Highlights
-
•
The cost-effectiveness (CE) of influenza vaccination varied between countries.
-
•
This was caused by differences in influenza epidemiology, HIV prevalence and unit costs.
-
•
CE of QIV depends on the countries' influenza B burden, CE thresholds and budgetary impact
-
•
QIV would only be cost-effective when high influenza attack rates were assumed.
-
•
Vaccine price of QIV has a high impact on the CE.
Keywords: Influenza, Cost-effectiveness, Vaccination, Trivalent, Quadrivalent, Dynamic transmission model
Abbreviations: GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; HIV, human immunodeficiency virus; IBS, individual based simulation; I$, international $; LMICs, low- and-middle income countries; NMB, net monetary benefit; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; QIV, quadrivalent influenza vaccine; SAR, symptomatic attack rate; TIV, trivalent influenza vaccine; WHO, world health organization; WTP, willingness-to-pay
Abstract
Background
To inform national healthcare authorities whether quadrivalent influenza vaccines (QIVs) provide better value for money than trivalent influenza vaccines (TIVs), we assessed the cost-effectiveness of TIV and QIV in low-and-middle income communities based in South Africa and Vietnam and contrasted these findings with those from a high-income community in Australia.
Methods
Individual based dynamic simulation models were interfaced with a health economic analysis model to estimate the cost-effectiveness of vaccinating 15% of the population with QIV or TIV in each community over the period 2003–2013. Vaccination was prioritized for HIV-infected individuals, before elderly aged 65+ years and young children. Country or region-specific data on influenza-strain circulation, clinical outcomes and costs were obtained from published sources. The societal perspective was used and outcomes were expressed in International$ (I$) per quality-adjusted life-year (QALY) gained.
Results
When compared with TIV, we found that QIV would provide a greater reduction in influenza-related morbidity in communities in South Africa and Vietnam as compared with Australia. The incremental cost-effectiveness ratio of QIV versus TIV was estimated at I$4183/QALY in South Africa, I$1505/QALY in Vietnam and I$80,966/QALY in Australia.
Conclusions
The cost-effectiveness of QIV varied between communities due to differences in influenza epidemiology, comorbidities, and unit costs. Whether TIV or QIV is the most cost-effective alternative heavily depends on influenza B burden among subpopulations targeted for vaccination in addition to country-specific willingness-to-pay thresholds and budgetary impact.
1. Introduction
Seasonal influenza has been estimated to cause between 3 and 5 million cases of severe illness and 250,000–500,000 deaths globally each year [1]. The elderly, very young children and people with specific health conditions are at highest risk of developing serious complications [2]. In addition, influenza imposes a significant economic burden involving health care costs and productivity losses. In low- and middle-income countries (LMICs), costs due to seasonal influenza may have a considerable economic impact, estimated at 2–6% of gross domestic product (GDP) per capita, compared to only 0.04–0.13% of GDP per capita in high income countries [3].
Annual vaccination is currently the most effective way of preventing influenza disease [1]. The commonly used trivalent influenza vaccines (TIVs) contain strains of two influenza A sub-types (H1N1 and H3N2) and one influenza B lineage (either Victoria or Yamagata), based on recommendations from the World Health Organization (WHO). Over the last decade, vaccine protection was regarded as sub-optimal in some years due to mismatches with the dominant circulating B lineage, or due to co-circulation of both B lineages in the same season [4]. In a response to this, quadrivalent influenza vaccines (QIVs) have been developed containing both B lineages (Victoria and Yamagata).
Previous cost-effectiveness analyses on influenza vaccination have had a focus on high-income countries with few economic studies of influenza vaccination in LMICs [3], [5], [6], [7]. For instance, in a recent paper QIV was found to be cost-effective in the United States [8]. Some LMICs are now considering whether seasonal influenza vaccination should be introduced in their vaccination programs and whether this should involve TIVs or QIVs. Significantly, cost-effectiveness outcomes are not directly transferrable between countries, due to differences in circulating strains, demographics, climate, co-morbidities, health care infrastructure and budgets. For example, a study in South Africa, a country with considerable human immunodeficiency virus (HIV) prevalence, estimated that the incidence of influenza-associated severe lower respiratory tract infections was 4–8 times higher in HIV-infected individuals as compared with HIV-uninfected individuals [9].
In this study we analyzed the cost-effectiveness of influenza vaccination with TIV and QIV in three communities: Agincourt, a low-income rural community in South Africa; Thai Nguyen, a middle-income urban community in Vietnam; and Albany, a high-income urban/rural community in Australia. For this purpose, individual based simulation (IBS) models for each of the three communities were developed and interfaced with a health economic analysis model, capturing the specific demographics and health profiles of each community. As circulation of the different influenza B lineages and corresponding TIV vaccine matches are hard to predict, we studied the impact of TIV and QIV using retrospective data, over the period 2003–2013 (11 seasons).
2. Methods
2.1. Model overview
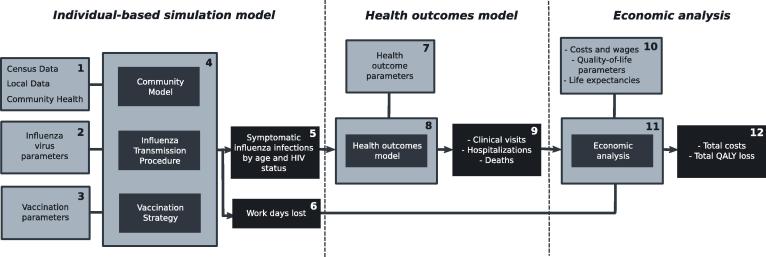
An overview of the analytic methodology used in this study is shown in Fig. 1; parenthesized numbers below refer to numbered items in the figure. Population and geographic data was used to build models for communities in South Africa, Vietnam and Australia (1). For each country, influenza strain circulation data was used to calibrate strain-specific influenza transmission parameters for the years 2003–2013 (2). For each of these combinations of communities and study years (39 in total), 3 different vaccination strategies were created: no vaccination, vaccination with TIV (using the actual influenza B strain present in the vaccine used in that country in that year), and vaccination with QIV (using both influenza B lineages) (3). For each combination of community, year and vaccination strategy (in total 117 scenarios) established individual based influenza spread simulation models (4) were used to assess the incidence of symptomatic influenza, stratified by age and HIV status (5). Influenza spread simulations also generated counts of work-days lost due to influenza (6).
Fig. 1.
Overview of the interfaced individual based simulation model and the health economic model. HIV: Human Immunodeficiency Virus; QALY: Quality-Adjusted Life Year. The numbers refer to the textual methodology overview given at the beginning of the Methods section.
These outputs, along with community, age, and HIV status-specific risk parameters (7) served as input to a health outcomes model (8), which generated numbers of clinical visits, hospitalizations and deaths due to influenza (9). Using cost and quality of life parameters (10), an economic analysis process (11) subsequently took the health outcomes counts, work-days lost and generated total costs and quality-adjusted life year (QALY) losses for each scenario. The differences between corresponding no-vaccination and vaccination scenarios served to calculate incremental cost-effectiveness ratios (ICERs) for the TIV and QIV vaccination strategies. Sensitivity analyses were performed to assess the robustness of results due to uncertainty in health outcome parameters, cost parameters and the stochastic nature of influenza spread.
