Abstract
Purpose
To evaluate choroidal and suprachoroidal changes following suprachoroidal injection of triamcinolone acetonide injectable suspension (CLS-TA), in eyes with macular edema due to retinal vein occlusion (RVO).
Design
Prospective cohort study within a randomized, controlled phase 2 clinical trial.
Methods
Enhanced-depth imaging optical coherence tomography (EDI-OCT) images were analyzed from 38 eyes of 38 treatment naïve patients with macular edema due to RVO, enrolled in the prospective Suprachoroidal Injection of Triamcinolone Acetonide with Intravitreal Aflibercept in Subjects with Macular Edema Due to Retinal Vein Occlusion (TANZANITE) study who received either a suprachoroidal injection of CLS-TA with an intravitreal injection of aflibercept (combination arm) or only an intravitreal injection of aflibercept (monotherapy arm) followed by monthly intravitreal aflibercept injections in both arms based on pro re nata (PRN) criteria.
Results
Macular choroidal thickness measured to the outer choroidal vessel lumen (vascular choroidal thickness, VCT), outer choroid stroma (stromal choroidal thickness, SCT), or inner scleral border (total choroidal thickness, TCT) showed no significant changes over 3 months in both study arms (P = 0.231–0.342). Eyes that received combination therapy showed a trend toward thickening of the suprachoroidal space (SCS) compared with monotherapy alone (13.4 µm vs 5.3 µm at 3 months; P=0.077). In the 15 eyes that demonstrated a visible SCS at baseline, the SCS expanded significantly after suprachoroidal CLS-TA injection (16.2 µm to 27.8 µm at 3 months; P=0.033).
Conclusions
Suprachoroidal injection of CLS-TA does not alter choroidal thickness in eyes with macular edema due to RVO, but may result in expansion of the SCS.
Keywords: aflibercept, drug delivery, retinal vein occlusion, suprachoroidal injection, suprachoroidal space, triamcinolone acetonide
Retinal vein occlusion (RVO) is the second leading cause of retinal vascular disease after diabetic retinopathy, with an estimated prevalence of 0.5% in people older than 50 years of age.1–4 Central and branch retinal vein occlusions (CRVO and BRVO) cause vascular congestion, endothelial damage, and release of inflammatory cytokines, which together contribute to the development of macular edema and vision loss in patients.5 Current treatments for macular edema from RVO include intraocular injections of anti-vascular endothelial growth factor (anti-VEGF) agents or steroids, although the latter have been relegated as second-line therapy due to concerns for cataract formation and intraocular pressure (IOP) elevation associated with intravitreal steroids.5–15
Recent advances in OCT technology have provided a better understanding of the choroidal-scleral junction, where the suprachoroidal space may be visible in some individuals.16, 17 The suprachoroidal space contributes to uveoscleral outflow dynamics,18 and has been a target for glaucoma drainage devices and for temporary buckling for retinal detachments using viscoelastics.19–21 The suprachoroidal space provides a novel route for drug delivery with access to the retina and choroid, while potentially limiting exposure to the anterior segment and minimizing risk for cataracts and glaucoma for corticosteroid delivery.17, 22–28 Ocular distribution studies in rabbits showed more than 10-fold higher concentrations of triamcinolone acetonide (TA) in the posterior segment of the eye after suprachoroidal delivery, compared with higher concentrations of TA in anterior segment tissues after intravitreal injections.28 Suprachoroidal administration of 0.2 mg TA was also as effective in reducing ocular inflammation in a pig model of uveitis as 2.0 mg intravitreally injected TA.26 In a phase 1/2 open-label study, a single suprachoroidal injection of 4 mg TA was well tolerated and showed possible efficacy in eight human subjects with noninfectious intermediate, posterior, or pan-uveitis, with no reports of steroid-related IOP elevation or need for IOP-lowering medication.6 These results in part led to the initiation of two randomized controlled phase 2 clinical trials evaluating the use of suprachoroidal TA injections in patients with non-infectious uveitis and RVO, and the planning of a third phase 2 trial in patients with diabetic macular edema.29
While past animal studies have evaluated choroidal morphologic changes following suprachoroidal drug delivery, these changes have not yet been described in humans.23, 25, 30 The goal of this study was to evaluate choroidal thickness (CT) changes over a 3-month period in a randomized phase 2 multicenter trial comparing intravitreal aflibercept with and without a single suprachoroidal administration of CLS-TA, a 40 mg/mL preservative-free, terminally sterilized ophthalmic aqueous injectable suspension of TA, in eyes with macular edema due to RVO.
Methods
Patient Selection
Enhanced depth imaging optical coherence tomography (EDI-OCT) images were obtained from all patients enrolled in the Suprachoroidal Injection of Triamcinolone Acetonide with Intravitreal Aflibercept in Subjects with Macular Edema Due to Retinal Vein Occlusion (TANZANITE) multicenter clinical trial (ClinicalTrials.gov, NCT02303184). The study was conducted in compliance with the Declaration of Helsinki, and informed consent was obtained from all patients prior to any study-specific procedures being performed. Institutional review boards from each site approved the study protocol prior to initiation in February 2015. Final data collection was completed for the primary outcome measure in March 2016.
