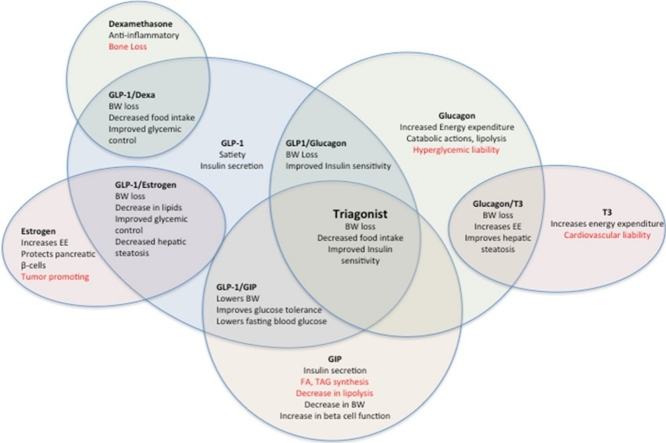
Graphical abstract
Keywords: GLP-1/Glucagon Co-agonism, Polypharmacology, Type 2 diabetes, Gip
Highlights
-
•
Unimolecular agonists targeting the receptors for GLP-1, GIP and Glucagon offer therapeutic value for the treatment of the metabolic syndrome.
-
•
Peptide-mediated nuclear hormone delivery is an innovative approach for novel pharmacotherapies.
Abstract
Chemical derivatives of the gut-derived peptide hormone glucagon-like peptide 1 (GLP-1) are among the best-in-class pharmacotherapies to treat obesity and type 2 diabetes. However, GLP-1 analogs have modest weight lowering capacity, in the range of 5–10%, and the therapeutic window is hampered by dose-dependent side effects. Over the last few years, a new concept has emerged: combining the beneficial effects of several key metabolic hormones into a single molecular entity. Several unimolecular GLP-1-based polyagonists have shown superior metabolic action compared to GLP-1 monotherapies. In this review article, we highlight the history of polyagonists targeting the receptors for GLP-1, GIP and glucagon, and discuss recent progress in expanding of this concept to now allow targeted delivery of nuclear hormones via GLP-1 and other gut hormones, as a novel approach towards more personalized pharmacotherapies.
1. Introduction
Diabetes mellitus is a devastating metabolic disease that has reached epidemic proportions worldwide. Type 2 diabetes (T2D) is the most common form of the disease, affecting 90–95% of diabetic patients, with 415 million affected individuals. Estimates suggest that by 2040 this number will rise to 642 million [1]. This rise in diabetes has severe economic consequences, since approximately 12% of the global health expenditure ($673 billion) is spent on diabetes and its complications, which includes hospital, outpatient, and therapeutic interventions [1]. This economic burden will continue to increase as the rates of diabetes are growing rapidly, especially in low- and middle-income countries, where more than 75% of diabetic individuals live [[2], [3], [4]]. Tragically, the World Health Organization (WHO) estimates that diabetes resulted in 1.6 million deaths in 2015, making it the 6th leading cause of death [5].
Diabetes mellitus is characterized by pathological failure to buffer against prolonged episodes of hyperglycemia, which ultimately leads to diabetes related complications. Microvascular complications include damage of the eyes (retinopathy), the nervous system (neuropathy) and the kidney (nephropathy), while macrovascular complications include coronary artery disease, cerebrovascular and peripheral vascular disease [6].
Cardiovascular disease is perhaps the most dangerous consequence of diabetes, despite the ravaging impact that neuropathy, nephropathy and retinopathy can have on diabetic individuals. The major contributor to type 2 diabetes is obesity, which is also gaining global prevalence [7,8].
Type 2 diabetes is managed with non-pharmacological interventions and various pharmacological treatment options. While lifestyle changes in diet and physical activity are the primary approach to treat individuals with diabetes, pharmacological options are usually required to meet target levels of blood glucose and glycolysated hemoglobin (HbA1c).
The most commonly used medicinal approaches to treat T2D include biguanides, sulfonylureas, thiazolidinediones, insulin, inhibitors of the sodium glucose cotransporter 2 (SGLT 2) or the dipeptidyl peptidase-4 (DPP-IV) enzyme, or mimetics targeting the receptor for glucagon-like peptide 1 (GLP-1) [9]. While all of these pharmacotherapies improve glucose handling, albeit with varying efficacy [[10], [11], [12]], many of these options have undesirable effects and some even possess a dose-dependent risk of causing hypoglycemia, such as insulin and sulfonylureas [[13], [14], [15], [16]]. Other side effects, such as weight gain, are also commonly described for the use of sulfonyluresas, insulin and thiazolidinediones, which limit their overall use in already obese individuals [16]. Collectively, there is a great demand for the development of safe and effective anti-diabetic agents with accompanied weight reduction and cardiovascular safety. In this review, we report recent advances in the development of unimolecular polyagonists, describe the underlying mechanisms identified in preclinical studies, and discuss potential translational relevance of these novel pharmacotherapies.
2. Physiology and pharmacology of GLP-1, GIP and glucagon
2.1. Glucagon-like peptide 1 (GLP-1)
Insulin secretion is controlled not only by glucose directly but also by insulinotropic factors like the incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) [17].
Glucagon-like peptide 1 is synthesized and secreted primarily from the intestinal L-cells after post- translational processing of proglucagon by prohormone convertase 1/3 (PC1/3). The proglucagon gene is not only expressed in the L-cells of the intestine, but also in the α-cells of the endocrine pancreas and neurons located in the caudal brainstem and hypothalamus [18]. GLP-1 is secreted in response to nutrient ingestion and acts as an insulinotropic hormone in a glucose-dependent fashion [19]. GLP-1 binds to the GLP-1 receptor (GLP-1R), a seven transmembrane G-protein-coupled receptor with high expression in pancreatic islets, the central nervous system and enteric neurons [20], but also in the lung, kidney, lymphocytes, macrophages [21], heart [22], and human coronary endothelial cells [23].
