Abstract
Objectives
The treatment of osteoporotic fractures is a major challenge, and the enhancement of healing is critical as a major goal in modern fracture management. Most osteoporotic fractures occur at the metaphyseal bone region but few models exist and the healing is still poorly understood. A systematic review was conducted to identify and analyse the appropriateness of current osteoporotic metaphyseal fracture animal models.
Materials and Methods
A literature search was performed on the Pubmed, Embase, and Web of Science databases, and relevant articles were selected. A total of 19 studies were included. Information on the animal, induction of osteoporosis, fracture technique, site and fixation, healing results, and utility of the model were extracted.
Results
Fracture techniques included drill hole defects (3 of 19), bone defects (3 of 19), partial osteotomy (1 of 19), and complete osteotomies (12 of 19). Drill hole models and incomplete osteotomy models are easy to perform and allow the study of therapeutic agents but do not represent the usual clinical setting. Additionally, biomaterials can be filled into drill hole defects for analysis. Complete osteotomy models are most commonly used and are best suited for the investigation of therapeutic drugs or noninvasive interventions. The metaphyseal defect models allow the study of biomaterials, which are associated with complex and comminuted osteoporotic fractures.
Conclusion
For a clinically relevant model, we propose that an animal model should satisfy the following criteria to study osteoporotic fracture healing: 1) induction of osteoporosis, 2) complete osteotomy or defect at the metaphysis unilaterally, and 3) internal fixation.
Cite this article: R. M. Y. Wong, M. H. V. Choy, M. C. M. Li, K-S. Leung, S. K-H. Chow, W-H. Cheung, J. C. Y. Cheng. A systematic review of current osteoporotic metaphyseal fracture animal models. Bone Joint Res 2018;7:6–11. DOI: 10.1302/2046-3758.71.BJR-2016-0334.R2.
Keywords: Metaphyseal Fracture, Animal Model, Osteoporosis, Systematic Review
Article Focus
Most osteoporotic fractures occur at the metaphyseal bone region but few models exist and the healing is poorly understood
Systematic review to identify and analyse the appropriateness of current osteoporotic metaphyseal fracture animal models
Key Messages
Complete osteotomy models are most commonly used and are best suited for the investigation of therapeutic drugs or noninvasive interventions The metaphyseal defect models are best suited for the study of biomaterials, which are associated with complex and comminuted osteoporotic fractures
Strengths and limitations
Updated review of currently available models
Due to the heterogeneity of the studies, pooled analysis was not feasible
Introduction
Osteoporosis is a major medical and socioeconomic threat characterized by a systemic impairment of bone mass, strength, and microarchitecture. The skeletal disorder predisposes patients to increased risk of fragility fractures. There are approximately 2.5 million osteoporotic fractures each year in the United States, with costs estimated at $15 billion USD in 2010 and projected to reach $25 billion USD by 2025.1 In 2000, an estimated 9.0 million osteoporotic fractures occurred worldwide, with the numbers continuously rising.2 The lifetime fracture risk of osteoporotic patients reaches as high as 40%,3 which is an important cause of morbidity and mortality in an ageing population.
The treatment of osteoporotic fractures is a major challenge, as bone healing is delayed due to the impaired healing properties with respect to callus formation, angiogenesis, and mineralization.4,5 Failure to unite results in pain, weakness, reduced mobility, and fixation failure; these complications are most common in elderly patients, which can lead to serious detrimental effects to overall health status. Enhancement of osteoporotic fracture healing is therefore critical as a major goal in modern fracture management. The development of an effective animal model for research is essential in this process.
Most osteoporotic fractures occur at the trabecular or the metaphyseal bone region,6 including the distal radius, proximal humerus, proximal femur, and vertebral bodies.7,8 Despite the evidence, most preclinical studies have concentrated on the healing of osteoporotic diaphyseal femur or tibia fractures with intramedullary fixation, often based on the model put forward by Bonnarens and Einhorn9 in 1984. However, it is well known that metaphyseal and diaphyseal fractures heal by completely different mechanisms.10 This animal model has therefore faced criticisms related to its clinical relevance, leading to the recent development of more appropriate models.