The study enrolled 46 patients diagnosed with macular edema due to RVO of ≤ 12 months duration, with best-corrected visual acuity (BVCA) of 20 to 70 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters and central subfield thickness (CST) > 310 µm as segmented and measured by the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) spectral domain optical coherence tomography (SD-OCT) instrument.31 Eyes were excluded if they received any prior intravitreal anti-VEGF injection for RVO, received a corticosteroid injection in the past 3 months, or had any uncontrolled ophthalmic condition other than RVO. Only one eye per patient was enrolled in the study.
Patients were randomized at baseline to receive a single 2 mg (50 µL) intravitreal injection of aflibercept followed by either a single suprachoroidal injection of 4 mg in 100 µL of CLS-TA suspension (combination arm) or a sham suprachoroidal injection (monotherapy arm). Suprachoroidal injections were administered using a proprietary syringe, which included an approximately 1-mm long, 30-gauge needle attached to a 1-mL syringe via a Luer lock, called a microinjector (Clearside Biomedical Inc., Alpharetta, GA).6 The CLS-TA suspension was injected 4–5 mm posterior to the limbus, approximately 200–300 µm anterior to the retina. After initial treatment, patients were examined on a monthly basis, and additional intravitreal aflibercept was given if there was any fluid with CST > 340 µm, any vision loss of 10 or more ETDRS letters from the prior visit, or vision loss of 10 or more letters accompanied by an increase in fluid of at least 50 µm. For subjects in the monotherapy arm, a sham suprachoroidal procedure was performed using a needleless hub on the microinjector to simulate the suprachoroidal injection procedure, while keeping all other components of the procedure as identical to the suprachoroidal injection in the combination arm, including the use of topical anesthetics.
Image Acquisition & Analysis
EDI-OCT images were acquired using a Spectralis SD-OCT and consisted of a single 20-degree horizontal line scan (~ 5.8 mm), centered on the fovea, taken in high-speed EDI-mode using the eye-tracking Automatic Real-Time (ART) mode with 100 frames captured per image. Automated image registration using Spectralis software was employed to ensure the same B-scan location at each visit for each study eye.
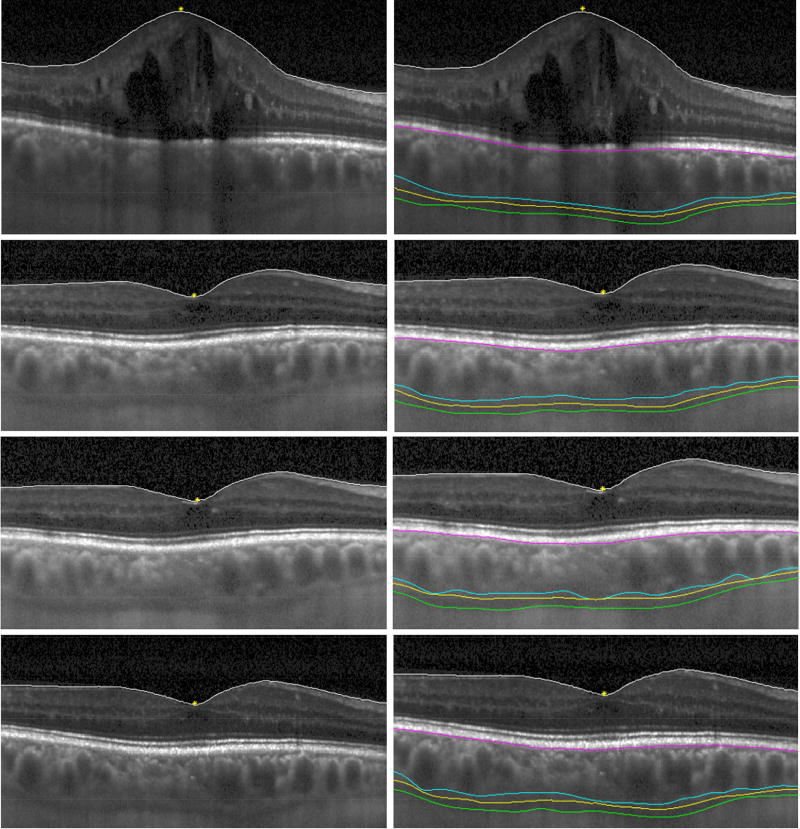
Deidentified EDI-OCT images were analyzed by two masked, independent graders (ASW, VSV) using the Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP) for semi-automatic segmentation of Bruch’s membrane and the choroidal-scleral junction.32 The choroidal-scleral junction was defined as 1) the outer border of the choroidal vessel lumen, 2) the outer border of the choroidal stroma, and 3) the inner border of the sclera, to measure the vascular choroidal thickness (VCT), stromal choroidal thickness (SCT), and total choroidal thickness (TCT), respectively (Figure 1).17, 33 The thickness of the suprachoroidal space (SCS) was determined from the difference between TCT and SCT. Thickness measurements were acquired at 0.005 mm intervals from 1.5 mm nasal to 1.5 mm temporal to the fovea, with 601 measurements per eye averaged across the central 3 mm segment around the fovea, which provides greater reproducibility than single-location measurements.33 Eyes that did not undergo EDI-OCT imaging, or had EDI-OCT images that were deemed ungradable due to poor image quality or to the choroidal-scleral junction being outside the scan area, were excluded from the analysis. Eyes were also graded subjectively for the presence of a visible SCS, defined as a distinct hyporeflective band between the choroidal stroma and sclera, as previously described.17
Figure 1.
