Abstract
Cocaine is a commonly abused recreational drug in the United States. An adult man developed non-specific pleuritic chest pain, pharyngitis and odynophagia after inhaling cocaine. Initial laboratory results revealed eosinophilia. Bronchoalveolar lavage also showed eosinophilia in the lavage fluid. These findings suggested the diagnosis of eosinophilic pneumonia. Chest imaging revealed scattered bilateral opacities and interstitial infiltrates. After initiation of systemic corticosteroids, the patient reported symptomatic resolution and radiographic clearance was achieved at 2 months follow up.
1. Introduction
Cocaine is one of the most commonly abused drugs in the United States. Despite having millions of chronic and occasional users, the reported cases of cocaine-induced eosinophilic pneumonia remain a handful [1,2]. Eosinophilic lung disease can be identified by an increased number of eosinophils in the lung tissue or BronchoAlveolar Lavage (BAL) fluid of a patient who has pulmonary symptoms or infiltrates on chest imaging.
2. Case presentation
A 59-year-old male presented to Emergency Department complaining of pleuritic chest pain for 10 days prior to presentation. The pain was described as reproducible and band like across the lower chest with worsening during deep inspiration. He also reported associated pharyngitis and odynophagia. The patient endorsed daily cocaine use, was an active smoker with a 12-pack years history, and occasional alcohol consumer. He was an employed electrician. He denied sick contacts, recent travel or other complaints prior to presentation. The patient had no history of lung disease and denied childhood asthma, he was born in Trinidad and Tobago but moved to the United States in his 20s. The patient reported having no pets or birds or mold in his apartment. There was no significant family history for cancer, connective tissue diseases or pulmonary diseases.
On physical examination the patient had red conjunctiva and denied chest tenderness upon palpation. Expiratory wheezes were auscultated in all lung fields with basilar rales and crackles noted at the bases.
