Abstract
Childhood obesity predicts adult cardiovascular risk. We hypothesized that the association between childhood body mass index (BMI) and adult carotid intima-media thickness (CIMT) may be modified by levels of adiponectin, an adipocytokine that connects body fatness with cardiovascular risk. The study sample included 1,052 adults (71% white and 29% black, 57% female) aged 23.8 to 43.5 years who were previously examined as children in the Bogalusa Heart Study cohort, with an average follow-up period of 26.5 (range 14.1 to 29.6) years. Childhood BMI, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and systolic blood pressure were standardized to age-specific z-scores. General linear models were used for data analyses. Childhood BMI (p = 0.034), low-density lipoprotein cholesterol (p <0.001), and systolic blood pressure (p = 0.005), along with adult adiponectin levels (p = 0.002) were associated with adult CIMT, adjusted for race, sex, adult age, and cigarette smoking. Further, adult adiponectin levels significantly modified the association between childhood BMI and adult CIMT (P for interaction = 0.0003) such that a significant association between childhood BMI and adult CIMT (p <0.0001) was only observed in those with adiponectin levels below the median. In conclusion, these results suggest that serum adiponectin levels modify the association between childhood obesity and adult atherosclerosis, which has implications for risk stratification and targeted intervention for obese children with low levels of adiponectin.
Childhood obesity is predictive of adult atherosclerotic diseases.1–3 Carotid intima-media thickness (CIMT), measured noninvasively by ultrasound, is a surrogate marker of atherosclerosis and is predictive of future atherosclerotic events.4 Accumulating evidence indicates that the consequences of childhood obesity are heterogeneous.5–8 Previously we reported that overweight children without accompanying cardiometabolic abnormalities tended to have CIMT in adult life similar to that seen in normal weight children and lower than what is observed in overweight children with cardiometabolic risk factors.5 However, the underlying mechanisms for the observed heterogeneity remain uncertain. Adiponectin is an adipocytokine secreted by adipocytes,9,10 and its levels in the blood are inversely associated with obesity, inflammation, insulin resistance, and CIMT.11–13 Whether adiponectin levels underlie the observed heterogeneity in the association between childhood obesity and CIMT in adult life is unknown. In the current study, this question was examined in the longitudinal cohort of the Bogalusa Heart Study.
Methods
The Bogalusa Heart Study is a long-term investigation of the natural history of cardiovascular disease conducted in a black-white (65% whites and 35% blacks) community in Bogalusa, Louisiana, begun in 1973 by Dr. Gerald S. Berenson. Nine cross-sectional surveys of children and adolescents aged 4 to 19 years and 11 cross-sectional surveys of adults aged 20 to 52 years who were examined during childhood and remained accessible were conducted in Bogalusa, Louisiana between 1973 and 2010. Linking these cross-sectional studies results in a longitudinal cohort with repeated measurements. Among the 1,203 participants who had their last examination in 2010, 1,052 adults (747 whites and 305 blacks; age range 23.8 to 43.5 years) had at least 1 examination in childhood and 1 in adulthood without missing values for any of the study variables. For those with more than 1 examination during childhood, the earliest value was used. The average follow-up is 26.5 years (range: 14.1 to 29.6 years).
All participants or their legal guardians in this study gave informed consent at each examination. Study protocols were approved by the Institutional Review Board of the Tulane University Health Science Center.
All surveys have followed the same standardized protocol since 1973. Study participants were instructed to fast for 12 hours before screening. Replicate measurements of weight and height were made, and the mean values were used to calculate body mass index (BMI, weight in kilograms divided by the square of height in meters).
Blood pressure levels were measured using a mercury sphygmomanometer on the right arm of participants in a relaxed, sitting position. Arm length and circumference were measured to ensure use of the proper cuff size. Blood pressure levels were reported as the mean of 6 replicate readings. The measurements were conducted by 2 trained and randomly assigned observers.