Semi-automated choroidal segmentation of EDI-OCT images before and after suprachoroidal triamcinolone acetonide and intravitreal aflibercept injection in an eye with ME due to RVO.
Images are shown before injection (top row), and at 1 month (second row), 2 months (third row), and 3 months (bottom row) after treatment, with (left column) and without (right column) segmentation boundaries, including the inner limiting membrane (white line), RPE/Bruch’s membrane complex (purple line), vascular choroidal thickness boundary (blue line), stromal choroidal thickness boundary (yellow line), and total choroidal thickness boundary (green line). The fovea is labeled with a yellow asterisk.
Statistical Analysis
A mixed repeated-measures analysis of variance (ANOVA) was used to compare changes between the treatment and control groups across time points in VCT, SCT, TCT, and SCS thickness. A quantitative criterion for SCS presence was determined using a receiver operating characteristic (ROC) curve and Youden’s index to measure the SCS thickness threshold value that provides maximal sensitivity and specificity for SCS visibility as qualitatively determined by trained graders. Intraclass correlation coefficients (ICC) were used to determine intergrader agreement of CT measurements. All statistical analyses were performed using SPSS (v.1.0.0.407, IBM Corp., NY). A p-value of < 0.05 was considered to be statistically significant.
Results
Demographics & Baseline Characteristics
Among the 46 participants enrolled in the prospective phase 2, TANZANITE, study, 38 subjects had EDI-OCT images that qualified for inclusion in this image analysis. Of these 38 subjects, mean age was 66 (range, 37–91) and 20 participants (52.6%) were male (Table 1). Thirty-three participants (86.8%) were Caucasian/Hispanic, 4 were African American, and 1 was Native American. There were no significant differences in age, sex, and racial distribution between the combination and monotherapy arms (Table 1). There were also no statistical differences in baseline visual acuity, or mean central VCT, SCT, and TCT between the two groups. There were also no significant difference in proportion of subjects with prior ocular surgery or laser therapy (P = 0.204).
Table 1.
Study Demographics
| All Subjects (n=38) |
Suprachoroidal CLS-TA + Intravitreal Aflibercept - Combination Arm (n=21) |
Intravitreal Aflibercept Only - Monotherapy Arm (n=17) |
P-value | |
|---|---|---|---|---|
| Age (years ± SD) | 65.7 ± 12.1 | 67.1 ± 9.0 | 64.1 ± 15.3 | 0.451 |
| Gender (male / female) | 20 / 18 | 13 / 8 | 7 / 10 | 0.343 |
| Race (CH / AA / AI) | 33 / 4 / 1 | 19 / 2 / 0 | 14 / 2 / 1 | 0.509 |
| Baseline VA (ETDRS letters) | 50.3 + 14.9 | 50.2 + 17.1 | 50.5 + 12.5 | 0.952 |
| Baseline CT (mean ± SE µm) | ||||
| VCT | 219.9 ± 14.2 | 234.9 ± 24.3 | 208.5 ± 15.0 | 0.369 |
| SCT | 248.9 ± 14.9 | 263.3 ± 25.4 | 233.5 ± 14.8 | 0.327 |
| TCT | 256.3 ± 15.0 | 272.9 ± 25.6 | 238.7 ± 14.5 | 0.262 |
| SCS Present (%) | 15 (39.5%) | 9 (42.9%) | 6 (35.3%) | 0.744 |
Abbreviations: CH, Caucasian/Hispanic; AA, African American; AI, American Indian; VA, visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; VCT, vascular choroidal thickness; SCT, stromal choroidal thickness; TCT, total choroidal thickness; n, number; SD, standard deviation; SE, standard error; SCS, suprachoroidal space.
