Abstract
Objectives
The diagnosis of periprosthetic joint infection (PJI) is difficult and requires a battery of tests and clinical findings. The purpose of this review is to summarize all current evidence for common and new serum biomarkers utilized in the diagnosis of PJI.
Methods
We searched two literature databases, using terms that encompass all hip and knee arthroplasty procedures, as well as PJI and statistical terms reflecting diagnostic parameters. The findings are summarized as a narrative review.
Results
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were the two most commonly published serum biomarkers. Most evidence did not identify other serum biomarkers that are clearly superior to ESR and CRP. Other serum biomarkers have not demonstrated superior sensitivity and have failed to replace CRP and ESR as first-line screening tests. D-dimer appears to be a promising biomarker, but more research is necessary. Factors that influence serum biomarkers include temporal trends, stage of revision, and implant-related factors (metallosis).
Conclusion
Our review helped to identify factors that can influence serum biomarkers’ level changes; the recognition of such factors can help improve their diagnostic utility. As such, we cannot rely on ESR and CRP alone for the diagnosis of PJI prior to second-stage reimplantation, or in metal-on-metal or corrosion cases. The future of serum biomarkers will likely shift towards using genomics and proteomics to identify proteins transcribed via messenger RNA in response to infection and sepsis.
Cite this article: A. Saleh, J. George, M. Faour, A. K. Klika, C. A. Higuera. Serum biomarkers in periprosthetic joint infections. Bone Joint Res 2018;7:85–93. DOI: 10.1302/2046-3758.71.BJR-2017-0323.
Keywords: Serum biomarkers, Periprosthetic joint infection, Hip, Knee, Joint arthroplasty
Article focus
Serum biomarkers remain a fundamental part of the evaluation and diagnosis of hip and knee periprosthetic joint infection. This article explores the evidence for promising new serum markers and explores factors affecting conventional serum testing, in order to enable clinicians to accurately interpret these tests.
Currently, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) continue their role as first-line screening tests for PJI. However, due to the lack of specificity, these tests are complemented by more specific synovial tests and microbiology findings, and should not be utilized alone in the diagnosis of PJI.
Other serum biomarkers have not demonstrated superior sensitivity and have failed to replace CRP and ESR as first-line screening tests.
Key messages
Our review helped identify factors that can influence serum biomarkers’ level changes; the recognition of such factors can help improve their diagnostic utility.
As such, we cannot rely on ESR and CRP alone for the diagnosis of PJI prior to second-stage reimplantation, or in metal-on-metal or corrosion cases.
D-dimer appears to be a promising biomarker, but more research is necessary.
Strengths and limitations
This review included the most recently published articles and the most up-to-date research on periprosthetic joint infection detection measures (within five years). However, we did not exclude landmark studies published before the selected window.
The literature review was comprehensive and included all serum biomarkers reported in the literature, their correlation with periprosthetic joint infection, and factors that can influence the serum biomarkers levels. However, the focus of the search was only for total hip and total knee arthroplasty studies; other forms of arthroplasty were excluded (shoulder or elbow).
Introduction
Periprosthetic joint infection (PJI) is a dreaded complication of total hip and knee arthroplasty. It poses a challenge to the orthopaedic surgeon with regards to prevention, diagnosis, and management. The diagnosis of PJI has been a subject of extensive research in recent years, as surgical management will require implant removal and possibly delayed reimplantation (two-stage revision).1–3 Therefore, having an accurate preoperative diagnosis is critical for surgical planning and managing patient expectations.4,5
In an effort to standardize the diagnosis of PJI, the Musculoskeletal Infection Society (MSIS) convened a workgroup in 2011 to issue diagnostic criteria for PJI;6 this was later modified in 2014.7 Some of the criteria included in this definition are only available to the surgeon intraoperatively (histology, presence of purulence) or postoperatively (microbiology), and therefore do not contribute towards a preoperative plan. Hence, more recent research has focused on preoperative criteria, such as synovial fluid tests (white blood cell count (WBC) and leucocyte esterase) and serological tests (conventional C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)). While synovial biomarkers have generally shown superior accuracy compared to serum biomarkers,8,9 serum tests remain the less invasive, first-line screening tool. The search continues for an ideal serum biomarker that offers high sensitivity and specificity to limit invasive and unnecessary joint arthrocentesis and extensive infection workup.
The purpose of this review is to summarize current evidence of the diagnostic accuracy of conventional serum biomarkers, and to explore the evidence for promising new serum markers. Furthermore, we explored factors affecting conventional serum testing, in order to enable clinicians to accurately interpret these tests.
Patients and Methods
Search strategy and selection criteria
We searched MEDLINE (1946 to 10 February 2017) and EMBASE (1974 to 08 February 2017). We used search terms that encompass all hip and knee arthroplasty procedures, as well as periprosthetic joint infection. The search also included an extensive list of biomarkers and statistical terms reflecting diagnostic parameters, such as sensitivity, specificity, and diagnostic odds ratio. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. After duplicates were removed, the search resulted in 556 articles. Only total hip and total knee arthroplasty studies were included; other forms of arthroplasty were excluded (shoulder or elbow). We largely selected articles published within the past five years, but did not exclude older articles that are commonly referenced and highly regarded. Articles evaluating synovial biomarkers were also excluded, as the focus of this review is limited to serum biomarkers.
Parameters of diagnostic performance
Interpreting studies of diagnostic tests requires an understanding of the many indicators of test performance. A brief introduction to these different indicators is therefore necessary. In the context of PJI, patients are grouped as either infected or noninfected. Such a dichotomization enables one to represent the comparison between a diagnostic test and its reference standard in a 2 × 2 contingency table (Table I). Common indicators derived from such a 2 × 2 table are the sensitivity and specificity of the test, the positive and negative predictive values, and the positive and negative likelihood ratios (Table II). None of these indicators validly represents the test’s discriminatory performance. Sensitivity is only part of the discriminatory evidence, as high sensitivity may be accompanied by low specificity. Additionally, no simple aggregation rule exists to combine sensitivity and specificity into one measure of performance.