Serum lipoprotein cholesterols and triglycerides were analyzed using the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN) which employs a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures. The laboratory utilized is monitored for precision and accuracy by the Lipid Standardization and Surveillance Program of the Centers for Disease Control and Prevention, Atlanta, Georgia. Serum adiponectin levels were measured using a commercial radioimmunoassay kit (Linco Research, St Charles, Missouri). On the basis of blind duplicate determinations on ~10% of the study samples, the intraclass correlation coefficient of reliability was 0.93 for serum adiponectin levels.
Ultrasound examinations were performed using a Toshiba Sonolayer SSH160A (Toshiba Medical, Tokyo, Japan), a 7.5 MHz liner array transducer on participants in the supine position with their head slightly extended and turned in the opposite direction of the carotid artery being studied. Images were recorded at the common carotid, carotid bulb (bifurcation), and internal carotid arteries bilaterally according to previously developed protocols for the Atherosclerosis Risk in Communities Study. Images were recorded on S-VHS tapes and read by certified readers from the Division of Vascular Ultrasound Research, Wake Forest University School of Medicine using a semiautomatic ultrasound image processing program developed by the California Institute of Technology Jet Propulsion Laboratory (Pasadena, California) according to standardized protocols. The mean of the maximum readings of 3 right and 3 left far walls for common, bulb, and internal segments was used to estimate CIMT.
Data analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). General linear models were used to estimate the difference in continuous variables between whites and blacks and between males and females after adjusting for age. The differences in categorical variables were tested by means of a chi-square test.
CIMT, triglyceride levels, and adiponectin levels were log-transformed to approximate a normal distribution. The association between childhood risk factors and adult CIMT were examined by using general linear models, adjusting for race, sex, adult age, smoking status, and adult adiponectin levels. Childhood BMI, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, and systolic blood pressure (SBP) were standardized to age-specified z-scores. The modification effect of adiponectin levels on the relationship between childhood BMI and adulthood CIMT was examined by including the interaction term between childhood BMI and adult adiponectin levels (at or above the median vs below the median that was adjusted for age, race, gender, and BMI). To illustrate the interaction effect, we depicted adult CIMT (least square means) by quartile of childhood BMI in the group with high and low adiponectin levels (above and below the median), separately, adjusted for the same covariates. To minimize the influence of different follow-up times in the total sample (14.1 to 29.6 years), we performed sensitivity analysis by including follow-up time as an additional covariate.
Results
In childhood, black girls had higher levels of HDL-C than white girls, and white girls had higher levels of LDL-C than white boys; white girls had higher levels of triglycerides than white boys and black girls (Table 1). In adulthood, white women had lower BMI than black women; men had higher levels of LDL-C, SBP, diastolic blood pressure, and triglycerides, and lower levels of HDL-C (only in whites) and adiponectin than women; blacks tended to have lower levels of LDL-C, triglycerides, and adiponectin, and higher levels of HDL-C and blood pressure than whites (Table 1). The mean CIMT measurements were larger for blacks compared with whites and for men compared with women (Table 1).
Table 1.
