Abstract
CA125 is the best ovarian cancer early detection marker to date; however, sensitivity is limited and complementary markers are required to improve discrimination between ovarian cancer cases and non-cases. Anti-CA125 autoantibodies are observed in circulation. Our objective was to evaluate whether these antibodies (1) can serve as early detection markers, providing evidence of an immune response to a developing tumor, and (2) modify the discriminatory capacity of CA125 by either masking CA125 levels (resulting in lower discrimination) or acting synergistically to improve discrimination between cases and non-cases. We investigated these objectives using a nested case-control study within the European Prospective Investigation into Cancer and Nutrition cohort (EPIC) including 250 cases diagnosed within 4 years of blood collection and up to 4 matched controls. Circulating CA125 antigen and antibody levels were quantified using an electrochemiluminescence assay. Adjusted areas under the curve (aAUCs) by 2-year lag-time intervals were calculated using conditional logistic regression calibrated towards the absolute risk estimates from a pre-existing epidemiological risk model as an offset-variable. Anti-CA125 levels alone did not discriminate cases from controls. For cases diagnosed <2 years after blood collection, discrimination by CA125 antigen was suggestively higher with higher anti-CA125 levels (aAUC, highest antibody tertile: 0.84 [0.76–0.92]; lowest tertile: 0.76 [0.67–0.86]; phet=0.06). We provide the first evidence of potentially synergistic discrimination effects of CA125 and anti-CA125 antibodies in ovarian early detection. If these findings are replicated, evaluating CA125 in the context of its antibody may improve ovarian cancer early detection.
Keywords: ovarian cancer, early detection markers, CA125, anti-CA125 antibodies, MUC16, autoantibodies
Introduction
CA125 (human mucin 16; MUC16) is produced by normal epithelial tissues, as well as epithelial cancers,1 and is the most extensively investigated marker for early detection of ovarian cancer. However, in prospective studies, CA125 only discriminates between ovarian cases and controls in blood samples collected maximally 1–2 years prior to diagnosis, as we recently demonstrated in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort,2 and as has been observed by others.3, 4 Thus, there has been interest in markers that might be complementary to CA125 for ovarian cancer early detection; possible candidates include tumor associated autoantibodies.5 These autoantibodies may be formed as a result of conditions including aberrant or over-expression of their corresponding antigen and tumor-related inflammation,6 and may reach detectable concentrations prior to the antigen themselves.7
Antibodies against CA125 are found in circulation. Autoantibodies to CA125 may serve as early detection markers, or may mask antigen detection by conventional assays, as has been demonstrated for both CA125 and another tumor-associated antigen, CA15.3.8, 9 Our aims were to investigate whether circulating anti-CA125 antibodies (1) can serve as early detection markers, providing evidence of an immune response to a developing tumor, and (2) modify the discriminatory capacity of CA125 by either masking CA125 levels (i.e., resulting in lower discrimination) or acting synergistically to improve discrimination between cases and non-cases. We evaluated these aims in an ongoing ovarian cancer nested case-control study within the EPIC cohort.
Methods
EPIC Cohort
The study protocol for EPIC10 and the full nested case-control study population2 used for this study have been previously described. Briefly, the EPIC cohort was established between 1992 and 2000 in 23 centers in 10 European countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom. More than 520,000 participants (367,903 women) were recruited at study baseline. Study participants completed questionnaires describing diet, reproductive history, menstrual factors, exogenous hormone use, as well as disease history, smoking, and alcohol use. A total of 226,673 women provided a blood sample at or near baseline. Follow-up for cancer outcomes and death is conducted via linkages with cancer and population registries with the exception of centers in Germany, Greece, and Naples, Italy; these centers utilize a combination of active follow-up, next-of-kin, and population registries. The study was approved by the ethics committee of the International Agency for Research on Cancer (IARC; Project 11-01), the University of Heidelberg (S-542/2012), and the ethical committees at the participating centers.