Choroidal Thickness Changes
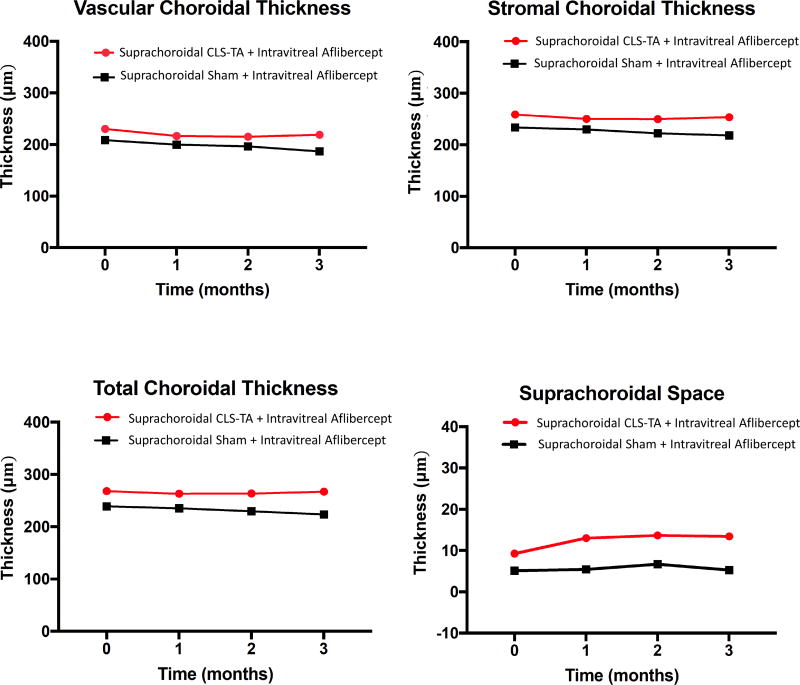
Mean central VCT, SCT, and TCT showed no significant change over the 3 months after treatment in either the combination arm which received both suprachoroidal CLS-TA and intravitreal aflibercept, or the monotherapy arm that received only intravitreal aflibercept (P=0.231–0.342, Figure 2). There was also no significant correlation between the change in retinal thickness and any choroidal thickness measurements in either arm or at any visit (P = 0.113 – 0.967), consistent with previous studies.34, 35 There was a very slight trend toward choroidal thinning using all three posterior boundary definitions, but none of these reached statistical significance (Figure 2, Table 2). The intergrader reliability was excellent across all three CT measures (ICC=0.94 – 0.99), with the posterior choroidal vessel boundary (VCT) demonstrating the least reproducibility (Table 2), which was consistent with published reports.33 There were no differences in median OCT acquisition time between subjects in the two arms at any time points (P = 0.671), and inclusion of image acquisition time as a co-variate showed no significant contribution from potential diurnal fluctuations (P = 0.712 among subjects, P = 0.122 between groups).
Figure 2.
Change in mean central choroidal thickness in eyes treated with suprachoroidal triamcinolone acetonide and intravitreal aflibercept (combination arm) versus intravitreal aflibercept only (monotherapy arm). Abbreviations: VCT, vascular choroidal thickness; SCT, stromal choroidal thickness; TCT, total choroidal thickness; SCS, suprachoroidal space; IVT, intravitreal, TA, triamcinolone acetonide)
Table 2.
Choroidal Thickness Comparisons
| VCT | SCT | TCT | SCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n=38 | Mean Thickness (µm) |
SE | ICC (95% CI) |
Mean Thickness (µm) |
SE | ICC (95% CI) |
Mean Thickness (µm) |
SE | ICC (95%CI) |
Mean Thickness (µm) |
SE |
| Suprachoroidal CLS-TA + Intravitreal Aflibercept (combination arm) | |||||||||||
| Baseline | 230.10 | 23.41 | 0.94 (0.85–0.97) | 258.80 | 24.37 | 0.99 (0.99–1.00) | 268.00 | 24.66 | 0.99 (0.99–1.00) | 9.27 | 2.91 |
| Month 1 | 216.60 | 17.79 | 250.00 | 18.73 | 263.00 | 18.93 | 13.03 | 3.23 | |||
| Month 2 | 215.10 | 17.46 | 249.80 | 18.69 | 263.50 | 18.67 | 13.69 | 3.28 | |||
| Month 3 | 218.80 | 18.79 | 253.60 | 20.70 | 267.00 | 20.65 | 13.43 | 3.20 | |||
| Intravitreal Aflibercept only (monotherapy arm) | |||||||||||
| Baseline | 208.50 | 15.00 | 0.94 (0.85–0.97) | 233.50 | 14.79 | 0.99 (0.99–1.00) | 238.70 | 14.51 | 0.99 (0.99–1.00) | 5.15 | 1.63 |
| Month 1 | 200.00 | 19.09 | 229.80 | 18.11 | 235.30 | 17.51 | 5.49 | 1.25 | |||
| Month 2 | 196.40 | 18.32 | 222.30 | 17.49 | 229.40 | 16.54 | 6.71 | 1.97 | |||
| Month 3 | 186.90 | 15.79 | 218.10 | 17.01 | 223.40 | 16.56 | 5.32 | 1.62 | |||
Abbreviations: VCT, vascular choroidal thickness; SCT, stromal choroidal thickness; TCT, total choroidal thickness; SCS, suprachoroidal space thickness; IVT, intravitreal; CLS-TA, preservative-free, terminally sterilized triamcinolone acetonide; n, number; SE, standard error; ICC, intra class correlation coefficient
Suprachoroidal Space Thickness Changes
The SCS thickness was calculated from the difference between the TCT and SCT, which are determined by the inner border of the sclera and outer border of the choroid stroma, respectively. Eyes that received both suprachoroidal CLS-TA and intravitreal aflibercept (combination arm) showed a trend toward thickening of the SCS compared with those that had aflibercept alone in the monotherapy arm (13.4 µm vs. 5.3 µm at 3 months; P=0.130; Figure 2, Supplemental Figure 1).