Characteristics of study participants by race and sex
| Variable | White | Black | p for Race Difference | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Males (N = 338) | Females (N = 409) | Males (N = 117) | Females (N = 188) | Males (N = 455) | Females (N = 597) | |
| Childhood (4–18 years) | ||||||
| Age (years) | 10.0 ± 3.3 | 9.9 ± 3.4 | 9.8 ± 3.0 | 9.5 ± 2.9 | 0.499 | 0.185 |
| BMI (kg/m2) | 17.6 ± 3.2 | 17.8 ± 3.7 | 17.6 ± 3.7 | 17.6 ± 3.9 | 0.694 | 0.494 |
| LDL cholesterol (mg/dL) | 88.2 ± 24.2 | 91.9 ± 25.2* | 89.9 ± 28.3 | 92.9 ± 23.9 | 0.974 | 0.947 |
| HDL cholesterol (mg/dL) | 65.1 ± 21.5 | 62.5 ± 21.1 | 68.0 ± 20.6 | 70.1 ± 20.3 | 0.143 | <0.001 |
| Triglycerides (mg/dL) | 70.0 ± 40.1 | 77.4 ± 37.5** | 64.2 ± 28.8 | 61.4 ± 22.3 | 0.158 | <0.001 |
| Systolic BP (mmHg) | 100.8 ± 9.8 | 99.5 ± 10.1 | 99.6 ± 12.2 | 98.4 ± 10.3 | 0.226 | 0.327 |
| Diastolic BP (mmHg) | 61.7 ± 8.2 | 61.9 ± 8.6 | 62.3 ± 8.6 | 60.9 ± 9.1 | 0.248 | 0.640 |
| Adulthood (19–52 years) | ||||||
| Age (years) | 36.6 ± 4.4 | 36.4 ± 4.3 | 36.8 ± 4.3 | 35.6 ± 4.9* | 0.721 | 0.036 |
| Number of Smokers | 99 (29.3%) | 114 (27.9%) | 41 (35.0%) | 54 (28.7%) | 0.245 | 0.830 |
| BMI (kg/m2) | 28.9 ± 5.5 | 28.2 ± 7.1 | 29.2 ± 6.9 | 30.9 ± 8.1 | 0.627 | <0.001 |
| LDL cholesterol (mg/dL) | 129.9 ± 34.5 | 124.1 ± 32.6* | 124.4 ± 41.6 | 114.1 ± 30.3* | 0.161 | <0.001 |
| HDL cholesterol (mg/dL) | 41.2 ± 12.1 | 50.6 ± 13.0** | 49.4 ± 15.9 | 52.2 ± 13.5 | <0.001 | 0.092 |
| Triglycerides (mg/dL) | 164.5 ± 129.8 | 122.0 ± 70.1** | 129.0 ± 108.0 | 88.8 ± 40.4** | <0.001 | <0.001 |
| Systolic BP (mmHg) | 118.0 ± 10.9 | 111.0 ± 11.1** | 128.5 ± 16.5 | 119.0 ± 16.0** | <0.001 | <0.001 |
| Diastolic BP (mmHg) | 80.2 ± 7.8 | 75.1 ± 8.3** | 86.8 ± 12.3 | 79.7 ± 11.2** | <0.001 | <0.001 |
| Adiponectin (µg/ml) | 7.5 ± 3.6 | 10.4 ± 4.6** | 6.9 ± 5.6 | 8.4 ± 4.4** | 0.003 | <0.001 |
| Carotid IMT(mm) | 0.86 ± 0.18 | 0.76 ± 0.12** | 0.90 ± 0.21 | 0.80 ± 0.16** | 0.006 | <0.001 |
Mean ± SD is presented unless otherwise stated.
p Values for continuous variables were adjusted for age.
Sex difference:
p < 0.05;
p < 0.01.
BMI = body mass index; LDL = low density lipoprotein; HDL = high density lipoprotein; BP = blood pressure; IMT = intima-media thickness.
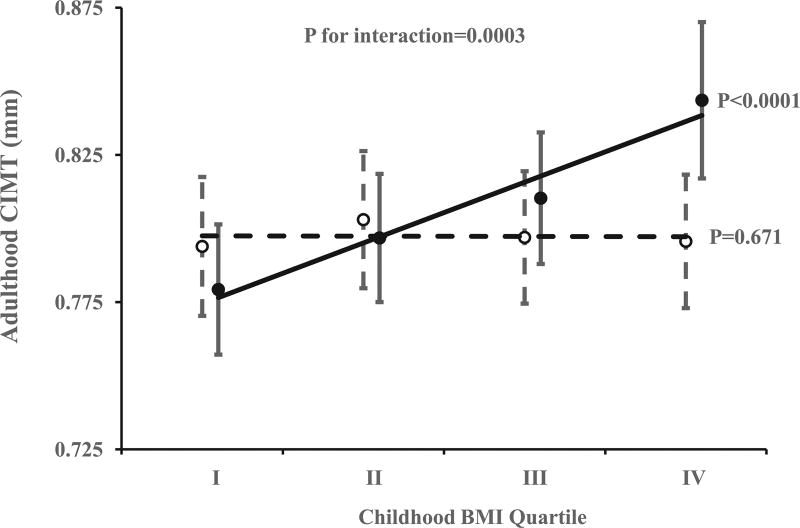
Significant childhood predictors of adult CIMT included BMI, LDL-C, and SBP; adiponectin levels in adulthood were also significantly associated with adult CIMT (Table 2). Significant interaction between childhood BMI and adult adiponectin levels on adult CIMT was identified. The association between childhood BMI and adult CIMT was only significant in the low adiponectin group (p <0.0001), but not in the high adiponectin group (p = 0.671; Figure 1). The results were similar after additional adjustment for duration of follow-up time.