Case and Control Selection
This study included 250 invasive epithelial ovarian cancer (EOC) cases diagnosed within 4 years of blood collection, plus up to 4 matched controls. Cases eligible for this study were diagnosed with incident invasive epithelial ovarian (International Classification of Disease (ICD) code: C569), fallopian tube (C570) or peritoneal cancer (C480, C481, C482, C488) after study baseline blood collection, and had data on tumor histology available from pathology reports or cancer registries. We restricted the study population for this study to cases diagnosed maximally 4 years after blood collection given the focus on early detection.
Controls were alive and cancer-free (except non-melanoma skin cancer) at the index date of their matched case. Controls were matched to cases on: recruitment center, and, at blood collection: age, time of day, fasting status, menopausal status, and current use of exogenous hormones, as well as menstrual cycle phase for premenopausal women, and were selected using incidence density sampling.
Laboratory Assays
CA125 antigen and anti-CA125 antibodies were quantified in serum or plasma in the Brigham and Women’s Hospital Obstetrics and Gynecology Genital Tract Biology Laboratory using electrochemoluminiscence detection (MS600 Reader, Meso Scale Discovery (MSD), Rockville, MD; CA125 antigen (MSD catalog number K151WC) and an anti-CA125 custom assay detecting human IgG1 antibodies to MUC16 purified from a human ovarian cell line (Meridian Life Sciences Inc., Memphis, TN). Anti-CA125 reagents were provided by Fujirebio Diagnostics, Inc. (Malvern, PA). Cases and matched controls were analyzed in the same analytic batch, alongside blinded quality controls; members of the laboratory staff were blinded to the case, control, or quality control status of the samples. The coefficients of variation (CV) for the blinded quality controls with values within the linearity range of each assay were calculated. For CA125, the interplate CV was 19%, the mean intraplate CV was 9%. For anti-CA125 anitbodies, the interplate CV was 35% and the mean intraplate was 14%. To account for variation in anti-CA125 antibody levels by laboratory batch, average batch recalibration was used as proposed by Rosner et al.11
Statistical Analyses
Levels of both CA125 and anti-CA125 antibodies were log transformed to achieve a more normal data distribution, and standardized to mean of 0. Spearman partial correlations, adjusted for matching factors, were used to examine correlations between CA125 and anti-CA125 in cases and controls. Tertiles of the biomarkers were defined in controls. Locally estimated scatterplot smoothing (LOESS) curves were used to describe mean levels of CA125 by anti-CA125 antibody levels in tertiles among cases and controls, by lag-time between blood collection and EOC diagnosis. Adjusted areas under the receiving operator characteristic curve (aAUCs) were calculated using conditional logistic regression models calibrated towards the absolute risk estimates from a pre-existing epidemiological risk model12 as an offset-variable. Discrimination of antigen was evaluated in tertiles of antibody levels, and vice versa. Heterogeneity in the discrimination of one marker by tertiles of the other marker was evaluated using a likelihood ratio test comparing models with and without an interaction term. Statistical analyses were conducted in SAS v. 9.3 (Cary, NC).
Results
The study population was median age 58 years (range: 30–78) at blood collection, and predominantly postmenopausal (72%). Cases were median 60 years (range: 31–79 years) at EOC diagnosis, and the majority were diagnosed with tumors of serous histology (54%) (Table 1).
Table 1.
Baseline characteristics, n (%) or median (range): EPIC ovarian cancer nested case-control study
| Cases n=250 |
Controls n=822 |
|
|---|---|---|
| Age at blood collection, years* | 58 (30–78) | 58 (30–77) |
| Ever full term pregnancy | 186 (79%) | 686 (88%) |
| Ever OC use | 96 (38%) | 386 (47%) |
| Postmenopausal at blood collection* | 181 (72%) | 585 (71%) |
| Ever menopausal hormone therapy use | 67 (27%) | 232 (28%) |
| Age at diagnosis, years | 60 (31–79) | |
| Time between blood collection and diagnosis, years | 1.84 (0–3.99) | |
| Histology | ||
| Serous | 134 (54%) | |
| Endometrioid | 23 (9%) | |
| Mucinous | 32 (13%) | |
| Clear Cell | 6 (2%) | |
| NOS/Other | 55 (22%) | |
| Stage at diagnosis | ||
| Localized (Stage I) | 39 (17%) | |
| Regional/Metastatic (Stage II or higher) | 188 (73%) |
matching factors
Anti-CA125 antibodies did not discriminate between EOC cases and controls, regardless of time between blood collection and diagnosis (e.g., cases diagnosed <6 months after blood collection, aAUC: 0.62 [95% confidence interval: 0.49–0.74]). Results were similar when we examined anti-CA125 antibodies within tertiles of CA125 (e.g., aAUCs cases diagnosed <2 years after blood collection: lowest tertile of CA125, aAUC: 0.53 [0.27–0.79]; highest tertile of CA125, aAUC: 0.59 [0.51–0.66]; phet=0.51).
