohol session. In combination treatment, NBQX was injected 5 min before ketamine. Ethanol intake was measured by weighting the bottle before and after the drinking session. Alcohol intake was calculated as grams of ethanol per kg of body weight.
The injection time points were based on previous studies showing antidepressant-like effect at the doses used [12,14]. In addition, we report the % of basal alcohol drinking as the amount of alcohol consumed on test day/mean alcohol consumption during 23 days x 100. This measure provides a less variable outcome by factoring out the individual differences that is typically manifested in this paradigm [16].
Behavioural Testing
Sucrose preference test, followed by locomotor activity test and forced swim test (FST) were performed at basal condition, i.e. before initiating alcohol drinking (test 1) as well as following alcohol exposure and drug treatments (test 2) in the same rats. The test battery 2 was performed in the same order as the test battery 1 (see timeline of the experimental procedures below).
A two-bottle choice procedure was used to determine the sucrose preference following initial 24 h adaptation period, during which each rat was provided two water bottles in its home cage. During the tests the animals had access to two bottles, one containing 1% sucrose and the other water only. After the 24 h exposure the amount of liquid consumed was measured and sucrose preference was calculated as the percentage of sucrose solution ingested relative to the total amount of liquid consumed: sucrose/(water + sucrose) x 100 [15,17]. Test 2 was conducted as test 1, starting 2 hours after the last alcohol drinking session.
An automated monitoring system (Actitrack system, Panlab, Spain) was used to measure locomotor activity as described previously [15]. Briefly, each animal was placed individually in the monitoring cage for 5 min and total locomotion was recorded. The arena was wiped with 1% acetic acid to avoid olfactory cues, before testing another animal. Again, test 2 was performed immediately after the sucrose preference test, followed by the forced swim test (FST) described below.
A modified FST [18] was used to measure the swimming activity (mobility) of the rats which consisted of swimming and climbing movements [12,19]. Briefly, each rat was placed into a 50 cm tall and 25 cm wide Plexi-glass cylinder filled with 30 cm of water (24±1°C). The session was video-taped and the swimming which included any active movement (as opposed to immobility) was scored by a blinded researcher.
For clarity of the experimental procedure a schematic of timeline is presented here:
It should be empathized that during the first test the animals were evaluated for their behavioural performance at baseline level and without any alcohol exposure, whereas the second test was following a withdrawal following chronic alcohol exposure. Thus, each group may serve as its own control for these behavioural evaluations.
Statistical analysis
Sucrose preference, locomotor activity and FST data were analyzed by repeated measure ANOVA (factor: drug treatment, repeated factor: day of measurement). For evaluation of alcohol intake, one-way ANOVA followed by Dunnet post-hoc test was employed. The analyses were performed using Statistica 13.2 (StatSoft, USA). A priori p value of less than 0.05 was considered statistically significant in all applied tests. Data is presented as mean ±SEM.
Results
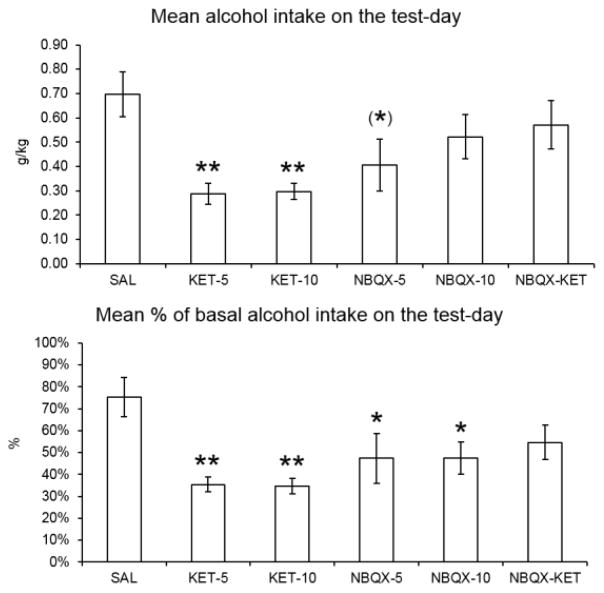
The effect of treatments on alcohol intake is shown in Figure 1. The results are presented as amount of alcohol consumed (g/kg bw) on the test day (Fig 1A) as well as % of basal drinking (Fig 1B). One way ANOVA revealed a significant effect of treatment on alcohol intake (g/kg bw): F(5,37)=3.002, p=0.023, Dunnet post-hoc test showed a significant effect of ketamine at 5 mg/kg dose (KET-5, p=0.007); 10 mg dose (KET-10, p=0.011) and a trend in the NBQX at 5 mg/kg dose (NBQX-5, p=0.069). Identical statistical analysis of the % values also revealed a significant effect of treatment: F(5,37)=3.344, p=0.014 and as expected, post hoc analysis indicated a higher statistically significant effects in both KET and NBQX groups: KET-5 (p=0.003); KET-10 (p=0.003), NBQX-5 (p=0.046) and NBQX-10 (p=0.021). Thus, both ketamine doses resulted in approximately 65% and NBQX (5 mg/kg) in approximately 50% reduction in the alcohol intake (Figs 1A and 1B). The 10 mg/kg NBQX resulted in approximately 30% reduction in alcohol intake that was not statistically significant. However, when comparing the % basal drinking, both doses of ketamine resulted in approximately 55% reduction (p <0.01), and both doses of NBQX resulted in approximately 38% reduction (p<0.05).