Urine toxicology at the time of admission was positive for cocaine and cannabis (THC), but negative for other illicit drugs. Laboratory studies revealed a white blood count of 9.2/μL with elevated serum eosinophils 11.8% (ref 0–7, Absolute Eosinophil Count: 1.1 cells/microL). HIV testing was negative.
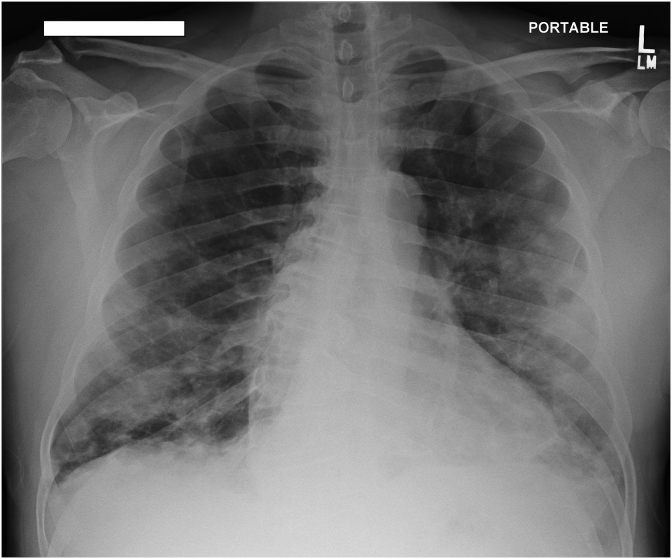
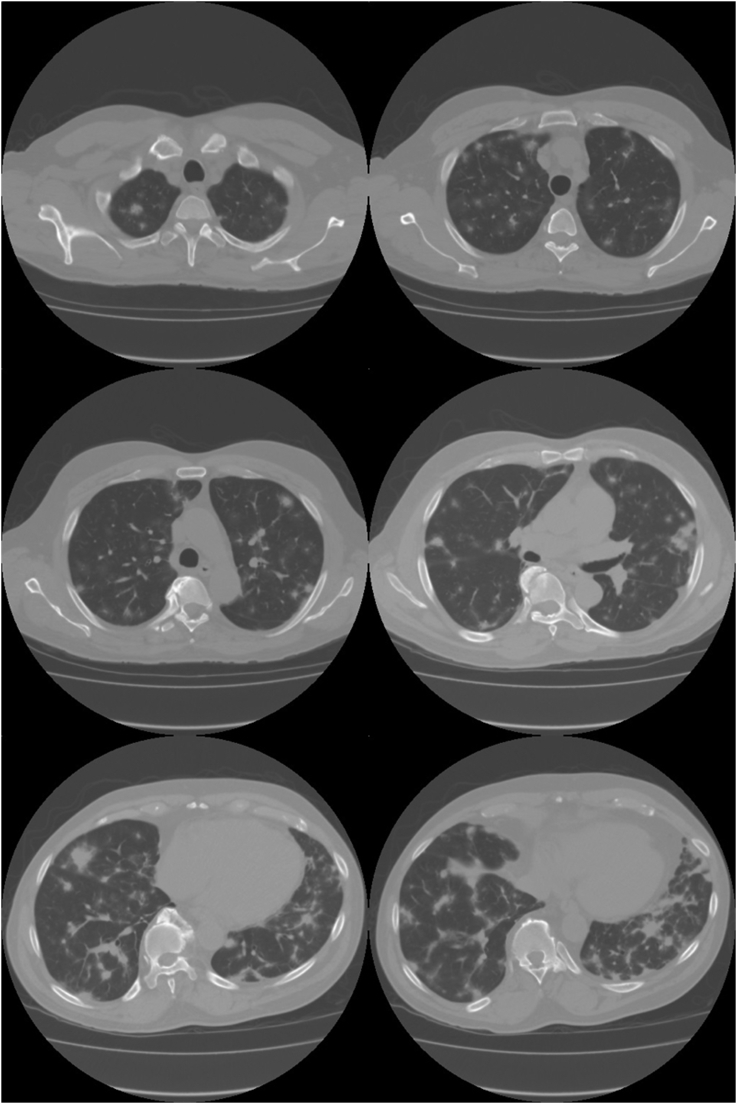
Chest X-ray showed bilateral nodular opacities in the lungs, most prominent within mid to lower lung zones, a stable cardio-mediastinal silhouette and no pleural effusion were noted (Fig. 1). Computed Tomography (CT) imaging of the chest revealed: small mediastinal lymph nodes with innumerable nodular densities in the pulmonary parenchyma scattered throughout both lungs, majority of which demonstrated a surrounding area of ground glass opacity (Halo sign). Some consolidations were also noted, the largest of which was located lateral to the left upper lobe and measured 3.6 × 3.1 cm with presence of air bronchograms (Fig. 2).
Fig. 1.
Chest X-ray showing bilateral nodular opacities in the lungs.
Fig. 2.
Chest Computed Tomography (CT) imaging revealing: small mediastinal lymph nodes with innumerable nodular densities in the pulmonary parenchyma scattered throughout both lung.
Urine Legionella, M. Pneumonia, and Aspergillus antibodies were negative. Procalcitonin level was negative.
Blood cultures revealed no growth after 5 days. Sputum analysis revealed normal flora but elevated WBC count (>25 in a low power field) and acid-fast bacilli (AFB) showed no organisms. CMV, ANA, Anti-GBM, Anti-centromere, anti-SCL-70, ANCA, myeloperoxidase, proteinas-3-antibody, atypical pANCA, perinuclear ANCA, Cytoplasmic AB, Histoplasma were all negative. Angiotensin was found to be within normal limits.
His eosinophil count peaked at 14.4 (WBC: 10.3, AEC 1.5). He underwent diagnostic bronchoscopy which evidenced normal mucosa, BAL showed a clear hazy fluid with 40 WBC 35% eosinophils, diagnostic testing of the aspirate was negative for bacteria, viral or AFB infection.
Our patient reported symptomatic improvement after the initial administration of intravenous steroids. Prior to discharge the patient was started on oral corticosteroids with an outpatient taper: 60mg daily with a 10mg weekly decrease, until reaching 10mg daily. After reaching 10mg daily, the patient completed 2 weeks of 10mg daily and 2 weeks of 5mg daily.