Levels of CA125 and anti-CA125 antibodies were not correlated in controls (Spearman r=−0.02). Correlations in cases differed by time between blood collection and diagnosis. We observed an inverse association between CA125 and anti-CA125 antibodies among cases diagnosed <1 year after blood collection and a weak positive correlation among cases diagnosed 1–4 years after blood collection (Table 2). The inverse association observed among cases with blood collection proximate to diagnosis is visualized on the LOESS plot (Figure 1a) showing that among cases diagnosed 0–1 year following blood collection lowest CA125 levels were observed in women with relatively high antibody levels (tertile 3, red line). CA125 levels among controls were consistent across all anti-CA125 tertiles (data not shown). Among women diagnosed within 2 years of blood collection, CA125 discrimination was strongest among women with relatively high anti-CA125 antibody levels (highest tertile of antibody: aAUC: 0.84 [0.76–0.92]) and lowest among women with low antibody levels (lowest tertile of antibody: aAUC 0.76 [0.67–0.86]; phet=0.06); Figure 1b). In a sensitivity analysis excluding women diagnosed <6 months prior to blood collection, aAUCs for CA125 were similar among women with high antibody levels (highest tertile: aAUC: 0.83 [0.74–0.92]), but somewhat lower among women with low antibody levels (aAUC: 0.71 [0.60–0.82]; phet=0.15).
Table 2.
Spearman partial* correlations between CA125 and anti-CA125 antibody levels in cases, by lag-time, and controls: EPIC ovarian cancer nested case-control study
| Time between blood collection and diagnosis |
Spearman r | |
|---|---|---|
| Cases | <0.5 year | −0.35 |
| <1 year | −0.22 | |
| 1–2 years | 0.24 | |
| 2–4 years | 0.12 | |
| Controls | n/a | −0.02 |
Adjusted for matching factors
Figure 1.
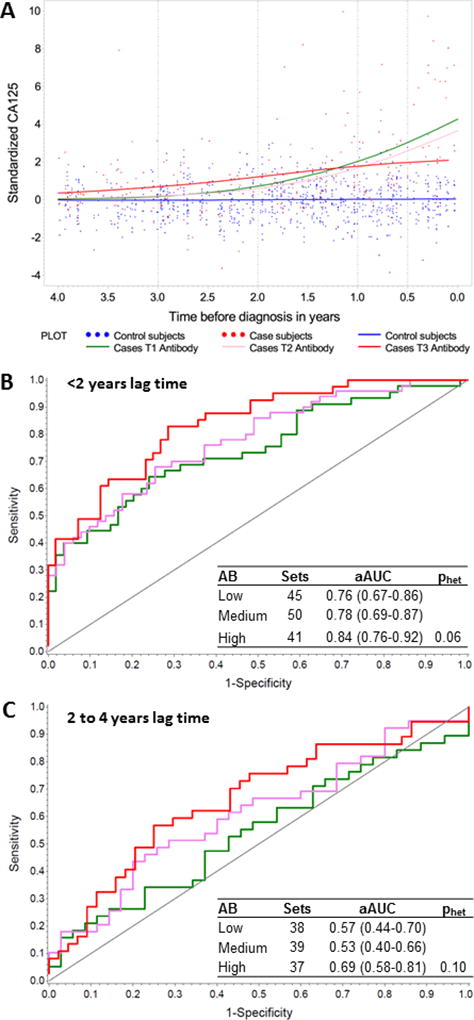
(a) LOESS plot showing distribution of log-transformed CA125 among controls [blue] and, among cases, with cases classified in tertiles of anti-CA125 antibody levels. Tertiles of antibody level are represented by green (tertile 1; lowest), pink (tertile 2), and red (tertile 3; highest); (b) aAUC for CA125 by antibody tertiles for cases diagnosed within 2 years of blood collection (c) and between 2 and 4 years of blood collection. Phet represents the heterogeneity between aAUCs in the first and third tertiles.