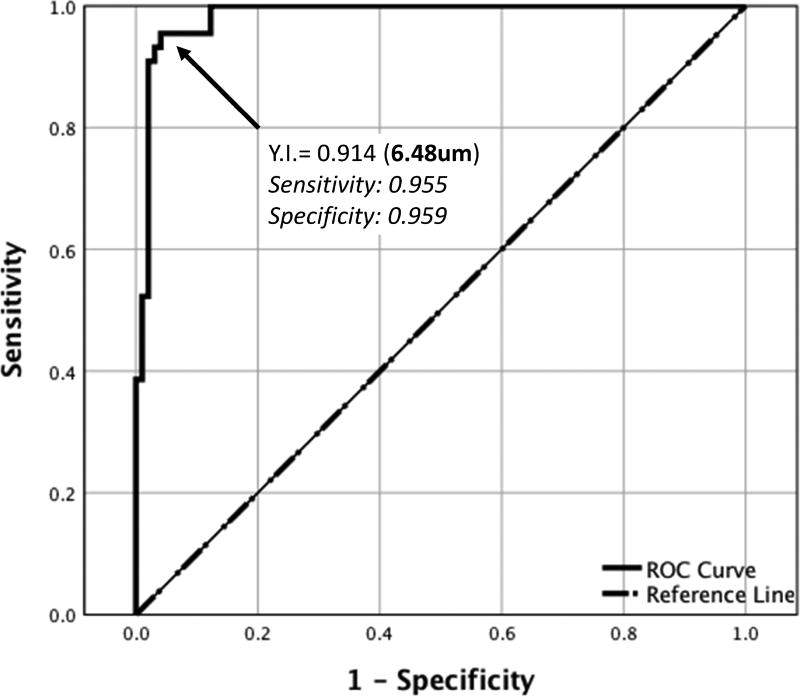
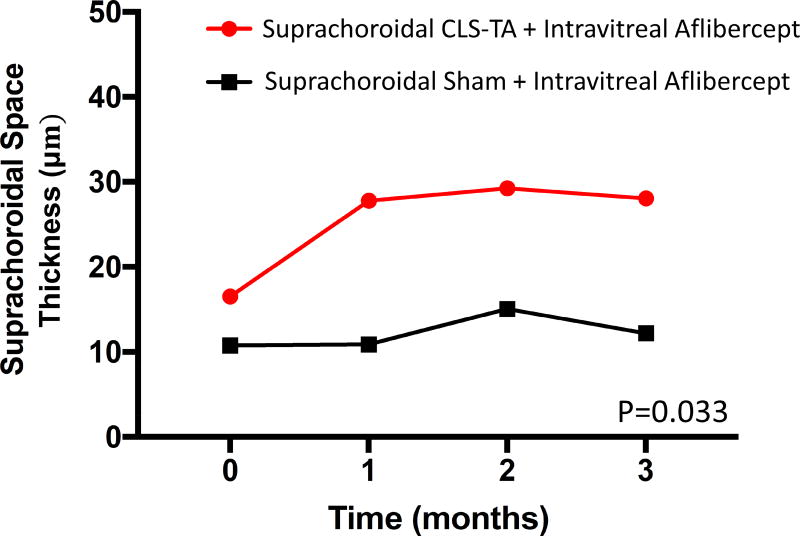
Using ROC analysis, the SCS was determined to be visible when SCS thickness was greater than 6.48 µm when averaged across the central 3-mm segment (Figure 3), based on a Youden’s Index of 0.914 (sensitivity = 0.955, specificity = 0.959). Using this criterion, 15 out of 38 study eyes (39.5%) were defined to have a discernible SCS. A subgroup analysis of only these eyes with a visible SCS showed mild but statistically significant SCS expansion in the combination arm, where mean SCS thickness increased from 16.2 ± 4.9 µm to 27.8 ± 3.8 µm at 3 months, compared to the monotherapy arm which remained essentially unchanged from 10.8 ± 4.4 µm at baseline to 10.2 ± 3.2 µm at 3 months (P=0.033, Figure 4).
Figure 3.
Receiver operating characteristic (ROC) curve using Youden’s Index (YI) to measure the threshold for determining the presence or absence of a visible suprachoroidal space.
Figure 4.