2.2. Individual based simulation models
2.2.1. Community models
The main characteristics of each community are shown in Table 1. Agincourt represents a low-income rural area in northern South Africa, with HIV prevalence at ∼16% in the adult population and a relatively low life-expectancy. The lower-middle income community of Thai Nguyen is located in north Vietnam near Hanoi and represents an urban setting and a relatively low HIV prevalence in adults (2.3%). Albany reflects a combined urban and rural community in Western Australia, representative of high-income countries with high life-expectancy and low HIV prevalence (0.2%). Current seasonal influenza vaccination coverage is moderate in Albany (20%), negligible in Agincourt (<2%) and absent in Thai Nguyen.
Table 1.
Main characteristics of the communities studied in this analysis.
| South Africa | Vietnam | Australia | |
|---|---|---|---|
| Community | Agincourt | Thai Nguyen | Albany |
| Community size | 40,400 | 74,000 | 29,400 |
| Setting | Rural | Urban, with large student population | Combined rural and urban |
| GDP/capita | I$1000 | I$5000 | I$42,500 |
| Life-expectancy [45], [46], [47] | 60.1 | 76.2 | 82.4 |
| HIV prevalence in adults [66], [67], [68], [69] | 16% | 2.3% | 0.2% |
| Vaccination coverage [70], [71] | 2% | 0% | 20% |
Each model was constructed using community-specific census and health data, and represents a community of individuals, each labelled with age (in bands 0–5, 6–12, 13–17, 18–24, 25–44, 45–64, 65+ years) and HIV status (see supplementary methods Table S1 for more details). Census and local government data was used to assign each individual to a number of contact groups (i.e. groups which the individual meets daily, including households, school classes, or groups of work colleagues). The size and overlapping memberships of contact groups is a key determinant of influenza spread, and these groups were constructed taking into account community-specific details including employment rate, workplace size, school attendance, number and size of schools, and household sizes. The IBS community models of Agincourt and Albany have been described in more detail previously [10], [11]. The community model of Thai Nguyen in Vietnam was developed using the same methodology as the other models and is described in detail in the supplementary material.
2.2.2. Influenza transmission
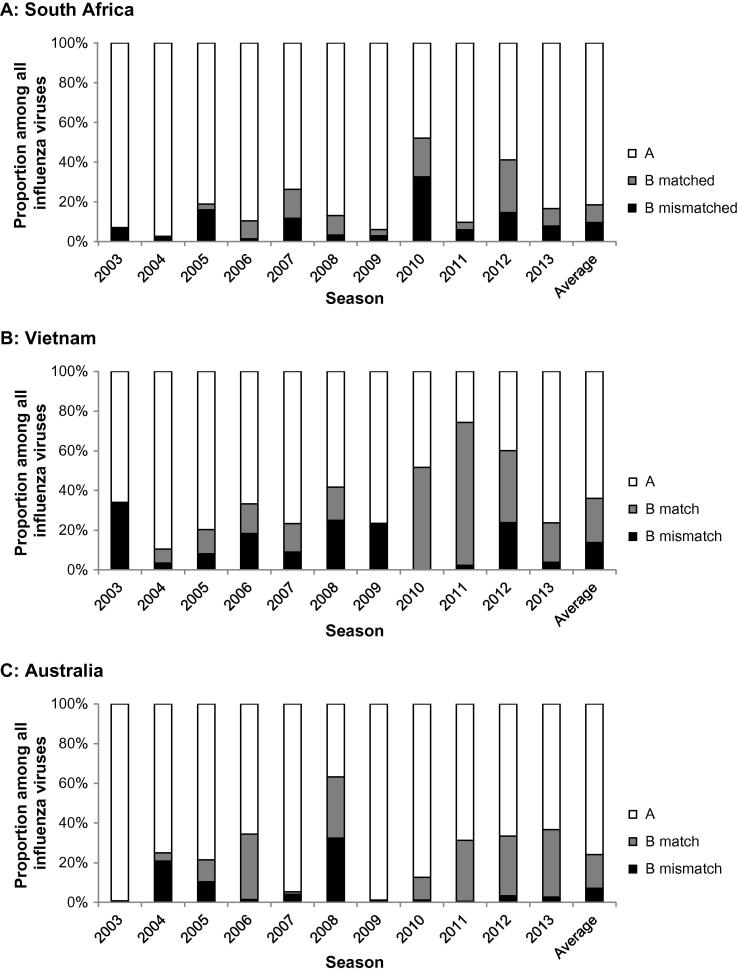
As the simulation software runs, individuals come into daily contact with other individuals in their contact groups, where influenza transmission from infectious to susceptible individuals may occur: a stochastic choice determines if transmission fails, or results in symptomatic or asymptomatic infection. The model is able to capture the infection history of each individual regarding infection status, i.e. susceptible, infected, infectious or immune (due to infection or vaccination). Separate infectivity status was recorded of each of the four seasonal influenza strains A(H3N2), A(H1N1), B Yamagata, and B Victoria. The output of the IBS-model consisted of the number of symptomatic influenza cases and number of work days lost. A work-day lost was deemed to have occurred when an individual who would have otherwise attended a workplace withdrew to their household, either due to influenza infection themselves, or because one or more children in the household was ill with influenza. Main input parameters of the IBS-model are listed in the supplementary methods Table S2. The annual attack rate of influenza infection in each unvaccinated community was set at 21% and annual symptomatic attack rate (SAR) at 5% [12], [13]. However, as the SAR of 5% has been determined in a setting where seasonal influenza vaccination already existed, we also performed analyses using a SAR of 10%. The transmissibility of each strain in each simulation year was calibrated to match the proportion of each strain occurring that year for each community (Fig. 2) [14], [15], [16], [17]. As no information on the lineages of influenza B infections was available for Vietnam, influenza B lineage data from Thailand was used to partition the Vietnamese B infection between the two lineages [18]. A more detailed description of the transmission parameter calibration process is given in the supplementary material.
Fig. 2.
Relative proportions of influenza A viruses and influenza B viruses stratified by matched and mismatched lineage (See Supplementary methods Table S4 for more details) by influenza season as used in the model. Split of influenza B by lineage in Vietnam was based on surveillance data from Thailand.
2.2.3. Vaccination
The number of vaccine doses used each year was assumed at 15% of the population and prioritized to vulnerable sub-groups: first to HIV-infected individuals, then to elderly aged 65+ years, and the remaining to children aged <5 years. Age-specific coverages are shown in supplementary methods Table S3. In the Agincourt and Albany communities, the Southern Hemisphere TIV vaccine composition as used in Australia and South Africa was assumed (Supplementary methods Table S4) [19]. In the case of VN, where currently no seasonal influenza vaccination occurs, the TIV recommendation for the previous northern hemisphere winter was used [19]. Vaccine efficacy was set at 65% for individuals aged <65 years and at 55% in people aged 65 and older [20], [21], [22]. No cross-strain vaccine protection was assumed; that is, vaccination with TIV did not provide any protection against the influenza B strain not included in the vaccine and vaccine efficacy for that strain was zero.