We observed suggestive differences in discrimination with longer time between blood collection and diagnosis (e.g., 2 to 4 years between blood collection and diagnosis, lowest tertile of antibody, aAUC: 0.57 [0.44–0.70]; highest tertile of antibody, aAUC: 0.69 [0.58–0.81]; phet=0.10; Figure 1c). Results were similar among cases with regional or metastatic disease (Stage II or higher) at diagnosis (data not shown); case numbers precluded an investigation among cases with localized disease (Stage I) at diagnosis (n=39).
Discussion
Tumor associated autoantibodies may be produced as an anti-tumor immune response to tumor-associated antigen (over)expression, mutations, and/or the products of post-translational processing, as well as the well-described inflammatory milieu of the tumor microenvironment.6 These antibodies are attractive as diagnostic biomarkers as generally they circulate at higher levels than their corresponding antigen, demonstrate higher stability over time,13, 14 and may be detectable at an earlier disease stage.7
In this exploratory study, we provide the first evidence that when circulating CA125 levels are examined in the context of anti-CA125 antibodies, their discriminatory ability for the early detection of EOC may be improved, with higher aAUCs for CA125 observed among women with higher anti-CA125 antibody levels. Among cases with short lag-time between blood collection and diagnosis, we observed lower circulating CA125 levels among women with higher anti-CA125 antibody levels, consistent with the hypothesis that higher antibody levels may mask detection of circulating antigen. Circulating immune complexes (CIC) to CA125 have been identified, and potential interference with conventional assays for CA125 demonstrated previously.8 This has also been observed for another mucin, MUC1 (CA15.3), with lower levels of circulating CA15.3 observed among ovarian cancer cases with the highest levels of MUC1 circulating immune complexes.9 In the current study, we evaluated IgG1 antibodies to CA125. Future studies should consider CA125/anti-CA125 CICs, and other immunoglobin isotypes such as IgM.
By themselves, anti-CA125 antibodies provided no discrimination in early detection models. This is consistent with one prior investigation in which CA125-IgM levels did not discriminate between prevalent cases and cancer-free controls.15 In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) study, antibodies against epitopes of another mucin MUC1, were evaluated with respect to OC early detection in samples taken on average 1 year before diagnosis.16 As independent markers, none of the anti-MUC1 antibodies predicted OC in the UKCTOCS; antibodies were not examined in the context of antigen levels.
We demonstrate that for CA125, the combination of antigen and antibody may offer better discrimination for OC early detection than CA125 alone. While novel, this investigation was limited by relatively small case numbers in each tertile of antibody level (e.g., <2 years between blood collection and diagnosis, n=41–50 cases per tertile). Additional investigations in prospective settings are required to confirm these findings.
Novelty and Impact.
In this prospective investigation using pre-diagnosis blood samples, anti-CA125 levels did not discriminate between ovarian cases and controls. However, discrimination of CA125 differed by levels of its antibody, with the highest discrimination among women with the highest antibody levels. CA125 and anti-CA125 may act synergistically for ovarian cancer early detection. If these findings are replicated, evaluating CA125 in the context of its antibody may improve ovarian cancer early detection.
Acknowledgments
This study was funded by the United States National Cancer Institute grant R01 CA158119.