There are very few of these newer models, and the healing of metaphyseal fractures is still poorly understood. The purpose of this systematic review was to identify and characterize the appropriateness of the available metaphyseal fracture animal models reported for osteoporosis research.
Materials and Methods
Search strategy
The Pubmed, Embase, and Web of Science databases (date last accessed 07 May 2017) were searched. The keywords used for the search criteria were “metaphys*” AND “animal model” AND “fracture” AND “osteoporo*”.
Search criteria
The inclusion criteria were: 1) preclinical studies, 2) use of animal model, 3) fractures performed at the metaphysis, and 4) study on fracture healing.
The exclusion criteria were: 1) review paper, 2) lack of osteoporosis induction, 3) no radiological imaging or histological analysis for fracture healing, 4) lack of control group, and 5) conference/abstract publication.
Selection of studies
Two independent reviewers performed the selection process on three databases. Each reviewer screened the titles and abstracts of each published study. Articles were selected based on the inclusion and exclusion criteria. Each article was reviewed and any disagreement was resolved by consensus and discussion.
Data extraction
For eligible studies, the two reviewers extracted information on: 1) animal used; 2) osteoporosis induction and method; 3) site and type of fracture; 4) type of fixation; 5) fracture healing results; and 6) up-to-date literature on the utility of the animal model.
Data analysis
Due to the large variation in animal models and methodology, a qualitative review was performed.
Results
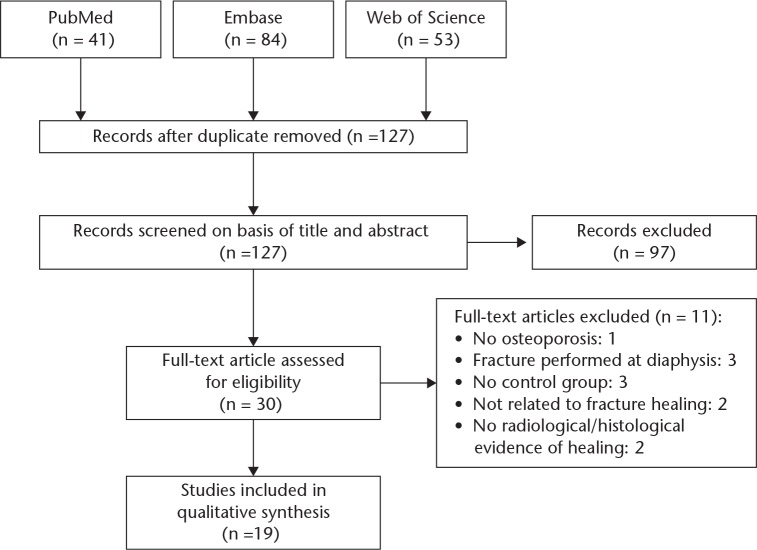
A total of 41, 84, and 53 studies were identified from PubMed, Embase, and Web of Science, respectively. All duplicate entries were removed, leaving 127 records. Each title and abstract was reviewed and 97 records were excluded. Upon detailed review of each study in full text, an additional 11 were excluded. One of these studies did not have induction of osteoporosis.11 Three studies performed fractures in the diaphysis.12-14 Three lacked a control group in the study design.15-17 Two studies were not related to fracture healing.18,19 Two studies did not have radiological imaging or histological analysis of fracture healing.20,21 Our results show a total of 19 studies for our systematic review (Fig. 1).
Fig. 1.
Flowchart of study selection
Characteristics of the papers
The 19 studies were published from 2010 to 2016 (Supplementary table i). All studies were preclinical studies with metaphyseal fracture models and induction of osteoporosis, performed in the rat,7,10,22-36 sheep,37 and goat.38
Induction and methodology of osteoporosis
All 17 rat studies performed bilateral ovariectomy to induce osteoporosis. One study performed ovariectomy on Chinese mountain goats and another study performed hypothalamic-pituitary disconnection (HPD) on adult Merino sheep. Supplementary table i summarizes the osteoporotic induction methods and confirmation of osteoporosis.