In addition to its insulinotropic effects, GLP-1 also inhibits glucagon secretion from pancreatic α-cells [24], delays gastric emptying [25], enhances β-cell proliferation while inhibiting β-cell apoptosis in rodents [26], decreases gluconeogenesis in the liver [27] and reduces hepatic lipid content [20]. GLP-1 also decreases body weight via centrally regulated inhibition of food intake, and GLP-1 fails to affect food intake in mice with CNS-specific deletion of the GLP-1 receptor [[28], [29], [30]]. In addition, GLP-1 has neuroprotective effects with pharmacological potential for the treatment of neurodegenerative diseases such as Alzheimer’s [31] and Parkinson’s disease (PD) [[32], [33], [34]]. Exendin-4 has further been shown to have neuroprotective effects in animal models of cerebral ischemia [35] and amyotrophic lateral sclerosis [36]. Moreover, GLP-1 has cardioprotective effects and exerts metabolic benefits in cardiomyocytes, blood vessels, immune cells, leading to attenuated development of atherosclerosis, beneficial reductions in systolic and diastolic blood pressure, reduced cholesterol plasma levels, and reduced ischemia-reperfusion injury [[37], [38], [39], [40]]. Additionally, GLP-1R mRNA transcripts are also expressed in the kidney and GLP-1 agonism in rodents with diabetic nephropathy reduces proteinuria and produces functional and histological improvement in the diabetic kidney [41]. Clinical data of pooled registration trials and results of large-sized cardiovascular outcome studies indicate that use of GLP-1R agonists, in addition to standard care, modestly improve albuminuria in T2D, beyond the effects of glycaemic control [42]. Especially the use of liraglutide in patients with T2D and high cardiovascular risk has resulted in lower rates of the development and progression of diabetic kidney disease than placebo [43]. GLP-1R mRNA was also detected at high levels in the rat lung and binds to receptors on the submucosal glands of the trachea and the smooth muscle of the pulmonary arteries, causing a significant increase in mucous secretion and pulmonary smooth muscle relaxation [44,45].
The insulinotropic and cardioprotective actions of GLP-1 make it a useful pharmacological tool in the fight against T2D. However, the therapeutic value of native GLP-1 is hampered by a relatively short half-life, less than two minutes in humans [46], due to degradation by dipeptidyl peptidase-4 (DPP-IV), which cleaves GLP-1 at its second N-terminal alanine 2 residue, thus yielding an inactive GLP-19-36 amide or GLP-19-37 [[46], [47], [48]]. In addition, neutral endopeptidase (NEP) has also been shown to deactivate GLP-1 in vivo [49,50]. To overcome the susceptibility for rapid DPP-IV degradation, synthetically-designed GLP-1 derivatives have been developed which possess a longer half-life due to amino acid substitutions at the N-terminus, or the addition of fatty acids which extend the half-life of GLP-1 through delayed clearance from the circulation [51]. These synthetic analogs are currently very popular in the treatment of diabetes.
Between 2005 and 2016, the FDA approved six synthetic GLP-1R agonists available for use in the United States to treat T2D: exenatide (Bayetta®, AstraZeneca, USA), liraglutide (Victoza®, Novo Nordisk), exenatide long-acting release (Bydureon®, AstraZeneca, USA), albiglutide (Tanzeum®, GlaskoSmithKline), dulaglutide (Trulicity®; Eli Lilly & Co), and lixisenatide (Lyxumia®, Sanofi). These analogs have extended biological half-lives and confer little to no risk of hypoglycemia. Chronic treatment of patients with T2D patients over 12–52 weeks with these different GLP-1 receptor analogs, such as liraglutide and exenatide, leads to a reduction in glycolysated hemoglobin (HbA1c) of 1.1-1.6% and up to 5% reduction in body weight [[52], [53], [54], [55], [56], [57]]. Besides the glucose lowering effect of all GLP-1 analogs, in 2017, the FDA approved liraglutide (Saxenda ®, 3 mg) for a second indication as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in overweight and obese adult patients [58]. Also in 2017, the FDA approved a new indication for liraglutide (Victoza ® 1,2/1,8 mg), for reducing the risk for myocardial infarction, stroke, and cardiovascular death in adults with type 2 diabetes who have established cardiovascular disease. Based on the results from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results—A Long Term Evaluation) trial, liraglutide reduced the risk of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke by 13% compared with placebo (p = 0.01), with an absolute risk reduction of 1.9% [59].
Currently, a new drug application for once-weekly semaglutide (Novo Nordisk) was submitted to the FDA in December 2016. Semaglutide is a fatty-acylated GLP-1 analog that has been shown to decrease body weight and to improve glycemia in preclinical trials with patients with type 2 diabetes [60]. Semaglutide is also under review by the European Medicines Agency, and the Japanese Pharmaceuticals and Medical Devices Agency [61].
Unfortunately, GLP-1 and its analogs induce adverse events, mostly of gastrointestinal character, such as nausea, vomiting and diarrhea [62]. These side effects occur in a dose-dependent manner, which generally limits the use of higher doses to drive greater weight loss [63]. An observed slight increase in heart rate in humans when treated with liraglutide and exenatide long-acting release (LAR) led to required cardiovascular outcome studies to demonstrate cardiovascular (CV) safety via randomized, controlled trials.
In light of the dose-limiting side effects, there is a desire for safer and more effective GLP-1 based analogs. In this review, we discuss the advantages of GLP-1 agonism in conjunction with other hormones, such as GIP and glucagon.