Table I.
2 × 2 contingency table
| Reference test (e.g. MSIS definition) |
||
|---|---|---|
| Infected | Noninfected | |
| New test: positive | True positive | False positive |
| New test: negative | False negative | True negative |
MSIS, Musculoskeletal Infection Society
Table II.
Commonly used test parameters in diagnostic studies
| Parameter | Formula | Definition |
|---|---|---|
| Sensitivity | TP / (TP + FN) | Proportion of positive result in infected patients |
| Specificity | TN / (TN + FP) | Proportion of negative result in noninfected patients |
| Positive predictive value (PPV) | TP / (TP + FP) | Proportion of infection among patients with a positive result |
| Negative predictive value (NPV) | TN / (TN + FN) | Proportion of no infection among patients with a negative result |
| Positive likelihood ratio (LR+) | Sensitivity / (1 - specificity) | Ratio of a positive result in infected patients to a positive result in noninfected patients |
| Negative likelihood ratio (LR-) | (1 - sensitivity) / specificity | Ratio of a negative result in infected patients to a negative result in noninfected patients |
| Accuracy | (TP +TN) / (TP + TN + FP + FN) | Global measure of performance |
| Youden’s index10 | Sensitivity + specificity - 1 | Global measure of performance |
TP, true positive; FP, false positive; FN, false negative; TN, true negative
Single indicators of test performance include accuracy, Youden’s index,10 receiver operating characteristic (ROC) curve, and the diagnostic odds ratio (DOR). Accuracy refers to the percentage of patients correctly classified by the test under evaluation. This percentage depends on the prevalence of the PJI in the study group whenever sensitivity and specificity are not equal, and it weighs false positive and false negative findings equally. Youden’s index is derived from sensitivity and specificity; as such, it is independent of prevalence.10 However, because Youden’s index is a linear transformation of the mean sensitivity and specificity, its values are difficult to interpret.10
The ROC curve is a plot of all pairs of sensitivity and specificity values for every cutoff value (Fig. 1). The shape of a ROC curve and the area under the curve (AUC) helps estimate the discriminative power of a test. The closer the curve is located on the upper-left hand corner and the larger the area under the curve, the better the test is at discriminating between infected and noninfected patients. AUC can have values between 0 and 1. A perfect test has an AUC of 1, whereas a nondiscrimination test has an AUC of 0.5. AUC is a global measure of diagnostic accuracy. For example, two different tests may have identical AUCs, but one can have significantly higher sensitivity, whereas the other can have significantly higher specificity. Global measures like AUC are used for general assessment and for comparison of two or more diagnostic tests. DOR is another global measure, and it represents the ratio of the odds of positivity in infected patients relative to the odds of positivity in noninfected patients. The value of DOR ranges from 0 to infinity, with higher values indicating better discriminatory test performance. A value of 1 means that a test does not discriminate between infected and noninfected patients.11
Fig. 1.
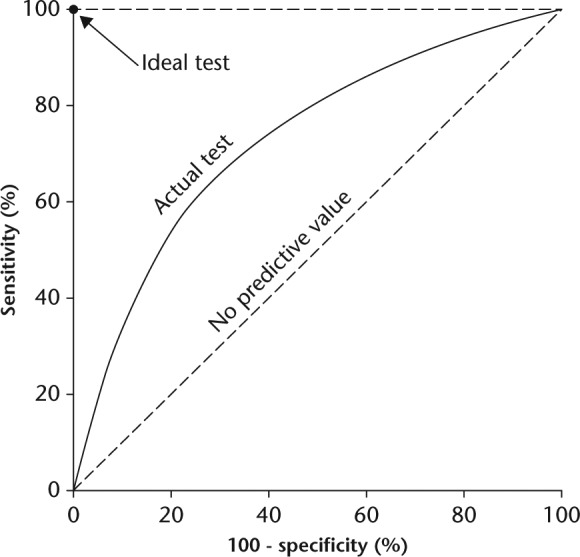
Receiver operating characteristic (ROC) curve
Results
Diagnostic efficacy
Erythrocyte sedimentation rate and C-reactive protein
ESR and CRP are by far the most commonly published serum biomarkers in PJI literature. ESR has been known to be elevated in inflammatory conditions since 1921, when Westergren12 recognized the usefulness of ESR in tuberculosis.13 Increases in fibrinogen and other normal plasma proteins, and the presence of abnormal circulating proteins derived from necrotic tissue, can enhance red-cell aggregation and accelerate the settling of erythrocytes, thus increasing the measured ESR. CRP is an archetype acute phase protein found in 1930 by Tillett and Francis.14,15 It is produced by the liver with maximum production 24 to 35 hours after inflammation onset. Studies using liver tissue cultures show that interleukin-6 (IL-6) is a massive inducer of CRP microRNA in liver cells. CRP binds Gram-positive and Gram-negative bacteria and stimulates their adhesion and phagocytosis by leucocytes. It is not a specific parameter for the presence of infectious induced inflammation, as it is also elevated in systemic autoimmune diseases such as rheumatoid arthritis, after trauma, or after surgery and tissue damage.16 On the other hand, its concentration decreases with the use of systemic corticosteroids.17
Due to their high sensitivity and routine accessibility, ESR and CRP currently remain as first-line screening tests for PJI. The articles included in this review showed that the sensitivity and specificity of ESR ranges from 42% to 94%, and 33% to 87%, respectively.18-24 CRP had sensitivities and specificities ranging from 74% to 94%, and 20% to 100%, respectively. If a positive ESR or CRP is considered the threshold for infection, the sensitivity is increased up to 97% but the specificity can be as low as 23%.21 On the other hand, if a positive ESR and a positive CRP is considered the threshold for infection, specificity is enhanced up to 93%, at the cost of sensitivity.20
Even in patients with inflammatory conditions, ESR and CRP are still helpful diagnostic tools. Cipriano et al25 aimed to evaluate the performance of ESR and serum CRP in diagnosing PJI in patients with inflammatory arthritis as opposed to noninflammatory arthritis. They found similar accuracy for both ESR and CRP in both inflammatory and noninflammatory groups (ESR AUC, 0.85 and 0.85, respectively; CRP AUC, 0.88 and 0.85, respectively). However, due to their modest specificity, relying solely on ESR and CRP in complex clinical scenarios is not appropriate.