2.3. Health outcomes model
Using the age and HIV-specific counts of symptomatic cases generated by the IBS model, counts of clinical visits, hospitalizations and deaths were generated using age- and country-specific probabilities for each community and vaccination strategy (Supplementary methods Table S5). We explicitly applied higher risks of clinical events for HIV-infected individuals with influenza disease as compared with non-HIV-infected individuals [9], [23]. As not all probabilities of a clinical event were available for each country, we also relied on data from countries in the same region. For instance, we used data on influenza-related clinical visits from Kenya [24] for Agincourt and data on hospitalizations and mortality from Thailand [25] for Thai Nguyen.
2.4. Economic analysis
2.4.1. Costs
In our health economic analysis, a societal perspective was taken, including health care costs as well as costs due to productivity losses. Healthcare costs included clinic visits with concomitant drug prescriptions, hospital admissions, vaccines and their delivery costs, while non-health care costs included productivity losses due to lost work days (Supplementary methods Table S6). All costs were expressed in International dollars (I$) and transformed to the base-year of 2013 using national consumer price indices [26], [27], [28]. International dollars adjust for differences in purchasing power, enhancing the comparability of monetary outcomes between LMICs and high-income countries. As the price of QIV was not available for the countries examined, we had to make an assumption. We assumed a price for QIV at 50% higher than the TIV price, similar to the price premium of QIV over TIV given by the US CDC [29]. Productivity losses due to influenza illness were calculated by multiplying the number of work days lost by average daily earnings in the community and applying the friction methods [30]. More details on conversion rates and cost inputs are presented in the supplementary material.
2.4.2. Health effects
Influenza-related QALY losses were quantified by summing up QALY losses due to influenza illness and premature mortality. QALY-losses for non-hospitalized and hospitalized case were obtained from the literature [31], [32]. Age-specific QALY losses due to influenza-related death were based on the national life expectancies [33], [34], [35] converted to QALYs using health-related quality-of-life estimates [36], [37], [38]. Notably, HIV+ individuals were assumed to have a lower life expectancy and health-related quality of life compared with HIV-uninfected individuals [39]. More details on QALY-loss inputs are presented in the supplementary material and Supplementary methods Table S7.
2.4.3. Cost-effectiveness analysis
Total costs and QALY losses of the no-vaccination, TIV and QIV alternatives were added together over the period 2003 to 2013 and ICER was calculated by dividing the incremental costs by the incremental QALYs. As the analysis was retrospective and vaccination occurred seasonally, we did not discount costs or health effects falling in the same year as vaccination. Future costs (e.g. lifelong productivity losses) were discounted to present values at 3% annually, while future health effects (e.g. preterm mortality) were not discounted [40].
2.5. Sensitivity analyses
We performed sensitivity analyses to determine the robustness of model outcomes and the impact of specific assumptions in both the simulation model and the combined health outcomes/economic analysis model. For each scenario a multivariate probabilistic sensitivity analysis (PSA) was conducted to assess the effect of parameter uncertainty. 1000 Monte-Carlo samples were created, with health outcomes and economic parameter values drawn from predefined distributions. This resulted in 1000 separate outcomes for total costs and total QALY loss per vaccination strategy. Details on interval ranges of the included economic parameters can be found in Supplementary methods Tables S5–S7. Net monetary benefits (NMBs) of each simulation were calculated for the three studied vaccination strategies (no vaccination, TIV, QIV) using the equation:
where λ is the willingness-to-pay (WTP) threshold. For each simulation it was analyzed which vaccination strategy was the most cost-effective (i.e. the one with the highest NMB) over a range of WTP thresholds. Cost-effectiveness acceptability curves (CEACs) were subsequently designed to present proportions of being the most cost-effective for each vaccination strategy. Finally, a univariate sensitivity analysis on the price difference between QIV and TIV was performed, by varying the price premium of QIV over TIV between 0% (QIV price equal to TIV price) to 100% over TIV (QIV price double of TIV price).
3. Results
3.1. Clinical outcomes
The clinical impact under the three alternatives of no-vaccination, TIV, and QIV is shown in Table 2 for SARs of 5% and 10%. Assuming a SAR of 5%, introduction of vaccination with TIV was found to reduce the incidence of symptomatic influenza in each community by 47.3–49.2%. These reductions were found to be similar across all age-groups (see supplementary results Figs. S1 and S2). Vaccination led to reductions in clinical visits, hospitalizations and deaths in the range 48.2–59.4% (Table 2). In Agincourt SA, the additional benefit of QIV over TIV was estimated to give a 12.1% reduction of symptomatic influenza and a 17.0% reduction of influenza-related mortality. In Thai Nguyen, the impact of QIV over TIV was estimated highest (22.5% reduction of symptomatic influenza and 27.6% reduction of influenza-related mortality) and in Albany lowest (1.2% reduction of symptomatic influenza and 2.3% of influenza-related mortality). Under the 10% SAR assumption scenario the additional benefit of QIV over TIV was estimated to be 9.5% for symptomatic influenza and 14.0% for influenza-related mortality in Agincourt; 8.9% for symptomatic influenza and 14.5% for influenza-related mortality in Thai Nguyen; and 2.0% for symptomatic influenza and 3.3% for influenza-related mortality in Albany.
Table 2.
Impact of vaccination with trivalent influenza vaccine and quadrivalent influenza vaccine on the incidence of symptomatic influenza cases, clinic visits, hospitalizations and deaths in the communities Agincourt (South Africa), Thai Nguyen (Vietnam) and Albany (Australia) over the period 2003–2013. NV: No vaccination, PY: Person years, QIV: Quadrivalent influenza vaccine, SAR: Symptomatic attack rate, TIV: Trivalent influenza vaccine.
| Community/vaccine alternativea | Symptomatic attack rate (%) per year (reductiona) | Clinical visit rate per 100,000 PY (reductiona) | Hospitalization rate per 100,000 PY (reductiona) | Mortality rate per 100,000 PY (reductiona) | ||||
|---|---|---|---|---|---|---|---|---|
| 5% SAR | ||||||||
| Agincourt (SA) | ||||||||
| NV | 5.0 | 607 | 26.0 | 4.07 | ||||
| TIV | 2.6 | (47.3%) | 279 | (54.1%) | 11.5 | (55.7%) | 1.83 | (55.1%) |
| QIV | 2.3 | (12.1%) | 232 | (16.7%) | 9.5 | (17.9%) | 1.52 | (17.0%) |
| Thai Nguyen (VN) | ||||||||
| NV | 5.0 | 389 | 43.5 | 2.01 | ||||
| TIV | 2.5 | (49.2%) | 178 | (54.4%) | 19.5 | (55.2%) | 0.88 | (56.5%) |
| QIV | 2.0 | (22.5%) | 130 | (26.6%) | 14.2 | (27.2%) | 0.64 | (27.6%) |
| Albany (AUS) | ||||||||
| NV | 4.9 | 1093 | 33.9 | 1.60 | ||||
| TIV | 2.6 | (47.6%) | 565 | (48.3%) | 14.7 | (56.8%) | 0.65 | (59.4%) |
| QIV | 2.5 | (1.2%) | 558 | (1.3%) | 14.3 | (2.3%) | 0.63 | (2.3%) |
| 10% SAR | ||||||||
| Agincourt (SA) | ||||||||
| NV | 10.1 | 1252 | 54.4 | 8.54 | ||||
| TIV | 7.2 | (28.6%) | 767 | (38.7%) | 32.0 | (41.2%) | 5.11 | (40.1%) |
| QIV | 6.5 | (9.5%) | 664 | (13.4%) | 27.4 | (14.4%) | 4.40 | (14.0%) |
| Thai Nguyen (VN) | ||||||||
| NV | 10.0 | 790 | 88.7 | 4.41 | ||||
| TIV | 7.6 | (24.1%) | 532 | (32.6%) | 58.7 | (33.9%) | 2.79 | (36.7%) |
| QIV | 6.9 | (8.9%) | 462 | (13.1%) | 50.6 | (13.8%) | 2.38 | (14.5%) |
| Albany (AUS) | ||||||||
| NV | 9.9 | 2259 | 73.9 | 3.78 | ||||
| TIV | 7.5 | (24.7%) | 1680 | (25.6%) | 45.8 | (38.1%) | 2.17 | (42.7%) |
| QIV | 7.3 | (2.0%) | 1645 | (2.1%) | 44.3 | (3.3%) | 2.10 | (3.3%) |
TIV versus NV, and QIV versus TIV.