2.2. Glucose-dependent insulinotropic polypeptide (GIP)
Glucose-dependent insulinotropic polypeptide (GIP) is synthesized in and secreted from enteroendocrine K- cells of the proximal small intestine in response to dietary lipids [[64], [65], [66]]. Additionally, CNS production of GIP has also been described in rodents [67], and GIP transcripts have been localized in pancreatic α-cells of rodents and humans [68]. Encoded by a proGIP precursor, post-translational processing by the prohormone convertase enzymes 1 and 3 (PC1/3) yields a mature 42 amino acid protein (GIP 1–42) [69]. In pancreatic α-cells and a subset of enteroendocrine K-cells however, differential processing of proGIP by PC 2 and C-terminal amidation by peptidyl-glycine α-amidating monooxygenase results in a 30 amino acid protein (GIP 1–30). The insulinotropic effects of this peptide seem to match GIP 1–42, but its extra-pancreatic actions are still elusive [70]. GIP’s major physiological role is targeting pancreatic islets to enhance insulin secretion under conditions of hyperglycemia [18], but GIP also stimulates the release of glucagon under conditions of hypoglycemia, thus serving as a bifunctional hormone that is capable of buffering against both extremes of glucose excursion [71]. In humans, intravenously administered GIP increases plasma insulin levels [72], but in some obese T2D patients, insulin secretion is blunted in response to GIP, indicating that impaired GIP signaling may be part of the mechanisms that underlie T2D [73]. In addition to its actions in modulating insulin release, GIP promotes pancreatic β-cell growth, differentiation, proliferation and survival in mouse models of diabetes [74]. GIP binds to the GIP receptor (GIPR), a class B G-protein-coupled receptor with high expression not just in pancreatic islets, but also in insulin-sensitive tissues involved in controlling systemic metabolism, such as the hypothalamus [75] and adipose tissue [76]. GIP has been stigmatized as an obesogenic factor, since GIP stimulates adipogenesis under in-vitro conditions [77], inhibits lipolysis [78] and stimulates lipogenesis [79]. Blocking GIP signaling in mice diminishes weight gain and improves glucose metabolism [[80], [81], [82], [83]]. Circulating levels of GIP also positively correlate with body weight, and thus are typically elevated in genetically- and diet-induced obese mice [[84], [85], [86]] and obese humans [[87], [88], [89]]. However, a growing body of new evidence suggests that GIP agonism has metabolic benefits. Mice overexpressing GIP or chronic GIPR agonism show improved glucose metabolism, without negative effects on body weight [90,91]. Other studies in pigs without functional GIP receptors show impaired glucose tolerance due to delayed insulin secretion, impaired insulinotropic effect of GIP and reduced β-cell-proliferation and reduced islet mass [92]. Analysis of several genome-wide association studies (GWAS), especially the Meta-Analysis of Glucose and Insulin-Related Traits Consortium (MAGIC), identified single nucleotide polymorphism (SNP) variants at the human GIPR locus (rs10423928) which were associated with impaired glucose tolerance [93]. Carriers of this GIPR rs10423928 A-allele showed decreased GIPR expression in islets and a blunted insulinotropic response resulting in elevated blood glucose following a post-oral challenge [94]. Such human studies thus support the idea that GIPR is a potential pharmacological target for the prevention and treatment of T2D.
Several GIPR antagonists have been used in rodents, suggesting that pharmaceutical inhibition of GIP might benefit obesity related metabolic disorders. These molecules evoke impaired glucose control [[95], [96], [97], [98]] and cause chronic weight loss in obese rodent models [80,81,99,100]. But these initially classified antagonists turned out to present cross-reactive antagonism at other receptors, for example at the glucagon receptor [95], are of low potency [[101], [102], [103]] or are partial agonists at the GIPR [97,[104], [105], [106]]. Thus, current data justify a redirection of manipulating the GIP system by increasing rather than diminishing GIP action using appropriately validated tools.
Besides using the GIP system for the treatment of metabolic diseases, GIP also influences bone remodeling and contains neuroprotective properties. In general, bone resorption is inhibited after nutrient ingestion. The GIPR is expressed in osteocytes, osteoblasts, and osteoclasts, as shown by both mRNA and protein expression [[107], [108], [109]]. GIP binds to GIPR in osteoblast cell lines at physiological concentrations, and stimulates collagen α(I) gene expression and alkaline phosphatase (ALP) activity, processes that are associated with bone formation [109]. In addition to promoting bone formation, GIP also reduces osteoclast differentiation [110] and bone resorption [107]. GIPR knockout mice exhibit reduced bone strength and increased trabecular (spongy) bone volume [111,112], further emphasizing the role of GIP in bone formation.
The GIPR is also expressed in the cerebral cortex, especially pyramidal cortical neurons [113], the hippocampus and olfactory bulb in rats and in the hippocampus and neocortex of humans [114]. Whether GIP crosses the blood-brain-barrier in physiologically relevant concentrations is still unclear; thus, the importance of central versus peripheral-produced GIP remains elusive. GIP enhances neuronal stem cell proliferation in the brain [113] and its property to enhance synaptic plasticity in the hippocampus is a mechanism considered to represent the cellular level of memory formation, making GIP analogs a promising target for the development of novel treatments of Alzheimer’s disease [115]. GIPR has also been localized in cells within the hypothalamus, pituitary, and adrenal cortex. GIPR activation increases plasma corticosterone in rodents [93], but the importance of the GIPR-hypothalamic-pituitary-adrenal axis has not yet been established for humans.
While offering numerous beneficial glucometabolic and neuroprotective effects, the therapeutic value of native GIP is hampered by its rapid degradation through N-terminal truncation by DDP-IV and undergoes rapid renal clearance [46,116,117]. Comparable to the chemical optimization approaches of GLP-1, several GIP analogs have been generated, for example by glycation, acetylation or acylation of the N- terminal tyrosine residue (Tyr1) [91,[118], [119], [120], [121], [122], [123], [124], [125], [126]] or by substitutions for the alanine residue (Ala2) via including clycine [118], N-methylglycine [127], serine [74] or homoalanine [127]. All these modifications offer protection from DPP-IV degradation, but with variable receptor affinity and activity. Both the acute and chronic effects of these GIP analogs are currently investigated in various animal models.
2.3. Glucagon
Glucagon is a 29 amino acid pancreatic peptide processed from proglucagon in pancreatic α-cells by PC2 [[128], [129], [130], [131]]. Glucagon is best recognized for its acute ability to increase blood glucose by increasing hepatic glucose production via enhanced glycogen breakdown and stimulation of gluconeogenesis [132], thus providing the major counter-regulatory mechanism for insulin in maintaining glucose homeostasis in vivo.
Glucagon acts via a seven-transmembrane G protein-coupled receptor consisting of 485 amino acids [133] and glucagon-binding sites have been identified in multiple tissues, including liver, brain, pancreas, kidney, intestine and adipose tissues [134,135].