Interleukin-6 (IL-6)
IL-6 is an inflammatory cytokine produced by stimulated monocytes and macrophages. It induces the production of acute-phase proteins, including CRP, and acts as a differentiating factor for B-lymphocytes and an activating factor for T-lymphocytes. A meta-analysis published in 2010 attracted attention to serum IL-6 as a potential superior diagnostic test compared with the conventional ESR and CRP.26 In this meta-analysis, the pooled sensitivity and specificity for IL-6 were 97% and 91%, respectively. Its DOR was higher than that for ESR and CRP. However, the data were mainly driven by one large and two smaller studies.27,28 More recent studies have found the diagnostic performance of serum IL-6 comparable to CRP. Glehr et al29 performed a prospective study including 124 revision arthroplasties and used MSIS criteria. IL-6 had a sensitivity, a specificity, and an AUC of 81%, 68%, and 0.80, respectively. CRP, in comparison, had an AUC of 0.9. Ettinger et al30 also used MSIS criteria in a prospective study to evaluate IL-6; with a cutoff value of 5.12 pg/ml, the authors found it to be 80% sensitive and 87.7% specific. Gollwitzer et al31 prospectively evaluated 35 patients undergoing revision total hip arthroplasty (THA) and total knee arthroplasty (TKA), including only purulent infection cases with Staphylococcus species, and compared them with aseptic loosening. Their criteria for infection included clinical exam (sinus tract), intraoperative cultures, histology findings, and CRP. Serum IL-6 had a sensitivity of 0.48, a specificity of 0.95, and an AUC of 0.687. Randau et al32 also performed a prospective study of 120 patients undergoing revision TKA and THA, and reported a sensitivity and specificity of IL-6 ranging from 49% to 79%, and 58% to 88%, respectively, depending on the cutoff value used. This variability and lack of consistency in the results have limited its implementation as a screening test to replace serum CRP and ESR.
White blood cell count (WBC)
While synovial WBC count and differential is one of the main diagnostic criteria for PJI, serum WBC count has very little utility. In a prospective study of 120 patients that underwent revision THA and TKA, Friedrich et al33 found a sensitivity of 21% and a specificity of 94% for serum WBC when using microbiology and histology data as a reference test. In another prospective study of 78 patients that underwent revision THA and TKA, Bottner et al27 found it to be 70% sensitive and 60% specific. Overall, these findings limit its utility in the diagnosis of PJI.
Procalcitonin
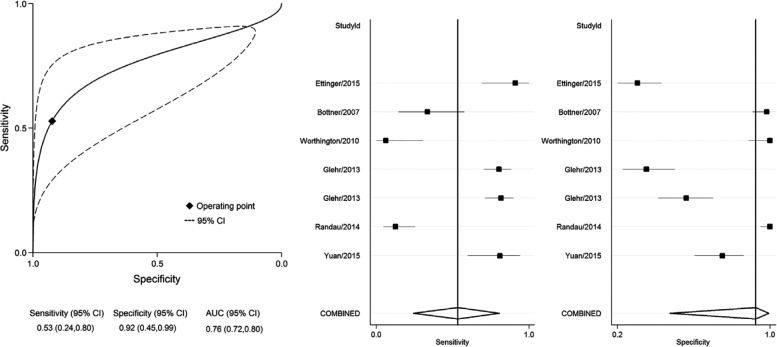
In the past 15 years, procalcitonin (PCT) has gained ground as a biomarker for sepsis.34 Procalcitonin is a protein produced by neuroendorcine cells and the parafollicular cells of the thyroid under physiological conditions. In sepsis, the main producers of PCT are macrophages and monocytic cells of different organs, especially the liver.15 PCT was found to have a high diagnostic accuracy for the identification of systemic infection.35 However, its diagnostic value for detecting PJI is uncertain. A recent meta-analysis helped to compile the results of six studies evaluating the diagnostic efficacy of PCT in PJI (Table III).36 PCT was found to have a pooled sensitivity, specificity, DOR, and AUC of 53%, 92%, 13, and 0.76 respectively (Fig. 2). Based on these results, serum PCT is not an ideal biomarker for the diagnosis of PJI because of its low sensitivity and small AUC.
Table III.