Change in mean central choroidal thickness in eyes with a visible suprachoroidal space treated with suprachoroidal triamcinolone acetonide, and intravitreal aflibercept (combination arm) versus aflibercept only (monotherapy arm). Abbreviations: VCT, vascular choroidal thickness; SCT, stromal choroidal thickness; TCT, total choroidal thickness; SCS, suprachoroidal space; IVT, intravitreal; CLS-TA, preservative-free, terminally sterilized triamcinolone acetonide.
Discussion
Suprachoroidal drug delivery is a relatively unexplored approach for the treatment of retinal diseases with the potential to maximize drug exposure to posterior segment tissues while also reducing drug exposure to the anterior portions of the eye, including the lens. In the phase 2 TANZANITE trial, subjects who received both suprachoroidal CLS-TA and intravitreal aflibercept in the combination arm achieved both greater visual and anatomic improvements over a 3-month period following treatment at baseline, as compared to subjects in the monotherapy control arm, who only received intravitreal aflibercept at baseline.36 In addition, suprachoroidal injections of CLS-TA were well tolerated with the potential to reduce the risk of glaucoma or cataract formation associated with intravitreal steroids.36 In this study, we evaluated choroidal and suprachoroidal changes in the central macula following a single suprachoroidal CLS-TA injection combined with intravitreal aflibercept, and found no significant thickness change in any choroidal layers. We observed a trend toward expansion of the SCS in the combination arm as compared to the monotherapy arm, which was statistically significant in eyes with a visible SCS (P=0.033). We suspect that the effect was best seen in eyes with a visible SCS because eyes with a thicker SCS at baseline are more likely to allow a small thickness change to be detected, and also eyes with a wider SCS may be more susceptible to SCS expansion following suprachoroidal injection. Because there was no control arm where vehicle-only was injected into the SCS, we cannot conclude whether the mild SCS expansion is a result of the physical volume effect of the suprachoroidal fluid injection or a pharmacologic effect of the CLS-TA drug suspension. Interestingly, this SCS expansion appeared to occur within the first month after suprachoroidal administration, and remained essentially unchanged for up to the 3 months of the trial. Since the first follow-up EDI-OCT images were not obtained until 1 month after treatment, during which time the injected fluid had likely dispersed into other tissues and the choroidal circulation,23–25 we cannot determine if a greater degree of SCS expansion might have been present in the immediate post-injection period. However, the persistence of the SCS expansion at 3 months is consistent with the 90-day window when TA concentrations are detectable in preclinical animal models, and the use of EDI-OCT imaging as a potential measure of sustained drug presence in the SCS warrants further exploration.37 Nevertheless, since data were not collected past the 3 month time period, it is unclear if this apparent expansion of the SCS will diminish over time. It is also important to note that while the reproducibility of CT measurements have been well studied, the reliability of SCS measurements have not been validated and such small changes may be susceptible to measurement variability. The inclusion of additional time points immediately after SCS injections, or additional scan patterns such as high-density macular cubes for volumetric measurements may allow more robust analyses of SCS changes in future studies.
The clinical utility of measuring CT in RVO patients remains unclear to date. Recent reports suggest that baseline CT may predict visual outcomes in RVO eyes.38 The choroid in eyes with macular edema due to RVO was found to be thicker than in fellow eyes, although choroidal thinning has also been noted following both intravitreal steroid and intravitreal anti-VEGF therapy including aflibercept.35, 38, 39 In this study, we noted no significant change in CT using three different posterior boundary definitions in either arm, suggesting that this single suprachoroidal CLS-TA injection may not affect the overall structure of the submacular choroid in eyes with RVO. Additional larger studies to evaluate CT changes after suprachoroidal injections for both RVO and other indications would help us better understand the utility of choroidal measurements. The randomized, masked, controlled phase 2, Suprachoroidal Injection of Triamcinolone Acetonide in Subjects with Macular Edema Following Non-Infectious Uveitis study (DOGWOOD; NCT02255032) has been completed, but the absence of a control arm that did not receive suprachoroidal CLS-TA and smaller sample size precludes a robust analysis of the choroidal effect from suprachoroidal injections from that clinical trial.
Observations from TANZANITE showed that 4 subjects in the combination treatment arm developed steroid-related IOP elevations, two of whom had pre-existing glaucoma. In the imaging analysis study, we evaluated the SCS thickness changes in these 4 subjects and found no significant difference with the other subjects who did not experience any increases in IOP (P = 0.285). Given the rapid tissue dispersion of agents injected into the SCS, any mild, subclinical thickness changes in the choroid or SCS are unlikely to impair aqueous humor production or outflow, in contrast to more clinically-apparent choroidal effusions that may occur in pathologic conditions.