3.2. Cost-effectiveness outcomes
Table 3 shows the effect of TIV and QIV on total influenza-related costs and QALY losses per person in each community over the period 2003–2013. Assuming a SAR of 5%, the ICER of TIV versus no vaccination was 1803/QALY gained in Agincourt, I$1064/QALY in Thai Nguyen and I$907/QALY in Albany. Increasing the SAR to 10% resulted in lower ICERs or even cost-savings (for instance, vaccination with TIV was estimated cost-saving as compared with no vaccination). When QIV was compared with TIV, the ICER was estimated at I$4183/QALY in Agincourt, I$1505/QALY in Thai Nguyen and I$82,669/QALY in Albany using a SAR of 5%. Assuming a SAR of 10%, the ICER decreased to I$1364/QALY in Agincourt, I$745/QALY in Thai Nguyen and I$28,419/QALY gained in Albany. More detailed results on the impact of vaccination on costs and QALY losses are presented in supplementary results Table S1 (5% SAR) and Table S2 (10% SAR). In Agincourt, highest QALY gains due to influenza vaccination were found, but least productivity losses were saved. In Albany, vaccination prevented relatively more healthcare costs and productivity losses.
Table 3.
Economic impact, health impact and cost-effectiveness of trivalent influenza vaccine and quadrivalent influenza vaccine in the communities Agincourt (South Africa), Thai Nguyen (Vietnam) and Albany (Australia) over the period 2003–2013.
| Outcome (per person) | NV | TIV | QIV | TIV-NV | QIV-TIV |
|---|---|---|---|---|---|
| 5% SAR | |||||
| Agincourt (SA) | |||||
| Total societal (I$) | 6.90 | 19.27 | 23.25 | 12.36 | 3.98 |
| Total QALYs lost | 0.01294 | 0.00609 | 0.00513 | −0.00686 | −0.00095 |
| ICER (I$/QALY) | 1803 | 4183 | |||
| Thai Nguyen (VN) | |||||
| Total societal (I$) | 9.35 | 13.61 | 14.98 | 4.26 | 1.37 |
| Total QALYs lost | 0.00782 | 0.00381 | 0.00290 | −0.00401 | −0.00091 |
| ICER (I$/QALY) | 1064 | 1505 | |||
| Albany (Aus) | |||||
| Total societal (I$) | 44.04 | 46.68 | 50.14 | 2.64 | 3.46 |
| Total QALYs lost | 0.00573 | 0.00282 | 0.00278 | −0.00291 | −0.00004 |
| ICER (I$/QALY) | 907 | 80,966 | |||
| 10% SAR | |||||
| Agincourt (SA) | |||||
| Total societal (I$) | 14.56 | 25.05 | 28.34 | 10.49 | 3.29 |
| Total QALYs lost | 0.02680 | 0.01688 | 0.01475 | −0.00992 | −0.00213 |
| ICER (I$/QALY) | 1057 | 1546 | |||
| Thai Nguyen (VN) | |||||
| Total societal (I$) | 20.38 | 23.12 | 23.87 | 2.74 | 0.75 |
| Total QALYs lost | 0.01603 | 0.01159 | 0.01039 | −0.00444 | −0.00120 |
| ICER (I$/QALY) | 617 | 620 | |||
| Albany (Aus) | |||||
| Total societal (I$) | 98.29 | 93.31 | 94.95 | −4.99 | 1.64 |
| Total QALYs lost | 0.01210 | 0.00847 | 0.00828 | −0.00363 | −0.00020 |
| ICER (I$/QALY) | CS | 8379 | |||
CS: Cost-saving, HCP: Healthcare payer’s perspective, NV: No vaccination, QIV: Quadrivalent influenza vaccine, SAR: Symptomatic attack rate, TIV: Trivalent influenza vaccine.