Characteristics of six studies evaluating procalcitonin (PCT) for the diagnosis of periprosthetic joint infection (PJI)
| Study | No. of patients | Study design | Cutoff | Joint | Reference test |
|---|---|---|---|---|---|
| Yuan et al,67 2015 | 75 | Prospective | 0.5 ng/ml | Hip | Histology, intraoperative findings |
| Ettinger et al,30 2015 | 77 | Prospective | 0.025 ng/ml | Hip, knee, shoulder | Histology, microbiology, intraoperative findings |
| Randau et al,32 2014 | 120 | Prospective | 46 ng/ml | Hip, knee | Histology, microbiology, intraoperative findings |
| Glehr et al,29 2013 | 124 | Prospective | 0.35 ng/ml; 0.055 ng/ml | Hip, knee | Histology, microbiology, intraoperative findings |
| Worthington et al,39 2010 | 46 | Prospective | 0.5 ng/ml | Hip | Microbiology |
| Bottner et al,27 2007 | 78 | Prospective | 0.3 ng/ml | Hip, knee | Histology, intraoperative findings |
Reproduced with permission from: Xie K, Qu X, Yan M. Procalcitonin and alpha-Defensin for Diagnosis of Periprosthetic Joint Infections. J Arthroplasty 2017;32:1387-1394. The reference numbers given in the ‘Study’ column correspond with the reference list in this paper, not the reference list given by Xie et al in their original study
Fig. 2.
Summary receiver operating characteristic curves and forest plot for procalcitonin. Reproduced with permission from: Xie K, Qu X, Yan M. Procalcitonin and alpha-Defensin for Diagnosis of Periprosthetic Joint Infections. J Arthroplasty 2017;32:1387-1394. CI, confidence interval; AUC, area under the curve.
Other biomarkers
Several other potential serum biomarkers have been identified in recent literature. One promising biomarker is D-dimer, a widely available serum biomarker that is known for its diagnostic utility of fibrinolytic activities in thromboembolic events, and that has demonstrated high sensitivity in the diagnosis of PJI.37 In an ongoing prospective cohort of 154 patients, Shahi et al37 found D-dimer to be have better sensitivity (97.7%) and specificity (93.6%) than ESR and CRP. D-dimer is an easily accessible assay in common routine practice, but more studies that reproduce these findings are needed to confirm its superiority to the conventional ESR and CRP.
Tumour necrosis factor-a (TNF-a) is another acute-phase inflammatory cytokine released by monocytes and macrophages that has been investigated in PJI. Two studies evaluated TNF-a using older PJI criteria27 and, more recently, MSIS criteria.30 Both showed similar diagnostic parameters: low sensitivity (43% and 35%) and high specificity (94% and 86%). Technical drawbacks include long processing times (over two hours) and the instability of the sample, which means it needs to be processed within 60 minutes of being drawn.27 Therefore, based on these results, TNF-a is not an ideal serum screening test for PJI.
Intercellular adhesion molecule-1 (ICAM-1) is a membrane glycoprotein that plays a key role in leucocyte migration and activation that has also been studied in the context of PJI. Drago et al38 studied ICAM-1 in a small sample of 52 patients undergoing revision hip or knee arthroplasty. Their definition of PJI depended largely on clinical signs and positive cultures. While they did not describe diagnostic parameters of ICAM-1, they found it to be significantly elevated in PJI patients compared with noninfected patients. Similarly, Worthington et al39 found ICAM-1 levels to be elevated in PJI patients. However, they did not specify which diagnostic parameters were used to define PJI. Further research quantifying its diagnostic performance is necessary before considering ICAM-1 as a clinically relevant serum biomarker for diagnosis of PJI.
Lipopolysaccharide-binding protein (LBP) is a polypeptide synthesized in hepatocytes when induced by IL-1, or synergistically by IL-1 and IL-6; the latter response can be enhanced by TNF-a.40,41 LBP facilitates bacterial lipopolysaccharide binding to CD14, which is present on monocytes and macrophages. Elevated LBP levels have been reported in neonatal early sepsis. While LBP may theoretically be a potential biomarker for PJI, two separate clinical studies showed little clinical utility with low sensitivity and specificity for PJI.30,33
Flow cytometry is another diagnostic method that has been investigated in the context of sepsis. This includes evaluating CD64 surface marker expression on neutrophils. CD64 can be used as a marker for neutrophil activation. While this has not been studied in PJI patients, Fjaertoft et al42 conducted a study to test whether CD64 level can differentiate between inflammation caused by bacterial infection versus inflammation brought on by surgical trauma. The authors found its expression to be significantly higher in infected patients, rather than those who underwent uncomplicated primary total hip arthroplasty (THA). The authors concluded that the expression of CD64 on neutrophils is a specific sign of bacterial infection. There are currently no studies comparing CD64 levels in noninfected arthroplasty patients with PJI patients.
Trends and factors affecting serum biomarkers
There is considerable variation in the literature with regards to appropriate thresholds for common serum biomarkers. In particular, ESR and CRP are nonspecific markers, and their measurement is affected by a variety of factors, including temporal trends, stage of inflammation (first-stage revision or explantation versus second-stage revision or reimplantation), patient factors (age, gender, underlying disease, medication), and implant factors (metal-on-metal bearing surfaces and corrosion).