Out of the 19 studies (Supplementary table i), nine performed analysis by micro-CT, pQCT (peripheral quantitative computed tomography), or DXA (dual-energy X-ray absorptiometry) to confirm osteoporotic induction. Seven studies were based on previous literature that confirmed osteoporosis. Three had created a new type of osteoporotic model, to simulate the early phase of osteoporosis.
Location and type of fracture
All fractures from the 19 studies were performed at the metaphyseal region of bones. Three were drill hole defect models, one was a partial osteotomy model, three performed fracture defect models, and 12 were complete osteotomy fracture models. The details are summarized in Supplementary table i.
Radiological and histological evidence of healing
All studies reported adequate fracture healing. Supplementary table i summarizes the radiological and histological findings for all 19 studies.
The interventions assessed with current osteoporotic animal models
The most commonly used osteoporotic metaphyseal fracture model was the bilateral osteotomy on the proximal tibia of ovariectomized rats, originated by Stuermer et al,10 which was used by 11 published studies.10,25-31,33,34,36 All 11 studies that used the bilateral osteotomy model investigated potential therapeutic drugs or noninvasive interventions. The unilateral complete osteotomy was used to investigate potential therapeutic agents to promote osteoporotic fracture healing. The three defect models enabled the study of biomaterials, while the three drill hole defect models were used to study biomaterials or therapeutic drugs. The partial osteotomy model has not yet been used to study interventions. Supplementary table i summarizes the details on the utility of each model.
Discussion
Previous models have concentrated on diaphyseal fractures, despite evidence that they heal by completely different mechanisms to metaphyseal fractures.39,40 Metaphyseal fractures heal in a rapid fashion. Chen et al41 have shown that there are several distinct histological stages in metaphyseal fracture healing with “cellular activation and differentiation, formation of woven bone, transformation of woven bone into lamellar bone and further remodeling”. On the other hand, diaphyseal fractures heal with a complex multistep process, in which both intramembranous and endochondral ossification occur to complete the process.39,41 Animal models using diaphyseal fractures are therefore considered not adequate for osteoporotic fracture research.10
Different osteoporotic induction methods were used in 19 studies in this systematic review. Most authors in this review have used the widely accepted ovariectomized rat model to produce this effect. The ovariectomized rat is the Food and Drug Administration (FDA) approved animal model to study osteoporosis.42 Rats are of low cost, require little maintenance, are easy and safe to handle, and have high reproducibility. It is known that rats reach sexual maturity at 2.5 months of age, and that their skeleton is considered mature after the age of 10 months.43 Both skeletally mature and immature rats can be used for the induction of osteoporosis. The use of the skeletally immature rat is appropriate for osteoporotic research as a low peak bone mass is achieved, which is a high-risk factor for human osteoporotic fractures.44 After ovariectomy in skeletally immature rats, the circulation of oestrogen is reduced and primary osteoporosis Type 1 and postmenopausal status are induced.45 In skeletally mature or aged rats, the process causes cancellous and endocortical bone loss, which exhibits primary osteoporosis Type 2 or senile osteoporosis.44,45 It is also well-established that osteoporosis occurs within two to three months postovariectomy, and studies have also shown that diet modifications can complement this process.10,46 Therefore, osteoporotic induction is adequate for the current rat models.
Osteoporotic models using larger animals, including goat and sheep, have also been described for osteoporotic research, but are considered to be second-line choices by the FDA. These models are less efficacious due to cost and availability, housing and spatial requirements, manageability, and reproducible results.47 Therefore, sample sizes are much lower compared with those in rodent models. However, these animals have the advantage of having haversian systems in bones that resemble those of humans.47 The current FDA-preferred osteoporotic induction method is by ovariectomy. In fact, the hypothalamic-pituitary disconnections performed by Bindl et al37 have unwanted side effects, including polydipsia and polyuria from diabetes insipidus.