Our findings provide novel insights into the ocular distribution and anatomic effects of suprachoroidal CLS-TA injection in human eyes with macular edema due to RVO. The suprachoroidal space influences both posterior and anterior segment disease processes, and continues to be a target for new interventions including glaucoma drainage devices, IOP monitoring, and injections of pharmacologic agents. Advantages of suprachoroidal drug administration include the reduction of anterior segment exposure, lower risk of cataracts and glaucoma, and potentially more targeted or sustained drug delivery for treatment of retinal conditions. Possible concerns include a learning curve for the more complex technique, inadvertent intravitreal administration, or failed delivery if the microneedle is not completely orthogonal to the ocular contour. Nevertheless, the suprachoroidal space remains a promising new route for ocular drug delivery that warrants further investigation.
Supplementary Material
Supplemental Figure 1: Scatterplots with linear regression line showing relationship between suprachoroidal space thickness at 1 month (left column), 2 months (middle column), and 3 months (right column) with baseline suprachoroidal space thickness in both the combination (top row) and monotherapy arms (bottom row).
Acknowledgments
A. Funding/Support:
Alex S. Willoughby: None
Vivian S. Vuong: National Center for Advancing Translational Sciences and NIH UL1TR000002.
David Cunefare: None
Sina Farsiu: NIH P30 EY005722.
Glenn Noronha: None
Ronald P. Danis: Research to Prevent Blindness, Inc. (New York, NY)
Glenn Yiu: by NIH K08 EY026101, the E. Matilda Ziegler Foundation for the Blind (Darien, CT), Barr Foundation for Retinal Research (Houston, TX), Alcon Research Institute (Fort Worth, TX), and the ARVO Foundation (Rockville, MD)
C. Other Acknowledgements: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentations:
None
Conflict of Interests:
AW: none
VV: none
GN: employee, patents, and equity at Clearside
RD: equity and employee relationship at EyeKor Inc.
DC: none
SF: patents in OCT imaging and analysis
GY: grants from Alcon, ARVO Foundation, E Matilda Ziegler Foundation, Genentech and personal fees for consultancy from Alimera, Allergan, Carl Zeiss Meditec, and Southern California Desert Retina.
B. Financial Disclosures:
Alex S. Willoughby: None
Vivian S. Vuong: None
David Cunefare: None
Sina Farsiu: patents in OCT imaging and analysis
Glenn Noronha: employee, patents, and equity at Clearside Biomedical, Inc. (Alpharetta, GA)
Ronald P. Danis: equity and employee relationship at EyeKor Inc. (Madison, WI)
Glenn Yiu: personal fees for consultancy from Alimera (Alpharetta, GA), Allergan (Dublin, Republic of Ireland), Carl Zeiss Meditec (Jena, Germany), and Southern California Desert Retina (Palm Desert, CA).
References
- 1.Ho M, Liu DT, Lam DS, Jonas JB. Retinal Vein Occlusions, From Basics to the Latest Treatment. Retina. 2016;36(3):432–48. doi: 10.1097/IAE.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–7. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 3.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–9.e1. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. discussion 141-3. [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56(4):281–99. doi: 10.1016/j.survophthal.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DA, Do D, Noronha G, Kissner JM, Srivastava SK, Nguyen QD. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Transl Vis Sci Technol. 2016;5(6):14. doi: 10.1167/tvst.5.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cekiç O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005;25(7):851–5. doi: 10.1097/00006982-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Chen YH, Wu PC, et al. Treatment of branch retinal vein occlusion induced macular edema in treatment-naïve cases with a single intravitreal triamcinolone or bevacizumab injection. Chang Gung Med J. 2010;33(4):424–35. [PubMed] [Google Scholar]
- 9.Cheng KC, Wu WC, Chen KJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema secondary to branch retinal vein occlusion. Eye (Lond) 2009;23(11):2023–33. doi: 10.1038/eye.2009.230. [DOI] [PubMed] [Google Scholar]
- 10.Moon J, Kim M, Sagong M. Combination therapy of intravitreal bevacizumab with single simultaneous posterior subtenon triamcinolone acetonide for macular edema due to branch retinal vein occlusion. Eye (Lond) 2016;30(8):1084–90. doi: 10.1038/eye.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF. Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye (Lond) 2005;19(1):65–71. doi: 10.1038/sj.eye.6701395. [DOI] [PubMed] [Google Scholar]
- 12.Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–60. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Kuppermann BD, Haller JA, Bandello F, et al. Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina. 2014;34(9):1743–9. doi: 10.1097/IAE.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 14.Javitt JC, Aiello LP, Chiang Y, Ferris FL, 3rd, Canner JK, Greenfield S. Preventive eye care in people with diabetes is cost-saving to the federal government. Implications for health-care reform. Diabetes Care. 1994;17(8):909–17. doi: 10.2337/diacare.17.8.909. [DOI] [PubMed] [Google Scholar]