Normal temporal trends
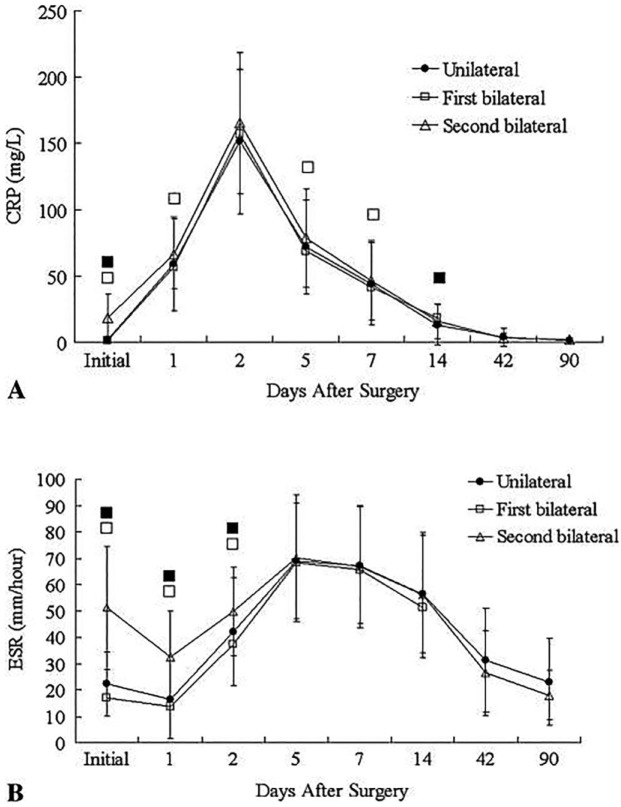
Time after index arthroplasty can have a confounding influence on ESR and CRP.20,43 In an effort to describe normal temporal trends of ESR and CRP, Park et al43 prospectively evaluated 320 total knee arthroplasties performed in 214 consecutive patients without developing infection for more than one year. They measured ESR and CRP at several timepoints and compared unilateral TKAs with staged bilateral TKAs. According to their results, CRP increased rapidly on the second day, then decreased rapidly from peak levels within two weeks, and reached normal levels by the 42nd day (Fig. 3). In contrast, ESR levels reached peaked levels on the fifth day, and remained increased elevated above 20 mm/hour on the 42nd day. It returned to close to preoperative levels on the 90th day. These temporal trends were similar between unilateral and staged bilateral TKAs.
Fig. 3.
Line graphs showing the temporal patterns of perioperative mean levels of A) C-reactive protein and B) Erythrocyte sedimentation rate (ESR) in unilateral, first knee bilateral, and second knee bilateral groups. Values with statistical significance are marked with a black box (■) for the comparisons between the unilateral and the second knee bilateral groups and with a white box (□) for the comparisons between the first knee bilateral and the second knee bilateral groups. Reproduced with permission from: Park KK, Kim TK, Chang CB, Yoon SW, Park KU. Normative Temporal Values of CRP and ESR in Unilateral and Staged Bilateral TKA. Clin Orthop Relat Res 2008;466:179-188.
Alijanipour et al44 also sought to define thresholds values for ESR and CRP in hip versus knee PJI, and for early postoperative and late-chronic PJI. In their institutional database of revision arthroplasty cases, the authors identified 108 hip PJI and 165 knee PJI cases based on the MSIS criteria. The authors used the four-week mark to separate early from late PJI. ESR values were not statistically different between hip and knee PJI, but median CRP values were higher in knee PJI (13.3 mg/dl) compared with hip PJI (7.3 mg/dl, p = 0.02). For ESR, the authors proposed a higher threshold of 54.5 mm/hour in early postoperative PJI, and 46.5 mm/hour in late-chronic PJI. For CRP, the threshold in early hip and knee PJI was 2.3 mg/dl, but in late-chronic PJI was 1.3 mg/dl for hips and 2.3 mg/dl for knees.
Trends prior to second-stage re-implantation
Two-stage revision arthroplasty of infected prosthetic joints is commonly performed in North America as definitive treatment of PJI.45,46 Determining whether a patient who has undergone the first stage of a staged revision arthroplasty is ready for reimplantation is a diagnostic dilemma. These patients have recently undergone explantation, typically in the six to eight weeks prior, which affects nonspecific inflammatory markers such as ESR and CRP. A progressively decreasing ESR and CRP have been considered favourable indicators for reimplantation in the absence of clinical signs of PJI.47
Ghanem et al48 retrospectively reviewed revision arthroplasty database at one institution and identified 109 consecutive patients who underwent two-stage revision arthroplasty. The authors measured serum ESR and CRP at a mean of 13 days prior to reimplantation (a mean of 94 days after first-stage explantation). The decision to proceed with the second-stage procedure was based on the absence of clinical signs of infection and on negative aspirate cultures. Using persistent PJI requiring further operation after the reimplantation procedure as an outcome measure, ESR and CRP both showed poor sensitivity and specificity (AUC of 0.5 and 0.54, respectively) in predicting persisting infection. The failure of ESR to emerge as a valuable marker in predicting persistent infection is not surprising, considering that normalization may not occur for at least three months.43 Similarly, Shukla et al49 reported poor accuracy of ESR and CRP prior to second-stage reimplantation of 87 patients with hip PJI. They found synovial fluid WBC count to be the best test for identifying infection (sensitivity, 78%; specificity, 96%). Kusuma et al50 showed that while ESR and CRP levels decreased between first stage and second stage, they were unable to identify an optimum cutoff value due to their poor diagnostic performance.
More recently, Hoell et al51 investigated the diagnostic accuracy of IL-6 prior to second-stage reimplantation. The authors reviewed 55 patients with hip and knee PJI defined by growing the same organism in at least two periprosthetic tissue cultures. Of those, 16 were found to have persistent infection (two positive cultures from at least three cultures at the time of reimplantation). Serum IL-6 had an AUC of 0.896 and an optimal cutoff value of ⩾ 13 pg/ml, indicating persistent infection; ⩽ 8 indicated absence of infection, and a range of nine to 12 was indeterminate. The authors also evaluated serum CRP, which was found to have an AUC of 0.704, higher than what has been reported in previous studies. When a cutoff value of > 2.5 mg/dl is used, CRP has a sensitivity of 43.7% and specificity of 92.3%. It is possible that serum IL-6 may have a role in deciding infection eradication prior to second-stage reimplantation, but more research is necessary to confirm these findings.