Current established and well-accepted parameters for osteoporosis assessment include the use of bone densitometry, such as dual-energy X-ray absorptiometry (DXA) scan, pQCT, and micro-CT, to evaluate the bone mineral density.43 Currently, DXA is the most widely validated technique to measure BMD (bone mineral density), which is the benchmark parameter for reference as defined by the World Health Organization (WHO). Other structural CT parameters can further assess and support architectural changes.
It is well known that the biomechanics and bone tissue quality of osteoporotic bone is significantly different to those of normal healthy bone. Most importantly, if osteoporotic fracture healing is the target of interest, the lack of induction of osteoporosis renders the model clinically irrelevant. Therefore, the induction of osteoporosis is essential in osteoporotic fracture studies.7,10,48
A few studies were simplified metaphyseal fracture models, including drill hole defects23,35,38 and partial osteotomy.37 Understandably, these drill hole defect and partial osteotomy models do not require fixation, and do not represent a clinically relevant scenario. The healing process is also very different from a complete fracture.38 Animal models that characterize a clinical fracture would need to create a complete discontinuity of the bone.48 On the contrary, simplified models with drill holes and partial osteotomies are easy to perform and have high percentages of success.23,37,38 Furthermore, drill hole defects have allowed the investigation of therapeutic drugs and biomaterials.23,38
All three bone defect models had complete discontinuity. These provide a clinically relevant model with the addition of osteoporosis and adequate stability with plate fixation similar to the clinical situation.48 Large metaphyseal defects are often accompanied with bone graft or substitutes during surgery, and the healing is evidently different to that of normal metaphyseal fractures.7 These models are created to best serve the study of biomaterials in the enhancement of osteoporotic fracture healing as stated by Alt et al.7 However, the use of implants with plates and screws increases the cost of the study and has potential complications, such as more technically difficult fixation.32
All 12 complete osteotomy models in this review had appropriate osteoporotic induction and complete discontinuity of bone during the osteotomy. However, the 11 studies by Stuermer et al,10,27,28,33 Kolios et al,25,26,29 and Komrakova et al30,31,34,36 performed bilateral proximal osteotomies on rats. Complete osteotomies are clinically relevant, but it is rare that both limbs are affected in clinical cases. Bilateral osteotomies may need to be avoided for ethical reasons if there is significant negative influence on the weight-bearing status of the animal during the healing phase, which would subsequently affect results.49 It would therefore be more appropriate for animal models to have a unilateral fixation instead. Ibrahim et al2 was the only study with a complete osteotomy unilaterally with fixation, but the authors did not comment on the success rate. Based on current literature, complete osteotomy models are appropriate for the investigation of potential therapeutic osteoporotic drugs and noninvasive interventions.
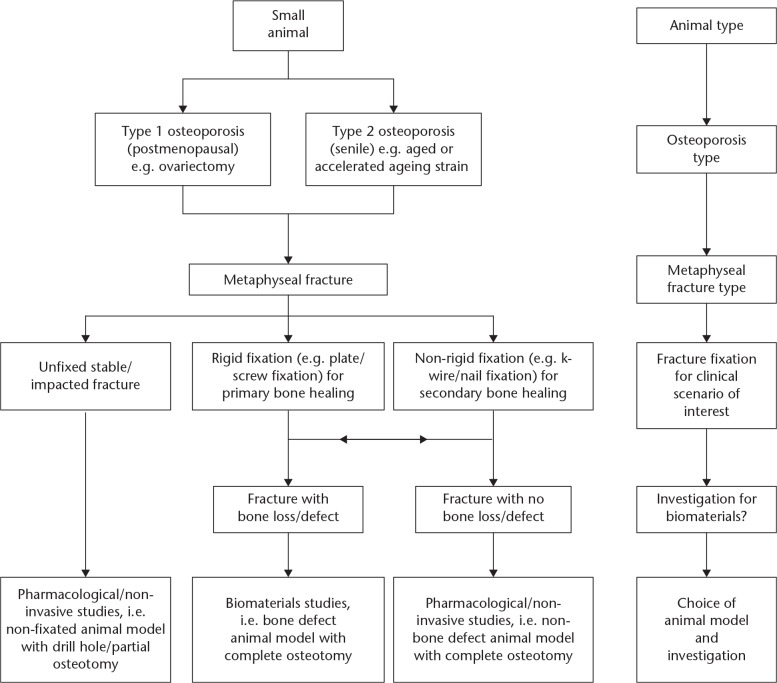
Although there are several metaphyseal models for the analysis of osteoporotic healing, there is room for improvement. Following analysis of the current models, we have derived a recommendation for future models. For a clinically relevant model, we propose that an animal model should satisfy the following criteria to study osteoporotic fracture healing: 1) induction of osteoporosis, 2) complete osteotomy or defect at the metaphysis unilaterally, and 3) internal fixation. Furthermore, in order to match clinical scenarios, we have created an algorithm for investigators to use in deciding the animal model of their interest (Fig. 2).