Table 2.
Regression of adult carotid intima-media thickness (log-transformed) on childhood risk factor variables (n = 1052; follow up time: 26.5 years)
| Variable | β | SE | p |
|---|---|---|---|
| Adult age (year) | 0.013 | 0.001 | <0.0001 |
| Black race | 0.044 | 0.011 | 0.0001 |
| Female sex | −0.091 | 0.011 | <0.0001 |
| Adult Smoking (yes) | 0.040 | 0.011 | 0.0003 |
| Childhood BMI | 0.011 | 0.005 | 0.034 |
| Childhood LDL cholesterol | 0.024 | 0.006 | <0.0001 |
| Childhood HDL cholesterol | 0.001 | 0.005 | 0.864 |
| Childhood triglycerides | −0.006 | 0.006 | 0.292 |
| Childhood systolic BP | 0.015 | 0.005 | 0.005 |
| Adiponectin (log-transformed) | −0.033 | 0.011 | 0.002 |
Childhood risk factor variables were standardized to age-specific Z-scores.
β = regression coefficient; SE = standard error; BMI = body mass index; LDL = low density lipoprotein; HDL = high density lipoprotein; BP = blood pressure; IMT = intima-media thickness.
Figure 1.
Adult carotid intima-media thickness (CIMT) in high adiponectin level group (open mark with dashed trend line) and in low adiponectin level group (closed mark with solid trend line) by childhood BMI quartile. Error bars show 95% confidence interval. p Values were adjusted for adult age, race, gender, cigarette smoking, and adiponectin levels.
Discussion
The current study demonstrated that childhood BMI was predictive of subclinical atherosclerosis in adulthood, and lower adulthood adiponectin levels were associated with increased CIMT. Further, adulthood adiponectin levels modified the association between childhood obesity and CIMT, suggesting that only in the presence of low levels of adult adiponectin was childhood BMI associated with adult CIMT.
As expected, childhood BMI, LDL-C, and SBP were all significantly associated with adult CIMT. These findings are consistent with earlier observations by us and others,1,3 and emphasize the importance of childhood risk factors for the development of atherosclerosis from childhood to adulthood.
A novel finding of the current study is that adult adiponectin levels modified the association between childhood BMI and adult CIMT. We have shown that the association between childhood overweight/obesity and adulthood CIMT is heterogeneous.5 Childhood overweight without elevated levels of LDL-C, triglycerides, blood pressure, and subdued levels of HDL-C did not have increased CIMT compared with those who had normal weight during childhood regardless of cardiometabolic profiles.5 However, the underlying mechanisms for the observed heterogeneity remain largely unexplored. Findings in the current study suggest that adiponectin levels may be an important intermediary for the observed heterogeneity between childhood overweight/obesity and adult CIMT. A recent study by Saarikoski et al. showed that childhood adiponectin levels were inversely associated with adult CIMT, consistent with a protective effect for higher adiponectin levels; however, the study did not examine the interaction between childhood BMI and adiponectin levels on adult CIMT.14
Adiponectin is an adipocytokine that plays a central role in the development of insulin resistance and inflammation.15,16 Adiponectin has an anti-inflammatory effect through actions on monocytes, natural killer cells and T and B lymphocytes, NF-κB, and interaction with tumor necrosis factor-alpha.17 It also inhibits macrophage-to-foam cell transformation and the proliferation of vascular smooth muscle cells.18 Through increased insulin resistance and inflammation, low adiponectin levels may promote development of endothelial dysfunction and thus increased endothelial damage in the vascular system. The current study provides novel evidence that adiponectin appears to play an important role in cardiometabolic health associated with obesity.