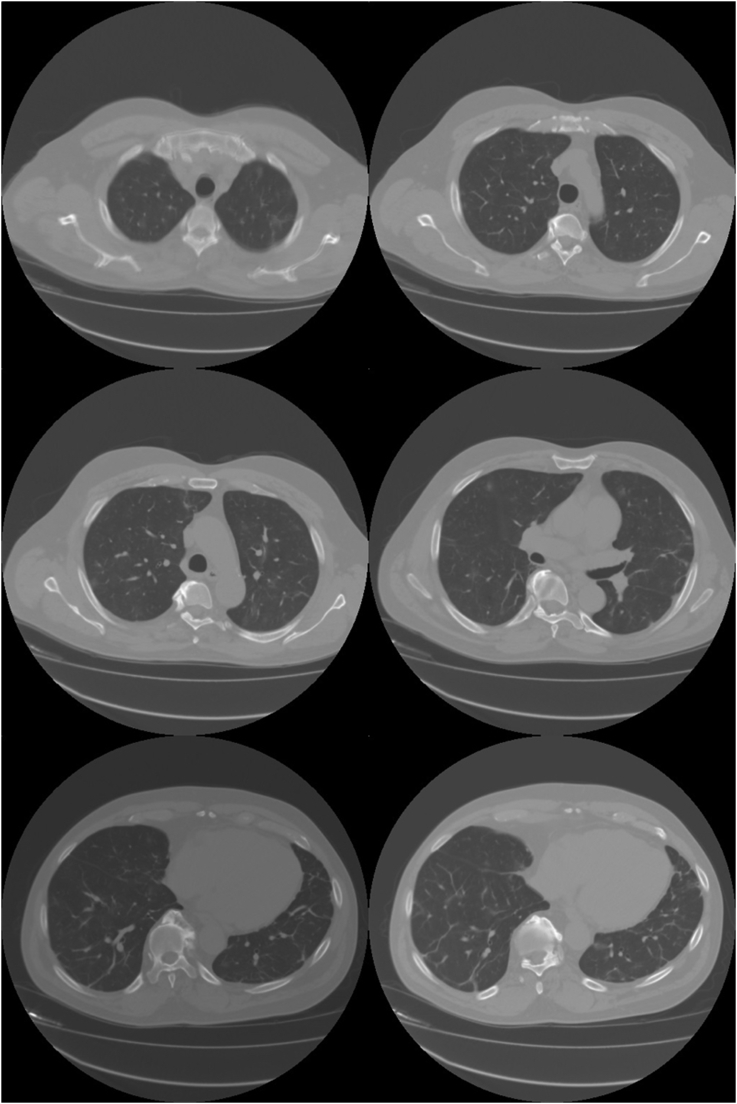
Outpatient PFTs revealed no obstruction or restrictive defect. Follow up CT imaging two months after discharge revealed resolution of previously noted nodular opacities but persistence of ground glass opacities with interval decrease in size. The patient reported abstinence from cocaine use at 2 months follow up (Fig. 3).
Fig. 3.
Follow up CT imaging two months after discharge and completing oral corticosteroid treatment revealed resolution of nodular opacities.
3. Discussion
The most frequent pulmonary complaints reported by cocaine users are: dyspnea, cough, sputum production and non-specific chest paint [3].
Pulmonary complications from cocaine use depend on the method of administration, dose, frequency of use and presence adulterant substances. The most common pulmonary complications of cocaine use are: pneumothorax, pneumo-mediastinum, pulmonary edema, pulmonary hemorrhage [4],bronchiolitis obliterans, hypersensitivity pneumonitis, pulmonary artery medial hypertrophy and thermal injuries to the airway [1,2,5].
Eosinophilic Pneumonia (EP) has been associated with the use of many drugs, including NSAIDs, antibiotics (minocycline, cephalosporins) and phenytoin. A list of possible causes of eosinophilic lung diseases is presented in Table 1.
Table 1.