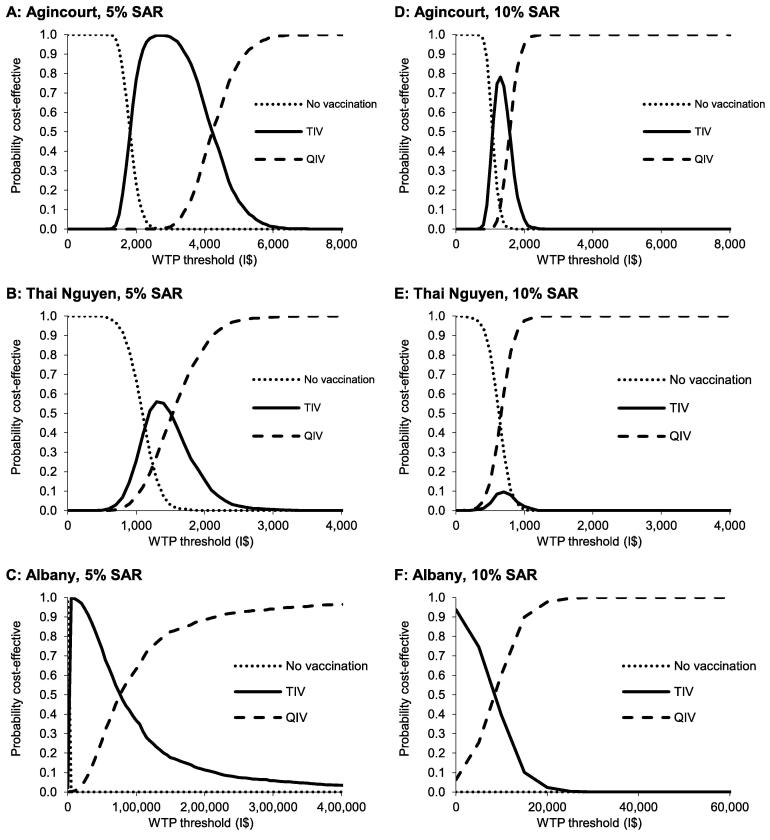
3.3. Sensitivity analyses
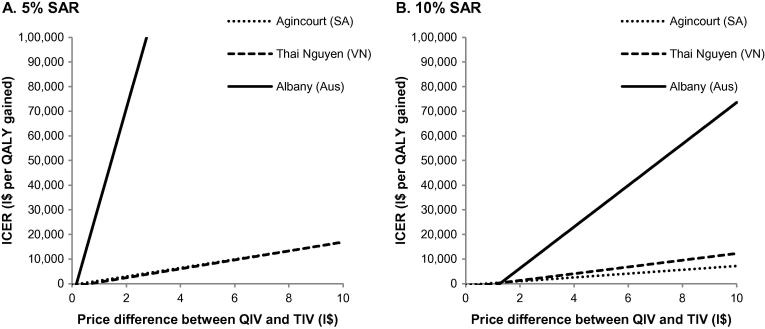
After using a PSA of 1000 Monte-Carlo samples, CEACs are presented in Fig. 3. These graphs show for each vaccination alternative the chance of being the most cost-effective over a range of WTP thresholds. Corresponding cost-effectiveness planes are shown in supplementary results Fig. S3. At a SAR of 5%, QIV had a >90% probability of being the most cost-effective alternative when the WTP threshold was above I$5200/QALY in Agincourt, I$2150/QALY in Thai Nguyen and I$220,000/QALY in Albany. The high WTP threshold in Albany can be explained by the small impact that was modelled for QIV as compared with TIV, resulting in more uncertainty. When a SAR of 10% was used, QIV had a >90% probability of being the most cost-effective alternative at a WTP threshold to I$1950/QALY, I$880/QALY and I$15,500/QALY for Agincourt, Thai Nguyen and Albany, respectively. A univariate sensitivity analysis of the vaccine price of QIV versus TIV is shown in Fig. 4. Overall, the price premium of QIV over TIV had a significant impact on cost-effectiveness results. With a SAR of 5%, the price premium of QIV should be lower than I$0.31 in Agincourt, I$0.67 in Thai Nguyen and I$0.16 in Albany to result in cost-saving ICERs. When a SAR of 10% was assumed, this price premium was allowed to increase to I$0.73 in Agincourt, I$1.05 in Thai Nguyen and I$1.26 in Albany.
Fig. 3.
Cost-effectiveness acceptability curves of implementing influenza vaccination with trivalent influenza vaccine and quadrivalent influenza vaccine in the communities Agincourt (South Africa), Thai Nguyen (Vietnam) and Albany (Australia) over the period 2003–2011, assuming a symptomatic attack rate of 5% (A–C) and 10% (D–F). Results are based on a probabilistic sensitivity analysis with 1000 simulations. I$: International dollar, NV: No vaccination, QIV: Quadrivalent influenza vaccine, SAR: Symptomatic attack rate, TIV: Trivalent influenza vaccine, WTP: Willingness-to-pay.
Fig. 4.
Univariate sensitivity analysis of the price premium of QIV over TIV when a SAR of 5% (A) and 10% (B) was assumed. 0% price premium means that the QIV price is equal to the TIV price, while 100% price premium means that the QIV price is double of the TIV price. Aus: Australia, ICER: Incremental cost-effectiveness ratio, I$: International$, QALY: Quality-adjusted life year, QIV: Quadrivalent influenza vaccine, SA: South Africa, SAR: Symptomatic attack rate, TIV: Trivalent influenza vaccine, VN: Vietnam.
4. Discussion
In this study the cost-effectiveness of influenza vaccination with TIV and QIV was modelled in communities in South Africa (Agincourt), Vietnam (Thai Nguyen) and Australia (Albany), having different demographics, economic status, health care resources, influenza epidemiology and HIV prevalence. We found that cost-effectiveness of QIV as compared with TIV differed significantly between countries, being the highest in Albany and lowest in Thai Nguyen. This difference was explained by greater influenza B co-circulation and vaccine mismatches in South Africa and Vietnam during the period 2003–2013 as compared to Australia. Highest QALY gains due to influenza vaccination were found in Agincourt, mainly due to a higher risk of influenza-related death in the HIV-infected population. In Albany, vaccination prevented more healthcare costs and productivity losses due to relatively higher hospital costs and daily wages. Sensitivity analyses demonstrated that the SAR and the price of QIV had a high impact on the ICER.
When a no-vaccination SAR of 10% was assumed the proportional reductions in SAR were lower than for the 5% scenario across all communities, and consequently proportional reductions in clinical visits, hospitalization and mortality were also lower. This is expected since influenza is more transmissible (i.e. has a higher R0) in the higher attack rate scenario, which makes all interventions proportionally less effective. This occurred particularly in the Thai Nguyen model where coverage among young children, who are disproportionally responsible for onward transmission, was higher than in other communities (see supplementary results for more explanation). Note however that while the proportional reductions are lower in the 10% SAR scenario compared to the 5% scenario, the absolute number of cases prevented is higher. Increase of the SAR from 5% to 10% improved the cost-effectiveness, explained by the increase of the absolute number of clinical events prevented using the same quantity of vaccine.
To decide whether influenza vaccination is cost-effective and, if decided yes, whether this should be TIV or QIV, depends on the country, influenza epidemiology and the countries’ WTP threshold. For none of the countries involved in this analysis an official cost-effectiveness threshold is available. For South Africa and Vietnam, however, a previously estimated threshold for LMICs in general of I$1045/QALY might be used [41], while a previous study in Australia used a threshold of I$32,900 (=Aus$50,000)/QALY [42]. Then, TIV would be cost-effective in Vietnam and Australia, while for South-Africa this depends on the attack rate. QIV would only be cost-effective when a high SAR of 10% was assumed. For Vietnam, also an official threshold of Thailand might be used, which has been estimated at I$8100 (=100,000 Thai Baht)/QALY [43]. In that case, QIV would be cost-effective independent of influenza attack rate assumptions.
However, a review of the role of cost-effectiveness on introducing human papillomavirus and rotavirus vaccination in LMICs has shown that budgetary impact is often deemed more important for implementing a vaccination program than cost-effectiveness [44]. Extrapolating the annual budget impact of TIV and QIV to a national scale in South Africa, the costs of TIV and QIV would be I$59 million and I$76, respectively, being 0.14% and 0.18% of the national healthcare budget as estimated by the WHO [45], [46], [47]. Historic case studies of implemented vaccines for children in South Africa showed that vaccine costs of pneumococcal vaccination and rotavirus vaccination were estimated at IS$66 million and IS$22 million, respectively, while the ICERs ranged between US$523–1347/QALY [48]. Although the annual budget impact and cost-effectiveness of influenza vaccination with TIV is still in line with for instance pneumococcal vaccination (I$1803/QALY), the ICER we estimated for QIV is significantly higher (I$4183/QALY). For Vietnam, nationwide vaccination costs would be estimated at I$63 for TIV and I$79 million for QIV, being 0.34% and 0.43% of the healthcare budget. Although currently no vaccines have been implemented structurally, cost-effectiveness analyses of rotavirus and hepatitis B for children were estimated at I$556/QALY and I$4/QALY, respectively [49], [50]. This could imply that in Vietnam other non-implemented vaccines might be of higher priority than influenza vaccination with TIV (I$1.064/QALY) or QIV (I$1505/QALY). It should be noted, however, that influenza requires annual vaccination, while pneumococcal and rotavirus vaccination confer longer-term immunity.