An accumulating body of evidence suggests that not all obesity is associated with cardiometabolic abnormality.5,19,20 Obesity without accompanying cardiometabolic abnormalities has been termed as “metabolically health obesity.” However, contributing factors for the metabolically health obesity phenotype are not very clear. Our study is consistent with previous observations that adiponectin and macrophage infiltration in the adipose tissue determines the cardiometabolic health associated with obesity.21,22 Many other factors, such as genetic profile, may play a role in obesity-related cardiometabolic disorders.23,24 Further studies are needed to explore additional contributing factors for obesity-associated cardiometabolic health consequences, which should have implications for prevention and treatment of obesity.
In conclusion, the current study demonstrates that childhood risk factors, including BMI, LDL-C, and SBP, are predictive of adult CIMT, underscoring the importance of addressing childhood cardiometabolic risk factors. Further, it suggests that adiponectin levels modify the association between childhood BMI and adult CIMT, indicating a potentially important role for adiponectin in the development of atherosclerosis from childhood to adulthood. Additional studies are needed to explore other contributing factors for the cardiometabolic health consequences of obesity.
Acknowledgments
We would like to acknowledge funding support by grants R01ES021724 from the National Institute of Environmental Health Sciences, R01AG016592 from the National Institute on Aging, and the contributions from the Bogalusa Heart Study volunteers in the current investigation. Shengxu Li is partly supported by grant 13SDG14650068 from American Heart Association and by grant P20GM109036 from the National Institute of General Medical Sciences.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and adult cardiovascular disease risk: a systematic review. Int J Obes (Lond) 2010;34:18–28. doi: 10.1038/ijo.2009.61. [DOI] [PubMed] [Google Scholar]
- 3.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter M, Sinclair H, Kunadian V. Carotid intima media thickness and its utility as a predictor of cardiovascular disease: a review of evidence. Cardiol Rev. 2016;24:70–75. doi: 10.1097/CRD.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Chen W, Srinivasan SR, Xu J, Berenson GS. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol. 2012;176(suppl 7):S142–S149. doi: 10.1093/aje/kws236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Soldatovic I, Sipetic-Grujicic S, Zdravkovic D. Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr. 2013;172:201–206. doi: 10.1007/s00431-012-1867-5. [DOI] [PubMed] [Google Scholar]
- 7.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of metabolically healthy obesity in children. Diabetes Care. 2014;37:1462–1468. doi: 10.2337/dc13-1697. [DOI] [PubMed] [Google Scholar]
- 8.Senechal M, Wicklow B, Wittmeier K, Hay J, MacIntosh AC, Eskicioglu P, Venugopal N, McGavock JM. Cardiorespiratory fitness and adiposity in metabolically healthy overweight and obese youth. Pediatrics. 2013;132:e85–e92. doi: 10.1542/peds.2013-0296. [DOI] [PubMed] [Google Scholar]