The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC-2009-AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), PI13/00061 to Granada;, PI13/01162 to EPIC-Murcia), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Abbreviations
- aAUC
adjusted areas under the receiver operating characteristic curve
- CA125
cancer antigen 125
- CA15.3
cancer antigen 15.3
- CI
confidence interval
- CV
coefficient of variation
- EOC
epithelial ovarian cancer
- EPIC
European Prospective Investigation into Cancer and Nutrition cohort
- IARC
International Agency for Research on Cancer
- MSD
Meso Scale Discovery
- MUC1
human mucin 1
- MUC16
human mucin 16
- UKCTOCS
United Kingdom Collaborative Trial of Ovarian Cancer Screening
Footnotes
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php
References
- 1.Haridas D, Ponnusamy MP, Chugh S, Lakshmanan I, Seshacharyulu P, Batra SK. MUC16: molecular analysis and its functional implications in benign and malignant conditions. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:4183–99. doi: 10.1096/fj.14-257352. [DOI] [PubMed] [Google Scholar]
- 2.Terry KL, Schock H, Fortner RT, Husing A, Fichorova RN, Yamamoto HS, Vitonis AF, Johnson T, Overvad K, Tjonneland A, Boutron-Ruault MC, Mesrine S, et al. A Prospective Evaluation of Early Detection Biomarkers for Ovarian Cancer in the European EPIC Cohort. Clin Cancer Res. 2016;22:4664–75. doi: 10.1158/1078-0432.CCR-16-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD, Bergan L, Thornquist MD, Scholler N, Kim N, O'Briant K, Drescher C, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G, Patriotis C, Pinsky PF, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer prevention research. 2011;4:365–74. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi JX, Qin JJ, Ye H, Wang P, Wang KJ, Zhang JY. Tumor associated antigens or anti-TAA autoantibodies as biomarkers in the diagnosis of ovarian cancer: a systematic review with meta-analysis. Expert Rev Mol Diagn. 2015;15:829–52. doi: 10.1586/14737159.2015.1035713. [DOI] [PubMed] [Google Scholar]
- 6.Zaenker P, Gray ES, Ziman MR. Autoantibody Production in Cancer--The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmunity reviews. 2016;15:477–83. doi: 10.1016/j.autrev.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Zaenker P, Ziman MR. Serologic autoantibodies as diagnostic cancer biomarkers--a review. Cancer Epidemiol Biomarkers Prev. 2013;22:2161–81. doi: 10.1158/1055-9965.EPI-13-0621. [DOI] [PubMed] [Google Scholar]
- 8.Cramer DW, O'Rourke DJ, Vitonis AF, Matulonis UA, Dijohnson DA, Sluss PM, Crum CP, Liu BC. CA125 immune complexes in ovarian cancer patients with low CA125 concentrations. Clin Chem. 2010;56:1889–92. doi: 10.1373/clinchem.2010.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourevitch MM, von Mensdorff-Pouilly S, Litvinov SV, Kenemans P, van Kamp GJ, Verstraeten AA, Hilgers J. Polymorphic epithelial mucin (MUC-1)-containing circulating immune complexes in carcinoma patients. British Journal of Cancer. 1995;72:934–8. doi: 10.1038/bjc.1995.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 11.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American Journal of Epidemiology. 2008;167:653–66. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Husing A, Fortner RT, Tjonneland A, Hansen L, Dossus L, Chang-Claude J, Bergmann M, Steffen A, Bamia C, Trichopoulos D, Trichopoulou A, et al. An epidemiologic risk prediction model for ovarian cancer in Europe: the EPIC study. British Journal of Cancer. 2015;112(Suppl):1257–65. doi: 10.1038/bjc.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. Journal of proteome research. 2008;7:1388–94. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–33. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandiera E, Zanotti L, Fabricio AS, Bucca E, Squarcina E, Romani C, Tassi R, Bignotti E, Todeschini P, Tognon G, Romagnolo C, Gion M, et al. Cancer antigen 125, human epididymis 4, kallikrein 6, osteopontin and soluble mesothelin-related peptide immunocomplexed with immunoglobulin M in epithelial ovarian cancer diagnosis. Clin Chem Lab Med. 2013;51:1815–24. doi: 10.1515/cclm-2013-0151. [DOI] [PubMed] [Google Scholar]
- 16.Burford B, Gentry-Maharaj A, Graham R, Allen D, Pedersen JW, Nudelman AS, Blixt O, Fourkala EO, Bueti D, Dawnay A, Ford J, Desai R, et al. Autoantibodies to MUC1 glycopeptides cannot be used as a screening assay for early detection of breast, ovarian, lung or pancreatic cancer. Br J Cancer. 2013;108:2045–55. doi: 10.1038/bjc.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]