Fig. 2.
Algorithm for small animal models for the investigation of osteoporotic fracture healing. Fracture types include complete osteotomy, bone defect, drill hole, and partial osteotomy.
Our past research has focused on osteoporotic fracture healing with a diaphyseal animal model.4,5,50 Our previous results show that osteoporotic healing was significantly delayed in terms of active callus formation, mineralization, angiogenesis and remodelling. However, a change in the animal model to a metaphyseal fracture following our new proposed criteria would provide a more accurate depiction of osteoporotic fracture healing. This is essential for quality studies, and for the establishment of future clinical interventions.
Footnotes
Author Contribution: R. M. Y. Wong: Principal investigator, Designing and carrying out the study, Writing the manuscript.
M. H. V. Choy: Co-first author, Reviewing the manuscript, Assisting in carrying out the study.
M. C. M. Li: Reviewing the manuscript, Assisting in carrying out the study.
K-S. Leung: Investigator, Designing and supervising the study, Reviewing the manuscript.
S. K-H. Chow: Investigator, Designing the study, Reviewing the manuscript.
W-H. Cheung: Investigator, Designing and supervising the study, Reviewing the manuscript.
J. C. Y. Cheng: Investigator, Designing the study, Overall supervision of the study, Reviewing the manuscript.
R. M. Y. Wong and M. H. V. Choy contributed to this article equally.
Conflicts of Interest Statement: The authors declare no conflict of interests.
Funding Statement
This project was supported by the Health and Medical Research Fund (HMRF), the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref: 04152406) and Asian Association for Dynamic Osteosynthesis (AADO) Research Fund (Ref: AADO-RF2016-2Y).
References
- 1. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res 2014;29:1667-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-1733. [DOI] [PubMed] [Google Scholar]
- 3. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi HF, Cheung WH, Qin L, Leung AH, Leung KS. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 2010;46:1299-1305. [DOI] [PubMed] [Google Scholar]
- 5. Cheung WH, Sun MH, Zheng YP, et al. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med Biol 2012;38:2120-2129. [DOI] [PubMed] [Google Scholar]
- 6. Larsson S. Treatment of osteoporotic fractures. Scand J Surg 2002;91:140-146. [DOI] [PubMed] [Google Scholar]
- 7. Alt V, Thormann U, Ray S, et al. A new metaphyseal bone defect model in osteoporotic rats to study biomaterials for the enhancement of bone healing in osteoporotic fractures. Acta Biomater 2013;9:7035-7042. [DOI] [PubMed] [Google Scholar]
- 8. Kherad M, Mellström D, Rosengren BE, et al. The number and characteristics of prevalent vertebral fractures in elderly men are associated with low bone mass and osteoporosis. Bone Joint J 2015;97-B:1106-1110. [DOI] [PubMed] [Google Scholar]
- 9. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res 1984;2:97-101. [DOI] [PubMed] [Google Scholar]
- 10. Stuermer EK, Sehmisch S, Rack T, et al. Estrogen and raloxifene improve metaphyseal fracture healing in the early phase of osteoporosis. A new fracture-healing model at the tibia in rat. Langenbecks Arch Surg 2010;395:163-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katae Y, Tanaka S, Sakai A, et al. Elcatonin injections suppress systemic bone resorption without affecting cortical bone regeneration after drill-hole injuries in mice. J Orthop Res 2009;27:1652-1658. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Tan H, Barrero M, et al. Sclerostin antibody treatment enhances fracture healing and increases bone mass and strength in non-fractured bones in an adult rat closed femoral fracture model. Osteoporos Int 2011;22:176. [Google Scholar]