Figure 1.
Effects of various treatments on A: alcohol intake (g/kg bw) and B: percent of baseline intake during the 90 min exposure period. Ketamine and NBQX in 5 and 10 mg/kg were administered 20 min prior to alcohol exposure. In combination studies, NBQX was administered 5 min prior to ketamine. Values are mean ± SEM, N=6–8/group. *p<0.05, **p<0.01 compared to control (SAL).
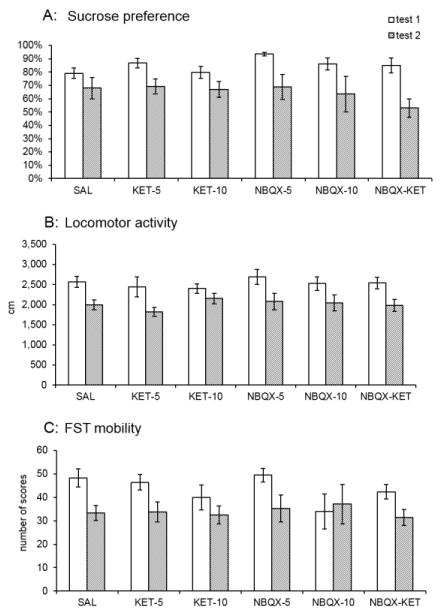
Sucrose preference data is shown in Figure 2A. Repeated measure ANOVA revealed significant effect for the day of measurement: F(1,27)=29.544, p<0.001, i.e., overall the animals consumed less sucrose on the second evaluation (test 2) compared to baseline (test 1). Thus, there was an overall decrease of approximately 20% in sucrose preference following alcohol intake. However, no treatment effect of any drug was observed.
Figure 2.
Effects of various treatments on behaviour at baseline (i.e., before any alcohol exposure) and following alcohol exposure. The behavioural tests were carried out following alcohol withdrawal. Values are mean ± SEM, N=6–8/group. The significant decrease of overall sucrose consumption, locomotor activity and swimming (mobility) scores in the FST in test 2 are clearly visible but not marked in the graph.
Locomotor activity data is shown in Figure 2B. Repeated measure ANOVA revealed significant effect for the day of measurement: F(1,40)=18.887, p<0.001, i.e., overall the animals had reduced locomotor activity on test 2 compared to test 1 by approximately 20%. Here also no treatment effect was observed.
Swimming (mobility) scores in the FST are shown in Figure 2C. Repeated measure ANOVA revealed significant effect for the day of measurement: F(1,39)=31.143, p<0.001, i.e., overall the animals swam less on test 2 compared to test 1. Thus, there was an overall decrease of approximately 25% in swimming score following alcohol intake. Again, no treatment effect was observed.
Discussion
The results of this study indicate that acute administration of low doses of both ketamine and NBQX can reduce ethanol intake in an animal model of alcohol drinking [15,20]. Our ketamine findings are in agreement with a recent report on its effectiveness in reducing alcohol intake in alcohol-preferring (P) rats [21]. Interestingly, we have also observed reduced methamphetamine self-administration by ketamine [22]. Thus, a wider role for glutamatergic receptors in drug addiction may be suggested.
Curiously, the effects of combination of the higher doses of ketamine and NBQX were not any different from NBQX alone. Although, combination of lower doses of NBQX and ketamine might reveal a different (e.g. additive) outcome, the current findings suggest that the interaction of NBQX with ketamine may differ depending on the behavioural effects of ketamine examined. Thus, NBQX may be effective in blocking the antidepressant effect of ketamine [14], but not necessarily the effects of ketamine on alcohol intake, suggesting that the mediators of ketamine effect as an antidepressant may be different from the mediators of its action in reducing alcohol intake. Nonetheless our data does call for more comprehensive dose-response studies involving not only ketamine and NBQX, but also other selective AMPA or kainate receptor antagonist on alcohol intake.