Differential diagnoses in eosinophilic lung diseases.
| Eosinophilic Lung Diseases |
|---|
Eosinophilic dysregulation
|
Infectious Causes
|
Miscellaneous
|
In the drug-induced EP model, the offending agent is retained in the pulmonary surfactant, which is then sequestered in the alveoli, leading to concentrations high enough to cause injury to the surrounding tissues [6]. Patients might not me forthcoming about over the counter or illicit drug and therefor special attention is required when drug-induced EP is suspected.
In normal individuals the eosinophil tissue-to-blood ratio is 100:1. When eosinophils are activated release of their granular contents can result in tissue injury and development of clinical findings (i.e.: eosinophilic lung disease) [7]. The lung is an eosinophil rich tissue owing to the in-situ production of eotaxin by alveolar macrophages, pulmonary endothelial cells, airway smooth muscle cells and alveolar epithelial cells [8].
Multiple mechanisms have been proposed for cocaine-induced lung damage: hypersensitivity reaction to crack cocaine [1], increased vascular tone leading to pulmonary hypertension, and polymorphonuclear neutrophils activation [5].
3.1. Clinical presentation
The first case report of cocaine induced eosinophilic pneumonia was reported in 1992 [1]. That patient presented with fever, bronchoconstriction, hypoxemia, and pulmonary infiltrates. Bronchioalveolar lavage fluid showed eosinophilia. Our case also had dyspnea and evidence of peripheral and bronchoalveolar eosinophilia. Oh Pi's patient presented with recurrent events after repeated cocaine use, all of which resolved with corticosteroid use. The resolution of symptomatic and radiographic findings after a course of steroids is a shared featured noted amongst our case and previous ones [1,2,5].
Because some diseases can present with prominent eosinophil infiltration in lung tissue without associated blood eosinophilia; Differentiating EP from crack lung can be difficult [7].The initial description of crack lung involved eosinophilia, pruritus and an elevated IgE level [9]. Crack lung, pulmonary hemorrhage, hypersensitivity pneumonitis and pulmonary eosinophilic diseases are radiographically indistinguishable from one and other [5].
Crack lung was described as an acute pulmonary syndrome consisting of diffuse alveolar damage and hemorrhagic alveolitis that occurs within 48 hours of smoking crack cocaine [10]. The presentation is associated with slowly progressive dyspnea, wheezing, a chronic cough, and also intermittent hemoptysis. Although our patient does not fit a crack lung syndrome for various reasons: patient used cocaine and not crack cocaine, his presentation was lacked common radiological findings seen in crack lung (I.e.: crazy paving) and lastly his clinical syndrome resolved within two months.
Other symptoms, such as fever, myalgias, and pleuritic chest pain, are common. Patients who have idiopathic acute eosinophilic pneumonia have normal or only slightly elevated blood eosinophil counts, whereas those who have drug-induced acute eosinophilic pneumonia often have moderately or highly elevated blood eosinophil counts. An eosinophil count in excess of 1000/mL should heighten the suspicion of drug-induced, as opposed to idiopathic, acute eosinophilic pneumonia. Chest radiograph findings in idiopathic and drug-induced acute eosinophilic pneumonia are similar [7].
3.2. Radiographic findings
The earliest radiographic findings are patchy interstitial infiltrates with Kerley B lines which progress to diffuse alveolar infiltrates with small or moderate sized pleural effusions [1,11].
HRCT may show diffuse areas of ground-glass attenuation, sometimes with well-defined nodular changes with patchy and random distribution or peripheral predominance [5,11].
3.3. Bronchoscopy findings
The visual appearance of the tracheal and bronchial mucosa and submucosa was normal to the sub-segmental level. Strong et al. also described a normal visual appearance of the tracheal and bronchial mucosa in their evaluation [2]. To date there is no indications for interval bronchoscopy follow up in cases of drug-induced EP.
3.4. Treatment
In the traditional description of drug-induced EP improvement can be achieved quickly by discontinuing the offending drug [12]. Steroids can be administered to speed the process of symptomatic recovery and resolution of hypoxemia and dyspnea [1,2,5]. Of the three previously reported cases of cocaine-induced EP two were successfully treated with corticosteroids and abstinence. This double-pronged approach aims to control the eosinophilic damage by decreasing inflammation in the airway. Table 2 provides a summary of all the reported cases of cocaine induced eosinophilic pneumonia.