- 13. Hyvönen PM, Karhi T, Kosma VM, Liimola-Luoma L, Hanhijärvi H. The influence of dichloromethylene bisphosphonate on the healing of a long bone fracture, composition of bone mineral and histology of bone in the rat. Pharmacol Toxicol 1994;75:384-390. [DOI] [PubMed] [Google Scholar]
- 14. Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun 2016;7:11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li KC, Chang YH, Yeh CL, Hu YC. Healing of osteoporotic bone defects by baculovirus-engineered bone marrow-derived MSCs expressing MicroRNA sponges. Biomaterials 2016;74:155-166. [DOI] [PubMed] [Google Scholar]
- 16. Ray S, Thormann U, Sommer U, et al. Effects of macroporous, strontium loaded xerogel-scaffolds on new bone formation in critical-size metaphyseal fracture defects in ovariectomized rats. Injury 2016;47(suppl 1):S52-S61. [DOI] [PubMed] [Google Scholar]
- 17. Baier M, Staudt P, Klein R, et al. Strontium enhances osseointegration of calcium phosphate cement: a histomorphometric pilot study in ovariectomized rats. J Orthop Surg Res 2013;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kampschulte M, Krombach GA, Richards DC, et al. Neovascularization of osteoporotic metaphyseal bone defects: A morphometric micro-CT study. Microvasc Res 2016;105:7-14. [DOI] [PubMed] [Google Scholar]
- 19. Nishizuka T, Kurahashi T, Hara T, Hirata H, Kasuga T. Novel intramedullary-fixation technique for long bone fragility fractures using bioresorbable materials. PLoS One 2014;9:e104603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng C, Alt V, Pan L, et al. Application of F-18-sodium fluoride (NaF) dynamic PET-CT (dPET-CT) for defect healing: a comparison of biomaterials in an experimental osteoporotic rat model. Med Sci Monit 2014;20:1942-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng C, Alt V, Pan L, et al. Preliminary evaluation of different biomaterials for defect healing in an experimental osteoporotic rat model with dynamic PET-CT (dPET-CT) using F-18-sodium fluoride (NaF). Injury 2014;45:501-505. [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim NI, Khamis MF, Mod Yunoh MF, et al. Targeted delivery of lovastatin and tocotrienol to fracture site promotes fracture healing in osteoporosis model: micro-computed tomography and biomechanical evaluation. PLoS One 2014;9:e115595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tao ZS, Zhou WS, Tu KK, et al. Effect exerted by Teriparatide upon Repair Function of β-tricalcium phosphate to ovariectomised rat’s femoral metaphysis defect caused by osteoporosis. Injury 2015;46:2134-2141. [DOI] [PubMed] [Google Scholar]
- 24. Thormann U, Ray S, Sommer U, et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013;34:8589-8598. [DOI] [PubMed] [Google Scholar]
- 25. Kolios L, Schumann J, Sehmisch S, et al. Effects of black cohosh (Cimicifuga racemosa) and estrogen on metaphyseal fracture healing in the early stage of osteoporosis in ovariectomized rats. Planta Med 2010;76:850-857. [DOI] [PubMed] [Google Scholar]
- 26. Kolios L, Daub F, Sehmisch S, et al. Absence of positive effect of black cohosh (Cimicifuga racemosa) on fracture healing in osteopenic rodent model. Phytother Res 2010;24:1796-1806. [DOI] [PubMed] [Google Scholar]
- 27. Stuermer EK, Sehmisch S, Daub F, et al. Raloxifene supports early fracture healing more than estrogen in ovariectomized rats. Osteologie 2013;22:290-297. [Google Scholar]
- 28. Stuermer EK, Komrakova M, Sehmisch S, et al. Whole body vibration during fracture healing intensifies the effects of estradiol and raloxifene in estrogen-deficient rats. Bone 2014;64:187-194. [DOI] [PubMed] [Google Scholar]