Historically, glucagon was used predominantly for the treatment of severe hypoglycemia [136]. Unfortunately, native glucagon possesses only poor solubility in aqueous solutions at or near physiological pH values. At concentrations suitable for its medicinal use, glucagon forms trimers, which lowers its solubility at physiological pH [137]. In acidic or alkaline aqueous solutions, its solubility is greater, but elicits a reduced chemical stability and biological potency. Thus, the glucagon emergency kit requires instant dissolution of lyophilized glucagon in an acidic solvent immediately prior to its use, a challenging task in a hypoglycemic emergency. Due to glucagon’s poor physical stability, short duration of action and limited aqueous solubility the investigation of its therapeutic potential in metabolic diseases has been difficult. Nevertheless, recent advances have altered the solubility of glucagon. To increase the aqueous solubility of glucagon at physiological pH, the substitution of asparagine (Asn) with asparic acid (Asp) at position 28 [138] or the COOH-terminal extension (CEX) of the glucagon sequence with a nine-amino acid COOH-terminal sequence derived from the GLP-1 paralog exendin-4 [138,139] is needed. Despite lower activity at the glucagon receptor relative to the native hormone [138], the evaluation of glucagon’s therapeutic potential has become possible in preclinical studies.
Glucagon’s diabetogenic nature resulting from its potent ability to enhance hepatic glucose output has led to various preclinical and clinical studies investigating the antihyperglycemic effect by inhibiting glucagon signaling. Such pharmacological approaches include the use of GCGR antagonizing peptides, small molecules monoclonal antibodies or antisense oligonucleotides. These studies have shown that inhibition of glucagon signaling improves glucose metabolism in mice, rats, rabbits, dogs and monkeys [131]. Notably, glucagon has additional beneficial metabolic effects beyond glucose buffering, including the modulation of lipid metabolism through activation of lipolysis and inhibition of lipid synthesis [140], regulation of energy intake [141], stimulation of brown fat thermogenesis [142], improvement of cardiac output [143] and inhibition of gastric motility [144].
Decades ago, repeated administration of glucagon was reported to yield improvements in rodent metabolism, especially lower body weight [145]. But the inherent risk of hyperglycemia, especially in patients with T2D, has complicated the translation of these observations to human study.
3. Unimolecular polyagonists- a new strategy for the treatment of the metabolic syndrome
3.1. GLP-1/Glucagon dual agonism
Many diseases cannot be sufficiently treated with a single drug regimen. Increasing the dose of a single hormone therapy can potentially increase the metabolic benefits of a drug but often coincides with an increased prevalence of unwanted off-target effects. The co-administration of several independent drugs, each having beneficial effects on metabolism, can decrease body weight beyond what can be achieved by a single hormone alone. Such combinatorial therapies are commonly used in the treatment of hypertension, dyslipidemia and diabetes, in order to meet target levels of blood pressure, lipids and glucose. Previously in the field of diabetes and obesity, loose co-administration of drugs were used to induce weight loss, such as the famous rainbow pills in the 1949′s [146], the combination of phentermine and fenfluramine (Phen/Fen) in the 1990′s [[147], [148], [149], [150], [151], [152], [153]] or Qsymia®, the combination of phentermine and topiramate [[154], [155], [156], [157], [158]]. While some of these pharmacotherapies achieved remarkable effects on metabolism, these combinations were not without risk and were not a pharmacological silver bullet against the obesity pandemic. However, in the last decade, we have witnessed a remarkable progress in the development of molecules that combine the beneficial effects of multiple key metabolic hormones into a single entity of enhanced potency and sustained action relative to the native hormones. The basic idea of these unimolecular multiagonists is that the simultaneous activation of different signaling mechanisms maximizes the metabolic benefits, minimizes adverse effects, and offers a more balanced pharmacokinetic action profile compared to loose co-administration of single hormones. A large number of unimolecular co-agonists are in preclinical and clinical development (Table 1). Several of these peptides, which will be discussed in more detail below, have shown synergistic metabolic action from mice to man and show overall good tolerability and improved safety.
Table 1.
Multiagonists Currently in Development.
| Target Receptors | Drug | Company | Status |
|---|---|---|---|
| GLP-1R/GCGR | HM12525A | Hamni Pharmaceuticals | Phase I |
| JNJ-54728518 | Janssen Pharmaceuticals | Phase I | |
| MEDI0382 | MedImmune | Phase II | |
| MK-8521 | Merck | Phase II | |
| NN9277 | Novo Nordisk | Phase I | |
| MOD-6030/1 | Prolor/OPKO Biological | Preclinical | |
| SAR425899 | Sanofi | Phase II | |
| VPD-107 | Spitfire Pharma | Preclinical | |
| TT-401 | Transition Therapeutics | Phase II | |
| ZP2929 | Zealand | Phase I | |
| GLP-1R/GIPR | CPD86 | Eli Lilly | Preclinical |
| LY3298176 | Eli Lilly | Phase I | |
| NN9709/MAR709/RG7697 | Novo Nordisk/Marcadia | Phase II | |
| SAR438335 | Sanofi | Phase I | |
| ZP-I-98 | Zealand | Preclinical | |
| ZP-DI-70 | Zealand | Preclinical | |
| GLP-1R/GCGR/GIPR | HM15211 | Hamni Pharmaceuticals | Preclinical |
| MAR423 | Novo Nordisk/Marcadia | Phase I |
In 2009, a new peptide was developed combining the glycemic and anorectic effects of GLP-1 with the lipolytic and thermogenic properties of glucagon [159], under the hypothesis that the insulinotropic effect of GLP-1 would restrain the hyperglycemic action of glucagon. Compared to its native hormones, this GLP-1/glucagon co-agonist exhibits improved pharmacokinetics and a superior time action. This co-agonist was designed on a glucagon backbone with six amino acids substituted by the respective GLP-1 residues [159]. Further modifications included the introduction of an aminoisobutyric acid (Aib) residue at position 2 of the peptide to yield protection from DPP-IV inactivation, forming a lactam bridge to boost GcGR agonism and to stabilize secondary structure, and pegylation of the C24 residue to prolong in vivo action and to delay renal clearance. Together these changes resulted in a molecule with nearly balanced co-agonism at both receptors and the necessary solubility and stability in physiological buffers [159]. In DIO mice, a once-weekly treatment with 70nmol/kg of this GLP-1/glucagon co-agonist normalizes body weight, improves glucose tolerance, lipid metabolism, and liver steatosis, all within four weeks of treatment (Fig. 1). In line with glucagon’s thermogenic capacity and GLP-1′s anorectic effect, the body weight loss is due to a loss of fat mass, decreased food intake, and increased energy expenditure. Notably, this co-agonist also decreases body weight and fat mass in GLP-1R KO mice, albeit with lower efficacy than in wild type mice, emphasizing that glucagon significantly contributes to the metabolic effects of this co-agonist.