Currently, there is no ideal serum biomarker with which to identify persistent infection prior to second-stage reimplantation. Research studies in this area have been hampered by the small sample size and the lack of a uniform definition for success or failure of second-stage procedures. Studies from our institution showed that MSIS criteria, the so-called benchmark for diagnosing PJI, are not useful for determining infection resolution of PJI after explantation.52,53 Frangiamore et al52 reported on 35 patients with antibiotic cement spacer before second-stage reimplantation with a minimum of one-year follow-up. When using the Delphi-based international consensus for success after treatment of PJI,54 MSIS had a sensitivity of 0 and a specificity of 89%. A second, larger study that evaluated 97 patients undergoing second-stage reimplantation also showed high specificity but low sensitivity for MSIS criteria for diagnosing persistent infection.53 While MSIS is clinically useful for ruling out persistent PJI at the time of reimplantation, the lack of its sensitivity precludes its use as a reference test for examining diagnostic tests. It is recommended that studies evaluating infection resolution should focus on long-term follow-up showing infection-free survival and lack of subsequent need for surgical intervention. However, even with such an approach, it is difficult to establish whether a patient develops an infection after reimplantation due to a recurrent infection or due to a new infection, especially when the culture results do not match.
Obesity and serum biomarkers
Obesity is a well-recognized risk for infection following total joint arthroplasty.55 In addition to being a risk factor, obesity has also been recognized as an inflammatory state.56 This is important to recognize when evaluating inflammatory markers for the diagnosis of PJI, as some obese patients without PJI may have false positive results. Motaghedi et al57 prospectively studied 60 patients undergoing THA to measure the inflammatory response by circulating levels of cytokines preoperatively and 24 hours postoperatively. The authors demonstrated enhanced cytokine reactivity and positive correlation between body mass index and IL-1β, IL-6, and TNF-a. Liu et al58 evaluated both ESR and CRP in obese and nonobese patients undergoing revision arthroplasty. Using MSIS criteria to identify PJI in their patient population, there was no difference in ESR and CRP values between obese and nonobese patients. However, using ROC curves, a higher cutoff value for diagnosing PJI in obese patients was found (3.6 mg/dl vs 1.4 mg/dl).
Periprosthetic fractures
Periprosthetic fractures can occur in patients with PJI. Superimposed PJI in the setting of fractures will certainly change surgical management, but also will be difficult to diagnose due to the acute inflammatory response associated with trauma. Chevillotte et al59 retrospectively evaluated 204 patients who sustained periprosthetic fractures after THA in order to characterize the increase in serum inflammatory markers and the positive predictive value for PJI. The authors measured serum levels of ESR, CRP, and WBC; infection was defined as the presence of the same organism on two or more cultures. The prevalence of PJI in periprosthetic fracture was 11.6%. ESR, CRP, and WBC were all poor at identifying infection in this population, with AUC ranging between 0.5 and 0.66. Based on their findings, the authors recommended against using elevated ESR and CRP as reliable predictors of PJI in the setting of periprosthetic fractures and, therefore, additional workup such as hip aspiration in those cases is not indicated based on these marker values alone.
Metal-on-metal bearing surfaces and corrosion
Metal-on-metal (MOM) THA and corrosion at the head-neck junction can lead to an adverse local tissue reaction (ALTR), which can present similarly to PJI and result in purulent-appearing synovial fluid.60,61 ALTR can be misleading and result in frequent false positive diagnosis of PJI in these patients, making the diagnosis of PJI more challenging. For example, studies have shown that synovial α-defensin, which is generally considered to be an accurate diagnostic test for PJI, is influenced by metallosis, leading to false positive results.62,63 Unfortunately, serum biomarkers were not found to be any more successful at identifying PJI in MOM THA. Yi et al64 found that ESR and CRP had positive predictive values of only 43% and 39%, with AUC of 0.88 and 0.85, respectively. While these markers were found to be somewhat sensitive, positive ESR and CRP in the setting of MOM should be interpreted with caution to avoid a false diagnosis of infection. With regards to corrosion at the metallic modular taper interfaces in THA, one study evaluated ESR and CRP levels in infected and noninfected patients who had dual taper modular stems.65 ESR and CRP were both found to have low sensitivity (57% and 29%, respectively) and high specificity (95% and 93%, respectively) for PJI. Again, this data shows limited utility of serum biomarkers in the context of corrosion.
Other related postoperative complications
When CRP is abnormally high after three weeks postoperatively or shows a bimodal pattern (elevation-depression-elevation), infection is suspected. However, a retrospective study examined 76 patients who had a bimodal pattern of CRP change, and found that only 18 patients had surgical site infection, while the remaining 58 patients were diagnosed with respiratory complications (pneumonia or upper respiratory infections), gastrointestinal (ileus or colitis), urinary tract infections, or deep venous thrombosis, or were thought to present with idiopathic elevations.66 Therefore, serologic marker thresholds and values should be interpreted in the context of the overall clinical presentation.
Conclusion
Serum biomarkers remain a fundamental part of the evaluation and diagnosis of hip and knee PJI. While certain synovial markers have proven to be superior diagnostic tools, serum biomarkers are less invasive screening tests that should be implemented prior to joint aspiration. Currently, ESR and CRP continue their role as first-line screening tests for PJI. However, due to the lack of specificity, these tests are complemented by more specific synovial tests and microbiology findings, and should not be utilized alone in the diagnosis of PJI. Other serum biomarkers have not demonstrated superior sensitivity and have failed to replace ESR and CRP as first-line screening tests. D-dimer appears to be a promising biomarker, but more research is necessary. Our review helped identify factors that can influence serum biomarkers level changes, and the recognition of such factors can help improve their diagnostic utility. As such, we cannot rely on ESR and CRP alone for the diagnosis of PJI prior to second-stage reimplantation, or in metal-on-metal or corrosion cases. The future of serum biomarkers will likely shift towards genomics and proteomics to identify proteins transcribed via messenger RNA in response to infection and sepsis.