The fact that low dose NBQX by itself could also reduce alcohol intake, strongly suggests that manipulation of glutamatergic receptors, other than NMDA might offer novel therapeutic targets. Such intervention could be of particular importance since the use of ketamine per se in AUD or other drug addiction is likely untenable as ketamine can exhibit reinforcing potential [19] and is capable of producing dissociative sensations and hallucinations in humans [23]. Although these adverse effects of ketamine are associated with relatively high dose, nevertheless they do pose a concern in actual use of ketamine in AUD [21]. Interestingly, a recent report advocates further evaluation of ketamine in alcohol withdrawal, particularly in emergency settings [24].
Our results also indicate that chronic alcohol resulted in depressive-like characteristic, underscored by expression of anhedonia (reduced consumption of sucrose) and helplessness, underscored by reduction in swimming in the FST. These effects were observed after few hours (in case of sucrose preference) or approximately 24 h after last alcohol intake. Thus, the depressive-like characteristics are likely due to withdrawal from alcohol [11]. A recent study by Holleran et al (2016) confirms a delayed depressive-like behaviour following alcohol withdrawal in mice. Interestingly, in this latter study, the effects of alcohol withdrawal in novelty-suppressed feeding test was reversed by ketamine but not memantine, another NMDA antagonist [25]. In our study, however, the behavioural effects induced by alcohol were not affected by ketamine and/or NBQX treatment, possibly due to dosing and/or the delay in assessing the behaviours following the drug treatments. It should be noted that ketamine and NBQX were injected once only in an acute dosing paradigm, so it is unlikely that neuroadaptive changes could occur in the glutamatergic system, which could mitigate their effects. Hence, it would be necessary to evaluate the effects of various doses of agonists and antagonists in a chronic paradigm to elucidate possible lasting and/or neuroadaptive changes.
To our knowledge though, this is the first report of low NBQX dose attenuating voluntary alcohol drinking in the “drinking in the dark” paradigm. Importantly, our data are in accordance with a previous study by Stephens and Brown (1999) who had shown that operant self-administration of ethanol could be impeded by NBQX (AMPA/kainate antagonist) and not by an AMPA only antagonist [26]. Another study by Wang et al (2012) reported that administration of NBQX into the dorsomedial striatum of rats reduced alcohol self-administration in an operant procedure [27]. More recently, effectiveness of a selective kainate antagonist on alcohol preference in rats was also demonstrated [28]. Moreover, both memantine as well as ketamine reduced alcohol intake under a fixed ratio 1 (FR1) schedule in Sardinian alcohol-preferring rats [29]. Hence, it would be prudent that future studies include evaluation of doses-response effects of selective kainate and/or other NMDA receptor subtype antagonists in drug seeking behaviour and particularly in various alcohol intake paradigms.
The importance of glutamatergic manipulation in AUD is further emphasized by recent findings where drugs that upregulate the expression of glutamate transporter in mesocorticolimbic circuit can reduce alcohol intake [9,10]. The mesolimbic and mesocorticolimbic systems are proven to be intimately involved in reward mechanism [7,30]. Thus, by normalizing the hyper-glutamatergic state in these circuits, it may be possible to counteract AUD as seen in genetic animal models of alcoholism [9,10]. Finally, both gender and age-related effects of glutamatergic manipulations in AUD have to be considered, as both these conditions can affect the responses to alcohol and drug treatments [31]. In fact, ketamine was shown to have differential effect on alcohol intake between male and female alcohol preferring (P) rats as the female rats were more sensitive to ketamine’s effect in that they responded with significantly higher reduction in alcohol intake compared to males [21].
In summary, our results indicate that both ketamine and NBQX at low doses reduce alcohol intake. Although none of the treatments affected behavioural consequences associated with alcohol withdrawal, our findings provide further validation of the significance of glutamatergic system manipulation, particularly on AMPA/kainate receptors in countering AUD.
Highlights.
Acute low dose ketamine reduced alcohol intake but not alcohol-related behavioural effects
Acute low dose NBQX reduced alcohol intake but not alcohol-related behavioural effects
NMDA or AMPA/kainate receptor antagonists may have treatment potential in alcohol use disorder
Acknowledgments
Funding
This study, in collaboration with investigators at Howard University in Washington, DC (supported by NIH/NIAAA R03AA022479, YT), was performed at Masaryk University as part of the projects number MUNI/A/1063/2016 and MUNI/A/1132/2017 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic.
The authors are grateful to Marcela Kucirkova and Jaroslav Nadenicek for their expert support in behavioural testing and excellent animal care.