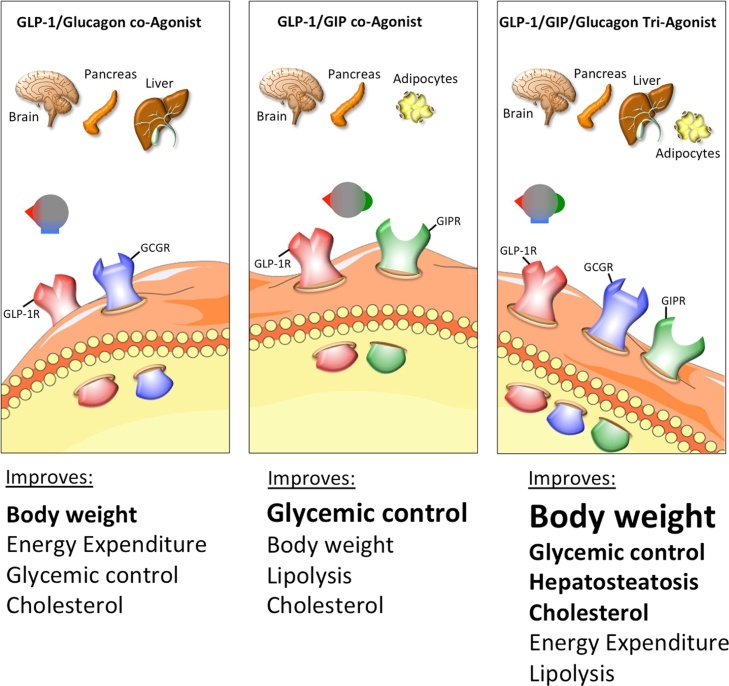
Fig. 1.
Schematic on the physiological effects of multiagonists targeting the receptors for GLP-1/Glucagon, GLP-1/GIP.
Maximal weight loss in the absence of hyperglycemia was achieved with a co-agonist comparably balanced for in vitro potency at murine GLP1R and GCGR [160], although the most effective ratio of GLP-1R and GCGR agonism may vary by species.
Interestingly, in DIO mice, the GLP-1/glucagon co-agonist restores leptin responsiveness when combined with pegylated leptin (PEG-leptin), thereby synergistically improving body weight loss [161]. Previously, the restoration of leptin sensitivity required the cessation of a HFD [162,163], however, the co-agonist was able to improve leptin sensitivity even in the presence of continued HFD. In these mice, liver and plasma triglycerides, cholesterol, insulin and leptin plasma levels improved after the treatment with the co-agonist, and the addition of PEG-leptin resulted in further improvements in these metabolic markers [161].
Nearly simultaneously to the development of this GLP-1R/GCGR co-agonist, a second GLP-1/GCGR co-agonist was engineered, but this time from the oxyntomodulin (OXM) peptide sequence. Oxyntomodulin is a 37 amino acid peptide released from intestinal L-cells in response to a meal. Oxyntomodulin activates both the GLP-1 and glucagon receptors, although with 10–100 fold lower affinity relative to the native hormones [164]. Oxyntomodulin reduces food intake and promotes weight loss in rodents and humans [[165], [166], [167], [168], [169]]. These observations prompted the development of the GLP-1R/GCGR polyagonist (Dual AG), based on the sequence of oxyntomodulin. The resulting Dual AG peptide is a full agonist of both target receptors, with comparable activity to native OXM [170]. In DIO mice, a two week treatment with 1.9 μmol/kg/day of Dual AG promoted superior weight loss, lipid lowering effects and improved glycemic control compared to a corresponding GLP-1R-selective agonist [170]. Dual AG also increases fatty acid oxidation and reduces hepatic steatosis [170].
Encouraged by the successful, independent development of different GLP-1/glucagon co-agonists, other single molecule polyagonists with enhanced metabolic efficacy were generated as anti-diabetic and anti-obesity therapeutic options.
In 2016, another GLP-1/glucagon co-agonist, MEDI0382, was tested in rodents and non-human primates. In DIO mice and cynomolgus monkeys, the co-agonist potently induces weight loss, reduces hepatic fat content and improves glucose metabolism [171].
One year later, another GLP-1/glucagon co-agonist was engineered on the exendin-4 sequence with included glucagon receptor agonistic activity while maintaining the potent GLP-1R activity and achieving sufficient in vivo stability. The introduction of a d-serine in position 2 of a chimeric peptide (peptide 7) and a lysine residue in position 14 modified by addition of palmitic acid at the ε- group using a γ-glutamic acid spacer provided a potent and balanced dual GLP-1/glucagon co-agonist. In DIO mice, a twice daily treatment with 50 μg/kg of this co-agonist showed significant glucose lowering effects with associated body weight and body fat mass reduction in murine models of diabetes and obesity over a treatment period of 33 days [172].
Additionally in 2017, a novel GLP-1R/GCGR dual agonist was developed with amino acid substitution at position 22, 23 and 25 by cysteine in glucagon and laurate maleimide conjugation to optimize the pharmacokinetic profile of this compound. In DIO mice, administration of 1000nmol/kg once every two days for a treatment period of one month normalized glucose tolerance, adiposity and improved plasma metabolic parameters, such as leptin, insulin and adiponectin [173].