Footnotes
Author Contribution: A. Saleh: Search strategy, Screening abstracts and selecting articles, Writing the manuscript.
J. George: Full-text article review, Data extraction.
M. Faour: Full-text article review, Data extraction.
A. K. Klika: Reviewing and revising the manuscript.
C. A. Higuera: Reviewing and revising the manuscript.
Conflicts of Interest Statement: The authors declare that there are no conflicts of interest.
Funding Statement
No funding was received for the production of this work. However, C. A. Higuera reports grants from KCI, Stryker, Mioscience, CD Diagnostics, and OREF, none of which relate to the current study. C. A. Higuera also reports consulting fees from KCI, Convatec, and Pfizer, none of which relate to the current study.
References
- 1. Lenguerrand E, Whitehouse MR, Beswick AD, et al. Revision for prosthetic joint infection following hip arthroplasty: Evidence from the National Joint Registry. Bone Joint Res 2017;6:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez-Perez M, Perez-Jorge C, Lozano D, et al. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model: An in vitro approach to the “race for the surface” theory. Bone Joint Res 2017;6:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills L, Tsang J, Hopper G, Keenan G, Simpson AHRW. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res 2016;5:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samara E, Moriarty TF, Decosterd LA, et al. Antibiotic stability over six weeks in aqueous solution at body temperature with and without heat treatment that mimics the curing of bone cement. Bone Joint Res 2017;6:296-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuenyongviwat V, Ingviya N, Pathaburee P, Tangtrakulwanich B. Inhibitory effects of vancomycin and fosfomycin on methicillin-resistant Staphylococcus aureus from antibiotic-impregnated articulating cement spacers. Bone Joint Res 2017;6:132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty 2011;26:1136-1138. [DOI] [PubMed] [Google Scholar]
- 7. Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty 2014;29:1331. [DOI] [PubMed] [Google Scholar]
- 8. Deirmengian C, Kardos K, Kilmartin P, et al. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res 2014;472:3254-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saleh A, Ramanathan D, Siqueira MBP, et al. The diagnostic utility of synovial fluid markers in periprosthetic joint infection: a systematic review and meta-analysis. J Am Acad Orthop Surg 2017;25:763-772. [DOI] [PubMed] [Google Scholar]
- 10. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-35. [DOI] [PubMed] [Google Scholar]
- 11. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129-1135. [DOI] [PubMed] [Google Scholar]
- 12. Westergren A. Studies of the suspension stability of the blood in pulmonary tuberculosis. Acta Med Scand 192;54:247-282. [Google Scholar]
- 13. Schulak DJ, Rayhack JM, Lippert FG, 3rd, Convery FR. The erythrocyte sedimentation rate in orthopaedic patients. Clin Orthop Relat Res 1982;167:197-202. [PubMed] [Google Scholar]
- 14. Tillet WS, Francis T. Serological reactions in pneumonia with non protein somatic fractin of pneumocoocus. J Exp Med 1930;52:561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prucha M, Bellingan G, Zazula R. Sepsis biomarkers. Clin Chim Acta 2015;440:97-103. [DOI] [PubMed] [Google Scholar]
- 16. Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunol Res 2013;56:131-142. [DOI] [PubMed] [Google Scholar]
- 17. Greenberg SB. Infections in the immunocompromised rheumatologic patient. Crit Care Clin 2002;18:931-956. [DOI] [PubMed] [Google Scholar]
- 18. Ghanem E, Antoci V, Jr, Pulido L, et al. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis 2009;13:e444-e449. [DOI] [PubMed] [Google Scholar]
- 19. Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One 2010;5:e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greidanus NV, Masri BA, Garbuz DS, et al. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg [Am] 2007;89-A:1409-1416. [DOI] [PubMed] [Google Scholar]
- 21. Costa CR, Johnson AJ, Naziri Q, et al. Efficacy of erythrocyte sedimentation rate and C-reactive protein level in determining periprosthetic hip infections. Am J Orthop (Belle Mead NJ) 2012;41:160-165. [PubMed] [Google Scholar]
- 22. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop 2011;35:1621-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McArthur BA, Abdel MP, Taunton MJ, Osmon DR, Hanssen AD. Seronegative infections in hip and knee arthroplasty: periprosthetic infections with normal erythrocyte sedimentation rate and C-reactive protein level. Bone Joint J 2015;97-B:939-944. [DOI] [PubMed] [Google Scholar]
- 24. Yi PH, Cross MB, Moric M, et al. The 2013 Frank Stinchfield Award: diagnosis of infection in the early postoperative period after total hip arthroplasty. Clin Orthop Relat Res 2014;472:424-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cipriano CA, Brown NM, Michael AM, et al. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg [Am] 2012;94-A:594-600. [DOI] [PubMed] [Google Scholar]
- 26. Berbari E, Mabry T, Tsaras G, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg [Am] 2010;92-A:2102-2109. [DOI] [PubMed] [Google Scholar]
- 27. Bottner F, Wegner A, Winkelmann W, et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg [Br] 2007;89-B:94-99. [DOI] [PubMed] [Google Scholar]
- 28. Di Cesare PE, Chang E, Preston CF, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg [Am] 2005;87-A:1921-1927. [DOI] [PubMed] [Google Scholar]
- 29. Glehr M, Friesenbichler J, Hofmann G, et al. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res 2013;471:2621-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis 2015;61:332-341. [DOI] [PubMed] [Google Scholar]
- 31. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg [Am] 2013;95-A:644-651. [DOI] [PubMed] [Google Scholar]