Footnotes
Statement of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Popp RL, Lovinger DM. Interaction of acamprosate with ethanol and spermine on NMDA receptors in primary cultured neurons. Eur J Pharmacol. 2000;394:221–231. doi: 10.1016/s0014-2999(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 2.Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgänsberger W, Schadrack J. The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology. 2001;40:749–760. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 3.Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, Gallop MA, Krstew EV, Lawrence AJ, Kiefer F. Acamprosate Produces Its Anti-Relapse Effects Via Calcium. Neuropsychopharmacology. 2014;39:783–791. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA Receptor GluR-C Subunit in Alcohol-Seeking Behavior and Relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Soto D, Altafaj X, Sindreu C, Bayés A. Glutamate receptor mutations in psychiatric and neurodevelopmental disorders. Commun Integr Biol. 2014;7:e27887. doi: 10.4161/cib.27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao PSS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. doi: 10.3389/fnins.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts-Wolfe DJ, Kalivas PW. Glutamate Transporter GLT-1 as a Therapeutic Target for Substance Use Disorders. CNS Neurol Disord Drug Targets. 2015;14:745–756. doi: 10.2174/1871527314666150529144655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getachew B, Hauser SR, Csoka AB, Taylor RE, Tizabi Y. Role of cortical alpha-2 adrenoceptors in alcohol withdrawal-induced depression and tricyclic antidepressants. Drug Alcohol Depend. 2017;175:133–139. doi: 10.1016/j.drugalcdep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacol Berl. 2013;230:291–8. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketamine Advocacy Group. Provider Directory. [accessed March 16, 2017];Dir US Provid Ketamine Ther Depress Bipolar PTSD Mood Disord. 2015 http://www.ketamineadvocacynetwork.org/provider-directory/
- 14.Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Ruda-Kucerova J, Babinska Z, Amchova P, Stark T, Drago F, Sulcova A, Micale V. Reactivity to addictive drugs in the methylazoxymethanol (MAM) model of schizophrenia in male and female rats. World J Biol Psychiatry. 2017;18:129–142. doi: 10.1080/15622975.2016.1190032. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin FL, Amit Z. Do taste factors contribute to the mediation of ethanol intake? Ethanol and saccharin-quinine intake in three rat strains. Alcohol Clin Exp Res. 1998;22:837–844. [PubMed] [Google Scholar]
- 17.Amchova P, Kucerova J, Giugliano V, Babinska Z, Zanda M, Scherma M, Dusek L, Fadda P, Micale V, Sulcova A, Fratta W, Fattore L. Enhanced self-administration of the CB1 receptor agonist WIN55,212-2 in olfactory bulbectomized rats: evaluation of possible serotonergic and dopaminergic underlying mechanisms. Front Pharmacol. 2014;5:44. doi: 10.3389/fphar.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacol Berl. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 19.Babinska Z, Ruda-Kucerova J. Differential characteristics of ketamine self-administration in the olfactory bulbectomy model of depression in male rats. Exp Clin Psychopharmacol. 2017;25:84–93. doi: 10.1037/pha0000106. [DOI] [PubMed] [Google Scholar]
- 20.Holgate JY, Shariff M, Mu EWH, Bartlett S. A Rat Drinking in the Dark Model for Studying Ethanol and Sucrose Consumption. Front Behav Neurosci. 2017;11:29. doi: 10.3389/fnbeh.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezvani AH, Levin ED, Cauley M, Getachew B, Tizabi Y. Ketamine differentially attenuates alcohol intake in male vs. female alcohol preferring (P) rats. J Drug Alc Res. 2017;2017:1–6. doi: 10.4303/jdar/236030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruda-Kucerova J, Babinska Z, Stark T, Micale V. Suppression of methamphetamine self-administration by ketamine pre-treatment is absent in the methylazoxymethanol (MAM) rat model of schizophrenia. Neurotox Res. 2017;32:121–133. doi: 10.1007/s12640-017-9718-9. [DOI] [PubMed] [Google Scholar]
- 23.Corlett PR, Honey GD, Fletcher PC. Prediction error, ketamine and psychosis: An updated model. J Psychopharmacol Oxf Engl. 2016;30:1145–1155. doi: 10.1177/0269881116650087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long D, Long B, Koyfman A. The emergency medicine management of severe alcohol withdrawal. Am J Emerg Med. 2017 doi: 10.1016/j.ajem.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, Rocco LER, Patel S, Winder DG. Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41:2062–2071. doi: 10.1038/npp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens DN, Brown G. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 1999;23:1914–1920. [PubMed] [Google Scholar]
- 27.Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci Off J Soc Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Nest D, Hernandez NS, Kranzler HR, Pierce RC, Schmidt HD. Effects of LY466195, a selective kainate receptor antagonist, on ethanol preference and drinking in rats. Neurosci Lett. 2017;639:8–12. doi: 10.1016/j.neulet.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabino V, Narayan AR, Zeric T, Steardo L, Cottone P. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. doi: 10.1016/j.bbr.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassareo V, Cucca F, Frau R, Di Chiara G. Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Front Behav Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]