Further confirming the translational value of the GLP-1/glucagon combination, loose low-dose co-infusion of GLP-1 and glucagon has been demonstrated to increase energy expenditure [174] and to decrease food intake in humans [52]. The translational potential of GLP-1R/GCGR co-agonism is further underscored by the many co-agonists currently being evaluated in clinical trials (Table 1).
3.2. GLP-1/GIP dual agonism
In 2013, a collaboration between the DiMarchi and Tschöp laboratories led to the development of a GLP-1/GIP co-agonist [175]. GIP was included to increase the insulinotropic effects of GLP-1 while the anorectic effects of GLP-1 would buffer against any possible obesogenic action that may or may not arise from GIP agonism. Beginning with the native glucagon sequence, amino acids from native GLP-1 and GIP were introduced and the resulting peptides were analyzed for activity at the GLP-1R and GIPR [175]. The final, balance co-agonist included an Aib residue at position 2 for the prevention of DPP-IV degradation, and the Cex extension to improve solubility and pharmacokinetics [175]. In diet-induce obese and hyperglycemic leptin-deficient mice, daily administration of the GLP-1/GIP co-agonist improves several hallmarks of the metabolic syndrome, such as obesity, hyperglycemia and dyslipidemia (Fig. 1) [175]. This co-agonist has shown great translational potential, with metabolic benefits translating from mice to non-human primates and humans. In cynomolgus monkeys, the acylated co-agonist was more efficient at reducing blood glucose levels and increasing plasma insulin than liraglutide [175]. In humans, the pegylated co-agonist was administered to healthy, non-diabetic volunteers. The co-agonist decreased plasma insulin and decreased blood glucose during a glucose infusion challenge, more effectively than liraglutide [175].
In a further clinical trial, a novel dual GLP-1/GIP co-agonist (MAR709/RG7697) (Table 1) was recently investigated after a single subcutaneous administration in healthy subjects. RG7697 was generally well tolerated up to 3.6 mg and the evidence of its glycaemic effect and pharmaocokinetic profile rendered this compound for further investigations in patients with T2D [176]. In a two week study, patients with T2D received once daily RG7697. The pharmacodynamic effect displayed dose-dependent reductions in fasting and postprandial plasma glucose, without increasing the risk of hypoglycaemia [177].
This GLP-1/GIP co-agonist was further developed by the addition of a fatty-acyl group. The fatty-acylated dual agonist, NNC0090-2746, was recently analyzed in a 12- week, randomized, placebo- controlled, double blind phase 2a trial, where T2D patients received 1,8 mg of the co-agonist subcutaneously once daily for 12 weeks. NNC0090-2746 significantly improved glycemic control, and reduced body weight, total cholesterol and leptin relative to placebo controls [178].
In addition to its metabolic action profile, GLP-1/GIP co-agonism has recently been shown to have neuroprotective effects in cell and mouse models of mild traumatic brain injury [179], in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model [180] and 6-hydroxydopamine (6-OHDA) brain lesion rat model of Parkinson’s disease [181] and in a rat model of Alzheimer’s disease [182] indicating that this polyagonist has also translational potential outside of regulating body weight and glucose metabolism.
The translational value of all these co-agonists needs to be proven in clinical settings, but already a number of compounds have proceeded into phase I and II clinical trials [183] (Table 1).
3.3. Unimolecular GLP-1/GIP/Glucagon tri-agonism
The success of the various GLP-1/GCG and GLP-1/GIP dual agonists inspired research towards the generation of a single molecule with balanced activity at all three target receptors. This concept of a GLP-1/GCG/GIP triple agonist was further spurred by the observation that the loose adjunct administration of GLP-1/GIP along with glucagon was superior in reducing body weight and to improve glycemia relative to treatment with the co-agonist alone [184]. The first GLP-1/GCG/GIP triple agonist was then engineered by introducing GCGR agonism to a GLP-1R/GIPR co-agonist [184]. Structure-activity relationship (SAR) studies identified key glucagon residues required for GCGR activity, and inclusion of these residues (Glu16, Arg17, Gln20, Leu27, and Asp28) resulted in a triple agonist [184]. In addition, the solubility and pharmacokinetics of the triple agonist were enhanced by including an Aib residue at position 2, a C-terminal CEX extension, and a palmitic acid via the Lys10 residue [184]. The activity of this tri-agonist at each of the three target receptors was validated using cell lines expressing a target receptor and a cAMP sensitive luciferase assay. In each of the three cell lines (GLP-1R expressing MIN6, GIPR expressing mouse 3T3-L1 apidocytes, and GCGR expressing rat hepatocytes), the tri-agonist displayed full activity and ten fold greater potency than the native ligand [184]. In DIO mice and rats, the tri-agonist lowered body weight to a greater extent than a matched dose of liraglutide [184]. In mice, the tri-agonist lowered food intake, reduced plasma insulin, increased plasma FGF21, lowered plasma cholesterol, and decreased hepatic lipid content, all more effectively than liraglutide (Fig. 1) [184]. Importantly, the tri-agonist also lowered fasting blood glucose levels and improved glucose tolerance, without inducing hypoglycemia. This could indicate that the GLP-1R agonism successfully counters the acute hyperglycemic effects of GCGR agonism, but it is also in keeping with the published results that chronic GCGR agonism promotes body weight loss and improvements in glucose tolerance [131]. In db/db mice, the tri-agonist prevents the excessive weight gain, without altering food intake [184]. Similarly, in ZDF rats, the tri-agonist decreases body weight, decreases fasting blood glucose, improves glucose tolerance, decreases glycosylated hemoglobin (HbA1C), and preserves islet cytoarchitechture [184]. Encouragingly, these beneficial effects were maintained for 3 weeks following treatment cessation, although body weight was regained [184]. In contrast, in lean mice the tri-agonist does not lower body weight, food intake or induce hypoglycemia [184], suggesting that the tri-agonist corrects metabolic dysregulation without inducing detrimental effects in metabolically healthy animals. The in vivo metabolic effects of the tri-agonist are dependent on agonism at each of the three targeted receptors, as deletion or blockage of any of the three targeted receptors results in blunted tri-agonist action[184].