- 32. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One 2014;9:e89045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedrich MJ, Randau TM, Wimmer MD, et al. Lipopolysaccharide-binding protein: a valuable biomarker in the differentiation between periprosthetic joint infection and aseptic loosening? Int Orthop 2014;38:2201-2207. [DOI] [PubMed] [Google Scholar]
- 34. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008;36:941-952. [DOI] [PubMed] [Google Scholar]
- 35. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206-217. [DOI] [PubMed] [Google Scholar]
- 36. Xie K, Qu X, Yan M. Procalcitonin and α-defensin for diagnosis of periprosthetic joint infections. J Arthroplasty 2017;32:1387-1394. [DOI] [PubMed] [Google Scholar]
- 37. Shahi A, Kheir MM, Tarabichi M, et al. Serum D-dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg [Am] 2017;99-A:1419-1427. [DOI] [PubMed] [Google Scholar]
- 38. Drago L, Vassena C, Dozio E, et al. Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. Int J Immunopathol Pharmacol 2011;24:433-440. [DOI] [PubMed] [Google Scholar]
- 39. Worthington T, Dunlop D, Casey A, et al. Serum procalcitonin, interleukin-6, soluble intercellular adhesin molecule-1 and IgG to short-chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci 2010;67:71-76. [DOI] [PubMed] [Google Scholar]
- 40. Geller DA, Kispert PH, Su GL, et al. Induction of hepatocyte lipopolysaccharide binding protein in models of sepsis and the acute-phase response. Arch Surg 1993;128:22-27. [DOI] [PubMed] [Google Scholar]
- 41. Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect 2006;8:946-952. [DOI] [PubMed] [Google Scholar]
- 42. Fjaertoft G, Håkansson LD, Pauksens K, Sisask G, Venge P. Neutrophil CD64 (FcgammaRI) expression is a specific marker of bacterial infection: a study on the kinetics and the impact of major surgery. Scand J Infect Dis 2007;39:525-535. [DOI] [PubMed] [Google Scholar]
- 43. Park KK, Kim TK, Chang CB, Yoon SW, Park KU. Normative temporal values of CRP and ESR in unilateral and staged bilateral TKA. Clin Orthop Relat Res 2008;466:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alijanipour P, Bakhshi H, Parvizi J. Diagnosis of periprosthetic joint infection: the threshold for serological markers. Clin Orthop Relat Res 2013;471:3186-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res 2004;428:35-39. [DOI] [PubMed] [Google Scholar]
- 46. Hoell S, Sieweke A, Gosheger G, et al. Eradication rates, risk factors, and implant selection in two-stage revision knee arthroplasty: a mid-term follow-up study. J Orthop Surg Res 2016;26;11:93-016-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res 2007;464:164-178. [DOI] [PubMed] [Google Scholar]
- 48. Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res 2009;467:1699-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shukla SK, Ward JP, Jacofsky MC, et al. Perioperative testing for persistent sepsis following resection arthroplasty of the hip for periprosthetic infection. J Arthroplasty 2010;25(suppl):87-91. [DOI] [PubMed] [Google Scholar]
- 50. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res 2011;469:1002-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoell S, Borgers L, Gosheger G, et al. Interleukin-6 in two-stage revision arthroplasty: what is the threshold value to exclude persistent infection before re-implanatation? Bone Joint J 2015;97-B(1):71-75. [DOI] [PubMed] [Google Scholar]
- 52. Frangiamore SJ, Siqueira MB, Saleh A, et al. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res 2016;474:1630-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res 2016;474:1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res 2013;471:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty 2005;20(suppl 3):46-50. [DOI] [PubMed] [Google Scholar]
- 56. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129-139. [DOI] [PubMed] [Google Scholar]
- 57. Motaghedi R, Bae JJ, Memtsoudis SG, et al. Association of obesity with inflammation and pain after total hip arthroplasty. Clin Orthop Relat Res 2014;472:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu JZ, Saleh A, Klika AK, Barsoum WK, Higuera CA. Serum inflammatory markers for periprosthetic knee infection in obese versus non-obese patients. J Arthroplasty 2014;29:1880-1883. [DOI] [PubMed] [Google Scholar]
- 59. Chevillotte CJ, Ali MH, Trousdale RT, et al. Inflammatory laboratory markers in periprosthetic hip fractures. J Arthroplasty 2009;24:722-727. [DOI] [PubMed] [Google Scholar]
- 60. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg [Am] 2012;94-A:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwon YM, Lombardi AV, Jacobs JJ, et al. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg [Am] 2014;96-A:e4, 1-6. [DOI] [PubMed] [Google Scholar]
- 62. Bonanzinga T, Zahar A, Dütsch M, et al. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A Prospective Study. Clin Orthop Relat Res 2017;475:408-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deirmengian C, Kardos K, Kilmartin P, et al. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg [Am] 2014;96-A:1439-1445. [DOI] [PubMed] [Google Scholar]
- 64. Yi PH, Cross MB, Moric M, et al. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin Orthop Relat Res 2015;473:498-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwon YM, Antoci V, Jr, Leone WA, et al. Utility of serum inflammatory and synovial fluid counts in the diagnosis of infection in taper corrosion of dual taper modular stems. J Arthroplasty 2016;31:1997-2003. [DOI] [PubMed] [Google Scholar]
- 66. Kim TW, Kim DH, Oh WS, et al. Analysis of the causes of elevated c-reactive protein level in the early postoperative period after primary total knee arthroplasty. J Arthroplasty 2016;31:1990-1996. [DOI] [PubMed] [Google Scholar]
- 67. Yuan K, Li WD, Qiang Y, Cui ZM. Comparison of procalcitonin and C-reactive protein for the diagnosis of periprosthetic joint infection before revision total hip arthroplasty. Surg Infect (Larchmt) 2015;16:146-150. [DOI] [PubMed] [Google Scholar]