- 29. Kolios L, Hoerster AK, Sehmisch S, et al. Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcif Tissue Int 2010;86:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Komrakova M, Weidemann A, Dullin C, et al. The impact of strontium ranelate on metaphyseal bone healing in ovariectomized rats. Calcif Tissue Int 2015;97:391-401. [DOI] [PubMed] [Google Scholar]
- 31. Komrakova M, Hoffmann DB, Nuehnen V, et al. The effect of vibration treatments combined with teriparatide or strontium ranelate on bone healing and muscle in ovariectomized rats. Calcif Tissue Int 2016;99:408-422. [DOI] [PubMed] [Google Scholar]
- 32. Thormann U, El Khawassna T, Ray S, et al. Differences of bone healing in metaphyseal defect fractures between osteoporotic and physiological bone in rats. Injury 2014;45:487-493. [DOI] [PubMed] [Google Scholar]
- 33. Stuermer EK, Komrakova M, Werner C, et al. Musculoskeletal response to whole-body vibration during fracture healing in intact and ovariectomized rats. Calcif Tissue Int 2010;87:168-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Komrakova M, Sehmisch S, Tezval M, et al. Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int 2013;92:509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McDonald MM, Morse A, Mikulec K, et al. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res 2012;30:1541-1548. [DOI] [PubMed] [Google Scholar]
- 36. Komrakova M, Stuermer EK, Werner C, et al. Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010;47:480-492. [DOI] [PubMed] [Google Scholar]
- 37. Bindl R, Oheim R, Pogoda P, et al. Metaphyseal fracture healing in a sheep model of low turnover osteoporosis induced by hypothalamic-pituitary disconnection (HPD). J Orthop Res 2013;31:1851-1857. [DOI] [PubMed] [Google Scholar]
- 38. Alt V, Cheung WH, Chow SK, et al. Bone formation and degradation behavior of nanocrystalline hydroxyapatite with or without collagen-type 1 in osteoporotic bone defects - an experimental study in osteoporotic goats. Injury 2016;47(suppl 2):S58-S65. [DOI] [PubMed] [Google Scholar]
- 39. Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater 2008;15:53-76. [DOI] [PubMed] [Google Scholar]
- 40. Sandberg OH, Aspenberg P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Joint Res 2015;4:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen WT, Han C, Zhang PX, et al. A special healing pattern in stable metaphyseal fractures. Acta Orthop 2015;86:238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone 1995;17(suppl):125S-133S. [DOI] [PubMed] [Google Scholar]
- 43. Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med 2008;58:424-430. [PMC free article] [PubMed] [Google Scholar]
- 44. Turner RT, Maran A, Lotinun S, et al. Animal models for osteoporosis. Rev Endocr Metab Disord 2001;2:117-127. [DOI] [PubMed] [Google Scholar]
- 45. Simpson AH, Murray IR. Main differences in osteoporotic fracture models: which should I use? Injury 2016;47(suppl 1):S15-S20. [DOI] [PubMed] [Google Scholar]
- 46. Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 1991;15:175-191. [DOI] [PubMed] [Google Scholar]
- 47. Reinwald S, Burr D. Review of nonprimate, large animal models for osteoporosis research. J Bone Miner Res 2008;23:1353-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheung WH, Miclau T, Chow SK, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury 2016;47(suppl 2):S21-S26. [DOI] [PubMed] [Google Scholar]
- 49. Auer JA, Goodship A, Arnoczky S, et al. Refining animal models in fracture research: seeking consensus in optimising both animal welfare and scientific validity for appropriate biomedical use. BMC Musculoskelet Disord 2007;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chung SL, Leung KS, Cheung WH. Low-magnitude high-frequency vibration enhances gene expression related to callus formation, mineralization and remodeling during osteoporotic fracture healing in rats. J Orthop Res 2014;32:1572-1579. [DOI] [PubMed] [Google Scholar]