Another iteration of a GLP-1R/GIPR/GCGR triple agonist has been manufactured by Hanmi Pharmaceuticals [185,186]. This tri-agonist, HM15211 (Table 1), is a glucagon analog with activity at all three target receptors, and is conjugated to the human aglycosylate Fc fragment to extend the circulating half-life [186]. This tri-agonist also induces greater body weight loss compared to liraglutide, and improves lipid metabolism and reduces hepatic steatosis [186].
Similarly, the tri-agonist YAG-glucagon is a modified glucagon peptide that is DPP-IV resistant and has full activity at all three target receptors, as shown by cAMP assays in target receptor transfected cell lines [187]. YAG-glucagon does not affect body weight or food intake in male NIH Swiss mice fed a high fat diet, although YAG-glucagon does improve glucose tolerance in these mice [187].
Other multi-agonists have been created utilizing antibody fragments. Syn-GIP-ZP is a tri-agonist created by fusing a dual GLP-1R/GCGR peptide (ZP) and GIP analog to the heavy and light chains of Synagis, an antibody known to have low immunogenicity in humans [188]. This tri-agonist has in vitro activity at all three target receptors, as measured by cAMP reporter assays in cell lines expressing the target receptors [188].
The prevalence and beneficial effects of all of these triple agonists underscore their potential as metabolic therapies. These multi- and tri-agonists can be more effective than their constituent monotherapies and are often more effective than currently available pharmacotherapies such as liraglutide. While these multi-agonists require additional mechanistic and safety analyses, they are poised to become effective new tools in the fight against diabetes, obesity, and metabolic dysregulation.
4. Peptide and hormone combinations
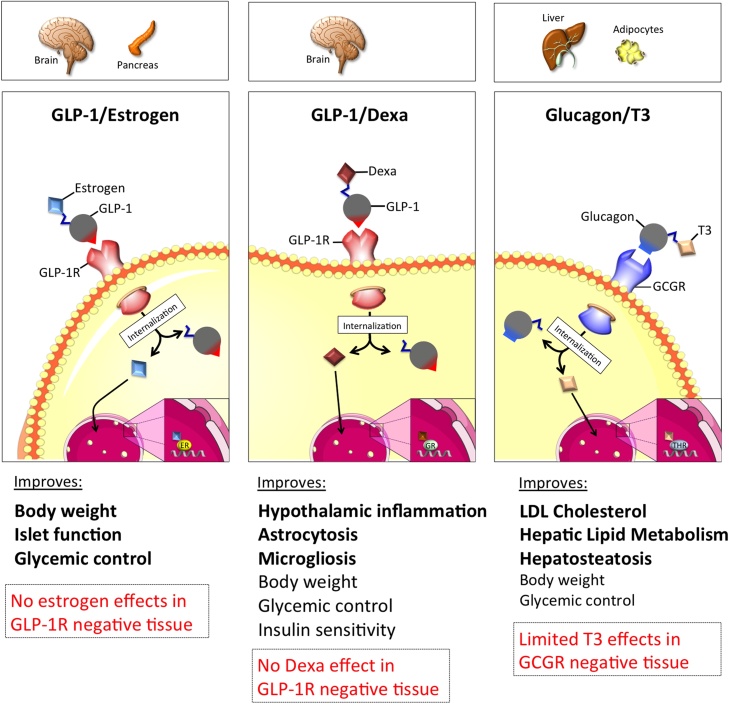
In addition to peptide/peptide multiagonists, peptide/nuclear hormone combinations can offer potential as anti-obesity therapeutics. Nuclear acting hormones, such as estrogen, dexamethasone, and T3 have profound effects on metabolism, but often also induce undesirable off-target effects due to their ubiquitous action profile [189]. The principle of a chimera between a peptide hormone and nuclear hormone is thus that the peptide hormone binds to its cell surface receptor and when internalized via the natural cell cycle pathway takes the nuclear hormone cargo with it, thereby restricting the nuclear hormone to only enter and act on cells that express the receptor for the peptide hormone (Fig. 2).
Fig. 2.
Schematic on the cell-selective delivery of estrogen, dexamethasone or T3 using GLP-1 or glucagon as the peptide hormone shuttle.
4.1. GLP-1 mediated delivery of estrogen
One example of a peptide/nuclear hormone combination is GLP-1 and estrogen. Estrogen has long been a potential anti-obesity and anti-diabetic therapy. Across species, females are protected from obesity and diabetes [[190], [191], [192]]. In vitro, estrogen protects pancreatic islets from proinflammatory cytokines, and protects against H2O2 and STZ-induced apoptosis [190]. In vivo, estrogen also decreases food intake while increasing energy expenditure [193]. Unfortunately, estrogen also has oncogenic and gynecological actions that preclude its use as a metabolic therapy. In order to overcome these liabilities, estrogen was conjugated to GLP-1, so that estrogen action was limited to cells that express the GLP-1 receptor, hypothetically circumventing undesired side effects. The GLP-1/estrogen conjugate consists of GLP-1 with the C terminal exendin-4 (Cex) extension, an aminoisobutyric acid at position 2 for protection from DPP-IV degradation, and a C-terminal lysine which attaches to estrogen via a stable ether bond. This conjugate shows full GLP-1 activity in vitro in cells that express the GLP-1R [194]. In MIN6 cells, the GLP-1/estrogen conjugate stimulates estrogen response elements to a greater extent than estrogen alone, demonstrating enhanced efficacy [194]. In DIO mice, the conjugate decreases fat mass without altering lean mass, which is due in part to a decrease in food intake [194]. The conjugate decreased the respiratory exchange ratio (RER) [194], indicating altered nutrient utilization. The conjugate also reduced plasma cholesterol, triglycerides, and free fatty acids, lowered plasma leptin, and reduced hepatosteatosis and hepatocellular damage [194]. In addition, the conjugate lowered hyperglycemia, improved glycemic control, and improved insulin sensitivity [194,195]. A CNS specific knockout of the GLP-1R completely abrogates the metabolic benefits of the GLP-1/estrogen conjugate [194], demonstrating the necessity of central GLP-1R agonism. In mice without estrogen receptors, the body weight loss is blunted [194]. Importantly, in ovariectomized (OVX) mice, the conjugate did not induce an increase in uterine weight [194,195] or stimulate tumor growth in estrogen-sensitive MCF-7 xenographs [194], suggesting the absence of estrogen effects in tissues lacking the GLP-1 receptor. Rats treated with the conjugate display reduced food reward behavior [196]. SPECT imaging in these rats reveals that the supramammillary nucleus (SUM) is the target site of the GLP-1/estrogen conjugate, a novel food-reward site within the CNS [196]. The GLP-1/estrogen conjugate also targets the LH and NTS, more classical energy balance regulating areas [196]. In summary, the GLP-1/estrogen conjugate shows great promise for targeting estrogen therapy. Due to the oncogenic potential of estrogen agonism, vigorous safety studies are required, however, early preclinical investigations have not revealed major off target effects. This conjugate has potentially translational value and should continue to be investigated as a metabolic therapy.