To date, the cost-effectiveness of QIV has been shown to be favorable in several high-income countries [7]. One other study also assessed the public health and economic impact of QIV versus TIV in Australia [51]. Excluding vaccination costs, they found that QIV would result in $I30.6 million (AUS$46.5 million) of savings to the society between 2002–2013. When we would extrapolate our results to the whole Australian population, we found savings of I$6.5 million over a similar period. This difference can be primarily explained by that in our analysis we used approximately three times lower probabilities of consulting a GP or being hospitalized due to influenza disease. Other differences were that we used a dynamic model instead of a static model, a lower coverage rate (15% vs. 17.5%) and lower hospitalization costs for the elderly population.
This study has its limitations. The annual attack rate of symptomatic influenza was kept constant at 5% (and 10%) over the 11 years in all three regions, whereas influenza incidence varies per year and per region. Furthermore, influenza peaks differently country-by-country, for example southern Vietnam has a tropical climate with year-round circulation [52], while our model of Thai Nguyen assumed a winter influenza season. This complicates the timing of vaccination and may affect vaccine protection, enhancing the risk of a vaccine mismatch or waning of the vaccine-induced protection. We used data from Thailand for Vietnam to partition influenza B between Victoria and Yamagata lineages, as data for Vietnam was absent. We chose Thailand because it is located in the same influenza transmission zone as considered by the WHO Global Influenza Surveillance and Response System [53]. Moreover, online available influenza laboratory data from WHO’s Flunet showed a comparable partition of influenza A/H1N1 and A/H1N3 over the period 2006–2013 for both countries (data prior 2006 was not available for Vietnam here) [54]. In Thailand, A/H3N2 contributed to 44% of the total influenza A positive samples, while this was 47% in Vietnam.
We did not apply cross-protection of TIV to the non-matched B lineage in our study. However, recent evidence suggests that such a cross-protection exists, that might reach up to 60–70% of the efficacy of the matched influenza strain [55], [56]. Including cross-protection in the analysis would increase the estimated ICERs of QIV as compared with TIV, as the relative impact of QIV over TIV is diminished. We assumed the northern hemisphere composition of TIV for Vietnam in our analysis. Vietnam has a relatively long latitudinal span, stretching up far above the equator, where also the community of Thai Nguyen is located. Moreover, it covers tropic and subtropical areas where the concept of hemispheres may not directly be applied [57]. Although the government eventually decides whether the northern or southern hemisphere vaccine is to be used and despite the limitation on the applicability of the hemisphere concept in a country like Vietnam, the WHO recently recommended the southern hemisphere vaccine for tropical Asian countries including the whole of Vietnam [58]. In supplementary methods Table S2, the B-strains of northern TIV (see Vietnam) and southern TIV (see South Africa and Australia) are presented by season, as well as the contribution of both B-lineages to influenza circulation. It shows that the B-strain included in TIV only differed between the northern and southern hemisphere vaccine during the seasons 2006 and 2008. Co-circulation of both B-lineages occurred in these two seasons, however, the southern hemisphere TIV matched the B-lineage that contributed highest to influenza circulation better than the northern hemisphere TIV (18.3% vs. 15.0% in 2006 and 25.0% vs. 16.7% in 2008). This would result in a slightly lower impact of QIV in Vietnam and a slight worsening of the cost-effectiveness, without changing our overall conclusions. Finally, the vaccine efficacy was assumed to be similar between HIV-infected and HIV-uninfected individuals, which might be regarded as a simplification as this is still uncertain for HIV-adults with low CD4 cell count [59].
With regard to clinical and health-economic outcomes, we used hospitalization and mortality incidences that were based on pneumonia and influenza diagnoses only, while influenza is also associated with other respiratory as well as cardiorespiratory complications [60]. This reflects a conservative approach, as the prevented number of hospitalizations and deaths might be higher. We ignored possible side-effects of the influenza vaccine, although serious adverse outcomes are rare and the influenza vaccine is generally regarded as safe, including in HIV-infected individuals [61]. We limited costs in our analysis to direct medical costs and productivity losses due to work absenteeism. Costs not involved in our analysis include, for instance, transport costs, over-the-counter medication/traditional medicine, and productivity losses due to influenza-related presenteeism. Finally, the results of the community models cannot be directly translated to a national setting. For example, the income level and access to healthcare differs considerably within a country, especially in SA.
Our use of community-specific individual based simulation models can be considered a major strength of this analysis. Use of such dynamic systems for cost-effectiveness analyses in infectious diseases interventions is nowadays considered as the preferred approach [62]. Furthermore, experience with previously developed community-based models has demonstrated that community characteristics, such as household size, co-morbidities and population age structure, strongly influence influenza spread and the effectiveness of potential influenza mitigation measures [63]. This illustrates that prevalence of co-morbidities that increase the risk of severe influenza-related complications, such as HIV for the Agincourt region, should ideally be evaluated in the context of a specific community model that represents the target country or target population within a country. A further strength of our analysis was the predominant use of data on clinical event probabilities and resulting costs based on country or region specific data sources.
For future research we encourage cost-effectiveness studies to include other groups at higher risk of influenza illness and its complications recommended by WHO such as children and pregnant women. For instance, children are generally known as key transmitters of influenza and targeting them could be indirectly effective in protecting vulnerable populations, the elderly and people with medical conditions, against influenza disease [64]. Vaccination of pregnant women has been urged as a priority by the WHO [65] and performing cost-effectiveness studies for these target-groups might inform decision makers which target-groups should be prioritized when distributing vaccines over the population.
5. Conclusion
Our study showed that the cost-effectiveness of vaccination with TIV and QIV varies by country and is dependent on influenza epidemiology, vaccine price, willingness-to-pay thresholds and budgetary impact. Given our assumptions on WTP-threshold and vaccine price, we found that vaccinating the most vulnerable populations, being HIV-infected before elderly and young children, with TIV was cost-effective in communities in Vietnam and Australia during the period 2003–2013. In the South African community, however, the cost-effectiveness of TIV was dependent on the assumed attack rate. QIV was cost-effective in all three communities when a high attack rate was assumed. We note that our cost-effectiveness estimates do not automatically apply to different settings/target groups with different vaccination coverages. Also, our study is based on historic data, which does not necessarily reflect future influenza circulation. Obviously, our interpretation should be considered next to the general context for influenza vaccination in which the notion always exists that worldwide coverage of vaccination is thought to increase irrespective of the specific vaccine applied, i.e. be it QIV or TIV.
Declaration of interest
This study was supported by the World Health Organization. The funder of this study defined the aim of the study, but had no role in study design, data collection, analysis and interpretation of the model outcomes. The funder had access to several drafts of the report and was able to provide comments on the content. The authors had final responsibility for the decision to submit for publication.