Table 2.
Reported cases of cocaine-induced eosinophilic pneumonia.
| Article | Time to Presentation since last cocaine inhalation | Clinical presentation | Diagnostic findings | Treatment | Follow up |
|---|---|---|---|---|---|
| McCornick [2007] | <24hours | Circulatory Shock | – | – | Passed away during hospitalization |
| Oh Pi [1992] | <24hours | Fever, bronchoconstriction, hypoxemia, Pulmonary Infiltrates | BAL Eosinophilia | Prednisone 30mg daily | 2.5 weeks |
| Strong [2003] | 1 week | Fever, sweats and productive cough | – | Prednisone 60mg daily with taper | 1 month |
| Present Case | 1 week | Pharyngitis, odynophagia and chest pain | BAL Eosinophila | Prednisone 60mg daily with taper | 2 months |
4. Conclusion
Cocaine induced eosinophilic pneumonia.
References
- 1.Oh P.I., Balter M.S. Cocaine induced eosinophilic lung disease. Thorax. 1992;47(6):478–479. doi: 10.1136/thx.47.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong D.H., Westcott J.Y., Biller J.A., Morrison J.L., Effros R.M., Maloney J.P. Eosinophilic “empyema” associated with crack cocaine use. Thorax. 2003;58(9):823–824. doi: 10.1136/thorax.58.9.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger N.A., Albin R.J. A review of the respiratory effects of smoking cocaine. Am. J. Med. 1989;87(6):664–668. doi: 10.1016/s0002-9343(89)80401-2. [DOI] [PubMed] [Google Scholar]
- 4.Dushay K.M., Evans S.K., Ghimire S., Liu J. Cocaine-induced diffuse alveolar hemorrhage: a case report and review of the literature. Rhode Island Med. J. 2016;99(8):34–36. (2013) [PubMed] [Google Scholar]
- 5.de Almeida R.R., de Souza L.S., Mancano A.D., Souza A.S., Jr., Irion K.L., Nobre L.F. High-resolution computed tomographic findings of cocaine-induced pulmonary disease: a state of the art review. Lung. 2014;192(2):225–233. doi: 10.1007/s00408-013-9553-6. [DOI] [PubMed] [Google Scholar]
- 6.Higashi Y., Nakamura S., Tsuji Y., Ogami C., Matsumoto K., Kawago K. A case of daptomycin-induced eosinophilic pneumonia and a review of the published literature. Int. Med. Tokyo Jpn. 2017 doi: 10.2169/internalmedicine.9010-17. https://www.jstage.jst.go.jp/article/internalmedicine/advpub/0/advpub_9010-17/_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen J.N. Drug-induced eosinophilic lung disease. Clin. Chest Med. 2004;25(1):77–88. doi: 10.1016/S0272-5231(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 8.Conroy D.M., Williams T.J. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir. Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissner D.G., Lawrence W.D., Selis J.E., Flint A. Crack lung: pulmonary disease caused by cocaine abuse. Am. Rev. Respir. Dis. 1987;136(5):1250–1252. doi: 10.1164/ajrccm/136.5.1250. [DOI] [PubMed] [Google Scholar]
- 10.Forrester J.M., Steele A.W., Waldron J.A., Parsons P.E. Crack lung: an acute pulmonary syndrome with a spectrum of clinical and histopathologic findings. Am. Rev. Respir. Dis. 1990;142(2):462–467. doi: 10.1164/ajrccm/142.2.462. [DOI] [PubMed] [Google Scholar]
- 11.McCormick M., Nelson T. Cocaine-induced fatal acute eosinophilic pneumonia: a case report. WMJ Off. Publ. State Med. Soc. Wis. 2007;106(2):92–95. [PubMed] [Google Scholar]
- 12.Toyoshima M., Sato A., Hayakawa H., Taniguchi M., Imokawa S., Chida K. A clinical study of minocycline-induced pneumonitis. Int. Med. Tokyo Japan. 1996;35(3):176–179. doi: 10.2169/internalmedicine.35.176. [DOI] [PubMed] [Google Scholar]