- 9.Liberale L, Bonaventura A, Vecchie A, Matteo C, Dallegri F, Montecucco F, Carbone F. The role of adipocytokines in coronary atherosclerosis. Curr Atheroscler Rep. 2017;19:10. doi: 10.1007/s11883-017-0644-3. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi N. Adipocytokines in cardiovascular and metabolic diseases. J Atheroscler Thromb. 2016;23:645–654. doi: 10.5551/jat.34918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue cross-talk. Cell Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92:3025–3032. doi: 10.1210/jc.2007-0619. [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola JM, Pahkala K, Viitala M, Ronnemaa T, Viikari J, Niinikoski H, Lagstrom H, Jula A, Simell O, Raitakari O. Association of adiponectin with adolescent cardiovascular health in a dietary intervention study. J Pediatr. 2015;167:353–360. e351. doi: 10.1016/j.jpeds.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 14.Saarikoski LA, Juonala M, Huupponen R, Viikari JS, Lehtimaki T, Jokinen E, Hutri-Kahonen N, Taittonen L, Laitinen T, Raitakari OT. Low serum adiponectin levels in childhood and adolescence predict increased intima-media thickness in adulthood. The Cardiovascular Risk in Young Finns Study. Ann Med. 2017;49:42–50. doi: 10.1080/07853890.2016.1226513. [DOI] [PubMed] [Google Scholar]
- 15.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Fu Z, Liu Z. Adiponectin and insulin cross talk: the microvascular connection. Trends Cardiovasc Med. 2014;24:319–324. doi: 10.1016/j.tcm.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15:221. doi: 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 19.Ferrannini E, Pilo A. Pattern of insulin delivery after intravenous glucose injection in man and its relation to plasma glucose disappearance. J Clin Invest. 1979;64:243–254. doi: 10.1172/JCI109445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo F, Garvey WT. Cardiometabolic disease risk in metabolically healthy and unhealthy obesity: stability of metabolic health status in adults. Obesity (Silver Spring) 2016;24:516–525. doi: 10.1002/oby.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 22.Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Insulin-sensitive obesity in humans—a ‘favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–124. doi: 10.1016/j.tem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Kilpelainen TO, Zillikens MC, Stancakova A, Finucane FM, Ried JS, Langenberg C, Zhang W, Beckmann JS, Luan J, Vandenput L, Styrkarsdottir U, Zhou Y, Smith AV, Zhao JH, Amin N, Vedantam S, Shin SY, Haritunians T, Fu M, Feitosa MF, Kumari M, Halldorsson BV, Tikkanen E, Mangino M, Hayward C, Song C, Arnold AM, Aulchenko YS, Oostra BA, Campbell H, Cupples LA, Davis KE, Doring A, Eiriksdottir G, Estrada K, Fernandez-Real JM, Garcia M, Gieger C, Glazer NL, Guiducci C, Hofman A, Humphries SE, Isomaa B, Jacobs LC, Jula A, Karasik D, Karlsson MK, Khaw KT, Kim LJ, Kivimaki M, Klopp N, Kuhnel B, Kuusisto J, Liu Y, Ljunggren O, Lorentzon M, Luben RN, McKnight B, Mellstrom D, Mitchell BD, Mooser V, Moreno JM, Mannisto S, O’Connell JR, Pascoe L, Peltonen L, Peral B, Perola M, Psaty BM, Salomaa V, Savage DB, Semple RK, Skaric-Juric T, Sigurdsson G, Song KS, Spector TD, Syvanen AC, Talmud PJ, Thorleifsson G, Thorsteinsdottir U, Uitterlinden AG, van Duijn CM, Vidal-Puig A, Wild SH, Wright AF, Clegg DJ, Schadt E, Wilson JF, Rudan I, Ripatti S, Borecki IB, Shuldiner AR, Ingelsson E, Jansson JO, Kaplan RC, Gudnason V, Harris TB, Groop L, Kiel DP, Rivadeneira F, Walker M, Barroso I, Vollenweider P, Waeber G, Chambers JC, Kooner JS, Soranzo N, Hirschhorn JN, Stefansson K, Wichmann HE, Ohlsson C, O’Rahilly S, Wareham NJ, Speliotes EK, Fox CS, Laakso M, Loos RJ. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet. 2011;43:753–760. doi: 10.1038/ng.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Day FR, Gustafsson S, Buchkovich ML, Na J, Bataille V, Cousminer DL, Dastani Z, Drong AW, Esko T, Evans DM, Falchi M, Feitosa MF, Ferreira T, Hedman AK, Haring R, Hysi PG, Iles MM, Justice AE, Kanoni S, Lagou V, Li R, Li X, Locke A, Lu C, Magi R, Perry JR, Pers TH, Qi Q, Sanna M, Schmidt EM, Scott WR, Shungin D, Teumer A, Vinkhuyzen AA, Walker RW, Westra HJ, Zhang M, Zhang W, Zhao JH, Zhu Z, Afzal U, Ahluwalia TS, Bakker SJ, Bellis C, Bonnefond A, Borodulin K, Buchman AS, Cederholm T, Choh AC, Choi HJ, Curran JE, de Groot LC, De Jager PL, Dhonukshe-Rutten RA, Enneman AW, Eury E, Evans DS, Forsen T, Friedrich N, Fumeron F, Garcia ME, Gartner S, Han BG, Havulinna AS, Hayward C, Hernandez D, Hillege H, Ittermann T, Kent JW, Kolcic I, Laatikainen T, Lahti J, Mateo Leach I, Lee CG, Lee JY, Liu T, Liu Y, Lobbens S, Loh M, Lyytikainen LP, Medina-Gomez C, Michaelsson K, Nalls MA, Nielson CM, Oozageer L, Pascoe L, Paternoster L, Polasek O, Ripatti S, Sarzynski MA, Shin CS, Narancic NS, Spira D, Srikanth P, Steinhagen-Thiessen E, Sung YJ, Swart KM, Taittonen L, Tanaka T, Tikkanen E, van der Velde N, van Schoor NM, Verweij N, Wright AF, Yu L, Zmuda JM, Eklund N, Forrester T, Grarup N, Jackson AU, Kristiansson K, Kuulasmaa T, Kuusisto J, Lichtner P, Luan J, Mahajan A, Mannisto S, Palmer CD, Ried JS, Scott RA, Stancakova A, Wagner PJ, Demirkan A, Doring A, Gudnason V, Kiel DP, Kuhnel B, Mangino M, McKnight B, Menni C, O’Connell JR, Oostra BA, Shuldiner AR, Song K, Vandenput L, van Duijn CM, Vollenweider P, White CC, Boehnke M, Boettcher Y, Cooper RS, Forouhi NG, Gieger C, Grallert H, Hingorani A, Jorgensen T, Jousilahti P, Kivimaki M, Kumari M, Laakso M, Langenberg C, Linneberg A, Luke A, McKenzie CA, Palotie A, Pedersen O, Peters A, Strauch K, Tayo BO, Wareham NJ, Bennett DA, Bertram L, Blangero J, Bluher M, Bouchard C, Campbell H, Cho NH, Cummings SR, Czerwinski SA, Demuth I, Eckardt R, Eriksson JG, Ferrucci L, Franco OH, Froguel P, Gansevoort RT, Hansen T, Harris TB, Hastie N, Heliovaara M, Hofman A, Jordan JM, Jula A, Kahonen M, Kajantie E, Knekt PB, Koskinen S, Kovacs P, Lehtimaki T, Lind L, Liu Y, Orwoll ES, Osmond C, Perola M, Perusse L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Rivadeneira F, Rudan I, Salomaa V, Sorensen TI, Stumvoll M, Tonjes A, Towne B, Tranah GJ, Tremblay A, Uitterlinden AG, van der Harst P, Vartiainen E, Viikari JS, Vitart V, Vohl MC, Volzke H, Walker M, Wallaschofski H, Wild S, Wilson JF, Yengo L, Bishop DT, Borecki IB, Chambers JC, Cupples LA, Dehghan A, Deloukas P, Fatemifar G, Fox C, Furey TS, Franke L, Han J, Hunter DJ, Karjalainen J, Karpe F, Kaplan RC, Kooner JS, McCarthy MI, Murabito JM, Morris AP, Bishop JA, North KE, Ohlsson C, Ong KK, Prokopenko I, Richards JB, Schadt EE, Spector TD, Widen E, Willer CJ, Yang J, Ingelsson E, Mohlke KL, Hirschhorn JN, Pospisilik JA, Zillikens MC, Lindgren C, Kilpelainen TO, Loos RJ. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]