PTdB’s position at the University of Groningen is financed by grants from the World Health Organization and various pharmaceutical companies, including those interested in the subject of matter. MJP and GM have received grants and honoraria from the World Health Organization and various pharmaceutical companies, including those interested in the subject of matter. CC has received grants from the World Health Organization and Sanofi Pasteur during the conduct of the study. NH, JK, TPLN, JM and CC have no competing interests. The Melbourne Centre has received funds from the IFPMA to support its influenza vaccine seed development work and IGB has shares in an influenza vaccine producing company.
Acknowledgements
We thank Christopher J. Gregory and colleagues of the Thailand MOPH-US CDC Collaboration for providing data on hospitalization and mortality rates in Thailand, and Kathleen Kahn and colleagues from NICD, for data on the Agincourt community. We thank Raymond Hutubessy and Jan Hendriks from the World Health Organization for providing comments on the study design and the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.12.073.
Contributor Information
Pieter T. de Boer, Email: p.t.de.boer@rug.nl.
Joel K. Kelso, Email: joel.kelso@uwa.edu.au.
Nilimesh Halder, Email: Nilimesh.halder@uwa.edu.au.
Thi-Phuong-Lan Nguyen, Email: ntplan75@gmail.com.
Jocelyn Moyes, Email: jossmoyes@gmail.com.
Cheryl Cohen, Email: cherylc@nicd.ac.za.
Ian G. Barr, Email: ian.barr@influenzacentre.org.
Maarten J. Postma, Email: m.j.postma@rug.nl.
George J. Milne, Email: George.Milne@uwa.edu.au.
Appendix A. Supplementary material
References
- 1.World Health Organization (WHO). Factsheet Influenza (Seasonal). Available from: <http://www.who.int/mediacentre/factsheets/fs211/en/> [Accessed March 1, 2015].
- 2.Centers for Disease Control and Prevention (CDC). Key Facts about Influenza (Flu) & Flu Vaccine. Available from: <http://www.cdc.gov/flu/keyfacts.htm> [Accessed May 1, 2015].
- 3.de Francisco Shapovalova N., Donadel M., Jit M., Hutubessy R. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine. 2015;33:6537–6544. doi: 10.1016/j.vaccine.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose C.S., Levin M.J. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81–88. doi: 10.4161/hv.8.1.17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peasah S.K., Meltzer M. Economic evaluation of influenza vaccination in preventing hospitalization in cardiovascular disease patients. Value Health. 2013;16:A283. [Google Scholar]
- 6.Ott J.J., Breteler J.K., Tam J.S. Influenza vaccines in low and middle income countries: a systematic review of economic evaluations. Hum Vaccines Immunother. 2013;9:1500–1511. doi: 10.4161/hv.24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer P.T., van Maanen B.M., Damm O. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2017;17:249–265. doi: 10.1080/14737167.2017.1343145. [DOI] [PubMed] [Google Scholar]
- 8.de Boer P.T., Crepey P., Pitman R.J. Cost-effectiveness of quadrivalent versus trivalent influenza vaccine in the United States. Value Health. 2016;19:964–975. doi: 10.1016/j.jval.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C., Moyes J., Tempia S. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis. 2013;19:1766–1774. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne G.J., Kelso J.K., Kelly H.A. A small community model for the transmission of infectious diseases: comparison of school closure as an intervention in individual-based models of an influenza pandemic. PLoS One. 2008;3:e4005. doi: 10.1371/journal.pone.0004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milne G.J., Halder N., Kelso J.K. Trivalent and quadrivalent influenza vaccination effectiveness in Australia and South Africa: results from a modelling study. Influenza Other Respir Viruses. 2015 doi: 10.1111/irv.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayward A.C., Fragaszy E.B., Bermingham A. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2:445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horby P., Mai le Q., Fox A. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the Ha Nam household cohort study I. Am J Epidemiol. 2012;175:1062–1074. doi: 10.1093/aje/kws121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAnerney J.M., Cohen C., Moyes J. Twenty-five years of outpatient influenza surveillance in South Africa, 1984–2008. J Infect Dis. 2012;206(Suppl 1):S153–S158. doi: 10.1093/infdis/jis575. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen Y.T., Graitcer S.B., Nguyen T.H. Vaccine. 2013;31:4368–4374. doi: 10.1016/j.vaccine.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr I.G., Jelley L.L. The coming era of quadrivalent human influenza vaccines: who will benefit? Drugs. 2012;72:2177–2185. doi: 10.2165/11641110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.The WHO Collaborating Centre for Reference and Research on Influenza. The WHO Collaborating Centre for Reference and Research on Influenza (VIDRL). Available from: <http://www.influenzacentre.org/> [Accessed 10/18, 2012].
- 18.Chittaganpitch M., Supawat K., Olsen S.J. Influenza viruses in Thailand: 7 years of sentinel surveillance data, 2004–2010. Influenza Other Respir Viruses. 2012;6:276–283. doi: 10.1111/j.1750-2659.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). WHO recommendations on the composition of influenza virus vaccines. Available from: WHO recommendations on the composition of influenza virus vaccines. [Accessed August 1, 2015].
- 20.Breteler J.K., Tam J.S., Jit M., Efficacy and effectiveness of seasonal and pandemic A (H1N1) influenza vaccines in low and middle income countries: a systematic review and meta-analysis. Vaccine. 2009;2013(31):5168–5177. doi: 10.1016/j.vaccine.2013.08.056. [DOI] [PubMed] [Google Scholar]
- 21.Simpson C.R., Ritchie L.D., Robertson C. Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: a retrospective observational cohort study. Lancet Infect Dis. 2012;12:696–702. doi: 10.1016/S1473-3099(12)70133-0. [DOI] [PubMed] [Google Scholar]
- 22.Widgren K., Magnusson M., Hagstam P. Prevailing effectiveness of the 2009 influenza A(H1N1)pdm09 vaccine during the 2010/11 season in Sweden. Euro Surveill. 2013;18:20447. [PubMed] [Google Scholar]
- 23.Cohen C., Moyes J., Tempia S. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PLoS One. 2015;10(3):e0118884. doi: 10.1371/journal.pone.0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emukule G.O., Khagayi S., McMorrow M.L. The Burden of Influenza and RSV among Inpatients and Outpatients in Rural Western Kenya, 2009–2012. PLoS One. 2014;9:e105543. doi: 10.1371/journal.pone.0105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmerman J.M., Chittaganpitch M., Levy J. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005–2008. PLoS One. 2009;4:e7776. doi: 10.1371/journal.pone.0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statistics South Africa. P0141 – Consumer Price Index (CPI), January 2015. Available from: <http://beta2.statssa.gov.za/publications/P0141/CPIHistory.pdf> [Accessed 03/01, 2015].
- 27.Australian Bureau of Statistics. 6401.0 – Consumer Price Index, Australia, Sep 2014. Available from: <http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/6401.0Sep%202014?OpenDocument> [Accessed March 1, 2015].
- 28.General Statistics Office of Vietnam. Consumer Price Index. Available from: <http://www.gso.gov.vn/default_en.aspx?tabid=625> [Accessed 03/01, 2015].
- 29.Centers for Disease Control and Prevention (CDC). Adult Influenza Vaccine Price List. Available from: <http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list> [Accessed February 5, 2015].