- 15.Hoerauf H, Feltgen N, Weiss C, et al. Reply. Am J Ophthalmol. 2016;169:292–3. doi: 10.1016/j.ajo.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Huynh E, Chandrasekera E, Bukowska D, McLenachan S, Mackey DA, Chen FK. Past, Present, and Future Concepts of the Choroidal Scleral Interface Morphology on Optical Coherence Tomography. Asia Pac J Ophthalmol (Phila) 2017;6(1):94–103. doi: 10.22608/APO.201698. [DOI] [PubMed] [Google Scholar]
- 17.Yiu G, Pecen P, Sarin N, et al. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA ophthalmology. 2014;132(2):174–81. doi: 10.1001/jamaophthalmol.2013.7288. [DOI] [PubMed] [Google Scholar]
- 18.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30(2):233–8. [PubMed] [Google Scholar]
- 19.Jordan JF, Engels BF, Dinslage S, et al. A novel approach to suprachoroidal drainage for the surgical treatment of intractable glaucoma. J Glaucoma. 2006;15(3):200–5. doi: 10.1097/01.ijg.0000212207.79899.85. [DOI] [PubMed] [Google Scholar]
- 20.Einmahl S, Savoldelli M, D'Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43(5):1533–9. [PubMed] [Google Scholar]
- 21.El Rayes EN, Oshima Y. Suprachoroidal buckling for retinal detachment. Retina. 2013;33(5):1073–5. doi: 10.1097/IAE.0b013e318287daa5. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Li X, Liu J, Han Y, Cheng L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J Control Release. 2015;203:109–17. doi: 10.1016/j.jconrel.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Chiang B, Venugopal N, Edelhauser HF, Prausnitz MR. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp Eye Res. 2016;153:101–109. doi: 10.1016/j.exer.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang B, Wang K, Ethier CR, Prausnitz MR. Clearance Kinetics and Clearance Routes of Molecules From the Suprachoroidal Space After Microneedle Injection. Invest Ophthalmol Vis Sci. 2017;58(1):545–554. doi: 10.1167/iovs.16-20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang B, Venugopal N, Grossniklaus HE, Jung JH, Edelhauser HF, Prausnitz MR. Thickness and Closure Kinetics of the Suprachoroidal Space Following Microneedle Injection of Liquid Formulations. Invest Ophthalmol Vis Sci. 2017;58(1):555–564. doi: 10.1167/iovs.16-20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Investigative ophthalmology & visual science. 2013;54(4):2483–92. doi: 10.1167/iovs.13-11747. [DOI] [PubMed] [Google Scholar]
- 27.Olsen TW. Suprachoroidal Drug Delivery: Unique New Observations. Invest Ophthalmol Vis Sci. 2015;56(8):4976. doi: 10.1167/iovs.15-17391. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Investigative ophthalmology & visual science. 2012;53(8):4433–41. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bethesda, MD: 2017. ClinicalTrials.gov. [Google Scholar]
- 30.Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PLoS One. 2012;7(10):e48188. doi: 10.1371/journal.pone.0048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–806. [PubMed] [Google Scholar]
- 32.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Optics express. 2010;18(18):19413–28. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuong VS, Moisseiev E, Cunefare D, Farsiu S, Moshiri A, Yiu G. Repeatability of Choroidal Thickness Measurements on Enhanced Depth Imaging Optical Coherence Tomography Using Different Posterior Boundaries. Am J Ophthalmol. 2016;169:104–12. doi: 10.1016/j.ajo.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto M, Yamashita M, Sakamoto T, Ogata N. Choroidal Blood Flow and Thickness as Predictors for Response to Anti-Vascular Endothelial Growth Factor Therapy in Macular Edema Secondary to Branch Retinal Vein Occlusion. Retina. 2017 doi: 10.1097/IAE.0000000000001566. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Yiu G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol. 2014;158(4):745–751 e2. doi: 10.1016/j.ajo.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campochiaro PA, Wykoff CC, Brown DM, et al. Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmology. 2017 doi: 10.1016/j.oret.2017.07.013. Submitted to. [DOI] [PubMed] [Google Scholar]
- 37.Edelhauser HF, Verhoeven RS, Burke B, Struble CB, Patel SR. Intraocular Distribution and Targeting of Triamcinolone Acetonide Suspension Administered Into the Suprachoroidal Space. Investigative Ophthalmology & Visual Science. 2014;55(13):5259–5259. [Google Scholar]
- 38.Rayess N, Rahimy E, Ying GS, et al. Baseline Choroidal Thickness as a Predictor for Treatment Outcomes in Central Retinal Vein Occlusion. Am J Ophthalmol. 2016;171:47–52. doi: 10.1016/j.ajo.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Esen E, Sizmaz S, Demircan N. Choroidal Thickness Changes after Intravitreal Dexamethasone Implant Injection for the Treatment of Macular Edema Due to Retinal Vein Occlusion. Retina. 2016;36(12):2297–2303. doi: 10.1097/IAE.0000000000001099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Scatterplots with linear regression line showing relationship between suprachoroidal space thickness at 1 month (left column), 2 months (middle column), and 3 months (right column) with baseline suprachoroidal space thickness in both the combination (top row) and monotherapy arms (bottom row).