- 30.Koopmanschap M.A. The friction cost method for measuring indirect costs of disease. J Health Econ. 1995;14:171–189. doi: 10.1016/0167-6296(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 31.van Hoek A.J., Underwood A., Jit M. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLoS One. 2011;6:e17030. doi: 10.1371/journal.pone.0017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollmann M., Garin O., Galante M. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS One. 2013;8:e60477. doi: 10.1371/journal.pone.0060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO). Mortality and global health estimates, Life expectancy, Life tables by country, South Africa. Available from: Life tables by country South Africa. [Accessed February 1, 2015].
- 34.Australian Bureau of Statistics. 3302.0.55.001 - Life Tables, States, Territories and Australia, 2011-2013. Available from: <http://www.abs.gov.au/> [Accessed February 1, 2015].
- 35.World Health Organization (WHO). Mortality and global health estimates, Life expectancy, Life tables by country, Viet Nam. Available from: <http://apps.who.int/gho/data/?theme=main&vid=61830> [Accessed 02/01, 2015].
- 36.Jelsma J., Hansen K., De Weerdt W. How do Zimbabweans value health states? Popul Health Metr. 2003;1:11. doi: 10.1186/1478-7954-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tongsiri S., Cairns J. Estimating population-based values for EQ-5D health states in Thailand. Value Health. 2011;14:1142–1145. doi: 10.1016/j.jval.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 38.McCaffrey N., Kaambwa B., Currow D.C., Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14:133. doi: 10.1186/s12955-016-0537-0. 016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson L.F., Mossong J., Dorrington R.E. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutubessy RCW(HO). Personal communication; 2015
- 41.B. WoodsP. RevillM. SculpherK. Claxton. Country-level cost-effectiveness thresholds: Initial estimates and the need of further research. Available from: <https://www.york.ac.uk/media/che/documents/papers/researchpapers/CHERP109_cost-effectiveness_threshold_LMICs.pdf> [Accessed 09/01, 2017]. [DOI] [PMC free article] [PubMed]
- 42.Newall A.T., Dehollain J.P. The cost-effectiveness of influenza vaccination in elderly Australians: an exploratory analysis of the vaccine efficacy required. Vaccine. 2014;32:1323–1325. doi: 10.1016/j.vaccine.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 43.The Subcommittee for Development of the National List of Essential Medicines. The threshold at which an intervention becomes cost-effective. Meeting of the Subcommittee for Development of the National List of Essential Medicine 9/2550. December 20, 2007, Nonthaburi, Thailand.
- 44.Newall A.T., Jit M., Hutubessy R. Are current cost-effectiveness thresholds for low- and middle-income countries useful? Examples from the world of vaccines. Pharmacoeconomics. 2014;32:525–531. doi: 10.1007/s40273-014-0162-x. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO). Countries/South Africa/Statistics. Available from: <http://www.who.int/gho/countries/zaf/en/> [Accessed 03/01, 2015].
- 46.World Health Organization (WHO). Countries/Viet Nam/Statistics. Available from: <http://www.who.int/countries/vnm/en/> [Accessed 03/01, 2015].
- 47.World Health Organization (WHO). Countries/Australia/Statistics. Available from: <http://www.who.int/countries/aus/en/> [Accessed 03/01, 2015].
- 48.Blecher M.S., Meheus F., Kollipara A. Financing vaccinations – the South African experience. Vaccine. 2012;30(Suppl 3):C79–C86. doi: 10.1016/j.vaccine.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 49.Tu H.A., Rozenbaum M.H., Coyte P.C. Health economics of rotavirus immunization in Vietnam: potentials for favorable cost-effectiveness in developing countries. Vaccine. 2012;30:1521–1528. doi: 10.1016/j.vaccine.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 50.Tu H.A.T., De Vries R., Woerdenbag H.J. Cost-effectiveness of universal hepatitis B immunization in Vietnam: application of cost-effectiveness affordability curves in health decision-making. Value Health. 2010;13:A250. doi: 10.1016/j.vhri.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Jamotte A., Chong C.F., Manton A. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16:630. doi: 10.1186/s12889-016-3297-1. 016-3297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saha S., Chadha M., Al Mamun A. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern Asia. Bull World Health Organ. 2014;92:318–330. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization (WHO). Influenza Transmission Zones. Available from: <http://www.who.int/csr/disease/swineflu/Influenza_transmission_zones.pdf?ua=1> [Accessed 1/12, 2017].
- 54.World Health Organization (WHO). Flunet. Available from: <http://www.who.int/influenza/gisrs_laboratory/flunet/en/> [Accessed 1/12, 2017].
- 55.DiazGranados C.A., Denis M., Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31:49–57. doi: 10.1016/j.vaccine.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 56.Tricco A.C., Chit A., Soobiah C. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. 7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Mello W.A., de Paiva T.M., Ishida M.A. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One. 2009;4:e5095. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization (WHO). Which vaccine formulation to use – Northern or Southern Hemisphere? Available from: <http://www.who.int/influenza/vaccines/tropics/vaccination_formulation/en/> [Accessed 1/12, 2017].
- 59.Remschmidt C., Wichmann O., Harder T. Influenza vaccination in HIV-infected individuals: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness and safety. Vaccine. 2014;32:5585–5592. doi: 10.1016/j.vaccine.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 60.Thompson W.W., Shay D.K., Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices – United States, 2013-2014. MMWR Recomm Rep. 2013; 62: p. 1–43. [PubMed]
- 62.Jit M., Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: a primer. Pharmacoeconomics. 2011;29:371–386. doi: 10.2165/11539960-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milne GJ, Baskaran P, Halder N, et al. Pandemic influenza in Papua New Guinea: a modelling study comparison with pandemic spread in a developed country. BMJ Open 2013; 3. doi: 10.1136/bmjopen,2012-002518. [DOI] [PMC free article] [PubMed]
- 64.Sugaya N. A review of the indirect protection of younger children and the elderly through a mass influenza vaccination program in Japan. Expert Rev Vaccines. 2014;13:1563–1570. doi: 10.1586/14760584.2014.951036. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization (WHO). Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–476. [PubMed] [Google Scholar]
- 66.UNAIDS. South Africa: HIV and AIDS estimates. Available from: <http://www.unaids.org/en/regionscountries/countries/southafrica> [Accessed 08/01, 2015].
- 67.Nguyen TPL. Local data supplied by T.P.L. Nguyen, Thai Nguyen University of Medicine and Pharmacy; 2015.
- 68.The Kirby Institute. HIV, viral hepatitis and sexually transmissble infections in Australia Annual Surveillance Report. 2014; Sydney, NSW, Australia; The Kirby Institute, UNSW.
- 69.AVERT. Australia HIV & AIDS Statistics. Available from: <http://www.avert.org/australia-hiv-aids-statistics.htm> [Accessed 05/01, 2015].
- 70.McAnerney J.M., Walaza S., Cohen A.L. Effectiveness and knowledge, attitudes and practices of seasonal influenza vaccine in primary healthcare settings in South Africa, 2010–2013. Influenza Other Respir Viruses. 2015;9:143–150. doi: 10.1111/irv.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Australian Institute of Health and Welfare. 2009 Adult Vaccination Survey: Summary results. 2011; PHE 135Cat. no. PHE 135. Canberra: AIHW.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.