4.2. GLP-1 mediated delivery of dexamethasone
Recently, a second example of a peptide/hormone combination, GLP-1/dexamethasone (GLP-1/Dexa), was engineered. Following the idea that coupling GLP-1 with the anti-inflammatory agent dexamethasone could selectively neutralize inflammatory-triggered processes in the hypothalamus and other GLP-1R positive tissues, the DiMarchi and Tschöp laboratories engineered a GLP-1/dexamethasone co-agonist [197]. The GLP-1/dexamethasone conjugate consists of GLP-1 with a C-terminal penicillamine (Pen) residue linked to dexamethasone. In DIO mice, a daily treatment with 100nmol/kg of GLP-1/Dexa reduces body weight, food intake and fat mass, improves glucose tolerance, reverses hypothalamic inflammation and ameliorates systemic inflammation within acute and chronic treatment settings. In contrast, in lean mice the co-agonist does not lower body weight, food intake or induce hypoglycemia [197], suggesting that the co-agonist primarily targets pathological processes driven by diet-induced obesity. Encouragingly, this GLP-1/Dexa co-agonist does not induce negative effects on bone integrity and HPA axis activity. Thus, GLP-1-directed glucocorticoid pharmacology represents a safe and efficacious therapy option for metabolic inflammation and obesity.
4.3. Glucagon-mediated delivery of thyroid hormone T3
Another peptide/hormone conjugate aims to address dyslipidemia and cholesterol metabolism by the targeted delivery of T3 using glucagon as the peptide carrier.
T3 increases energy expenditure and fatty acid oxidation, and influences cholesterol metabolism [[198], [199], [200]]. However, T3 off-target effects include cardiac hypertrophy, tachycardia, bone resorption, and muscle wasting [[201], [202], [203], [204], [205]]. In order to avoid these detrimental T3 effects, T3 was conjugated to DPP-IV resistant glucagon via a gamma-glutamic acid spacer, thus limiting the T3 effects to tissues expressing the glucagon receptor, which is mainly the liver and white adipose tissue [206]. This conjugate has similar activity in vitro at the glucagon receptor as native glucagon, and also induces the transcriptional activity of thyroid hormone response elements in HepG2 cells expressing GCGR [206]. In DIO and LDLR−/− mice fed a high cholesterol diet, the conjugate lowers total cholesterol, reduces circulating triglycerides, and lowers hepatic cholesterol and hepatocellular vacuolation [206]. The conjugate further improves glucose tolerance, lessens hyperglycemia, and increases insulin sensitivity [206], indicating that the hyperglycemic liability of glucagon receptor agonism is counter-balanced by T3 activity. All of the beneficial effects are lost in mice globally lacking the glucagon receptor, or in mice lacking the thyroid hormone receptor B isoform in the liver [206]. The weight loss activity of the conjugate is mediated partially through increased browning of white adipose tissue and an increase in whole body expenditure, consistent with T3 action on this tissue [206]. Notably, the conjugate shows an improved action profile relative to T3 treatment on both the cardiovascular system and bone metabolism [206].
5. Conclusions
Over the last years, a series of unimolecular multiagonists have been developed and their metabolic efficacy to improve body weight and glycemia has been demonstrated to translate from diet- and genetically induced obese rodents to non-human primates and humans. Several unimolecular dual and triple agonists are in clinical evaluation and as of today, the improved metabolic efficacy doesn’t seem to compromised by reduced safety, thus emphasizing that these m
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by the Alexander von Humboldt Foundation, the Helmholtz Alliance ICEMED & the Helmholtz Initiative on Personalized Medicine iMed by Helmholtz Association, and the Helmholtz cross-program topic “Metabolic Dysfunction.” This work was further supported by grants from the German Research Foundation DFG-TS226/1-1, DFG-TS226/3-1, the <gs5>European Research Council ERC AdG HypoFlam</gs5> no. 695054 and the <gs6>German Center for Diabetes Research</gs56> (DZD e.V.).
References
- 1.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators G.B.D.R.F., Forouzanfar M.H., Alexander L., Anderson H.R., Bachman V.F., Biryukov S. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 5.2017. The Top 10 Causes of Death Fact Sheet. (Accessed November 28 2017) http://www.who.int/mediacentre/factsheets/fs310/en/index1.html. [Google Scholar]
- 6.Association A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–S48. [PubMed] [Google Scholar]
- 7.Apovian C.M. The clinical and economic consequences of obesity. Obes. Rev. 2013;19:s219–28. [PubMed] [Google Scholar]
- 8.Bray G.A., Kim K.K., Wilding J.P.H. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 9.Trasher J. Pharmacologic management of type 2 diabetes mellitus: available therapies. Am. J. Cardiol. 2017;120:S4–S16. doi: 10.1016/j.amjcard.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Bolen S., Feldman L., Vassy J., Wilson L., Yeh H.C., Marinopoulos S. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes Mellitus. Ann. Intern. Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 11.Peters A. Incretin-based therapies: review of current clinical trial data. Am. J. Med. 2010;123:S28–37. doi: 10.1016/j.amjmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Bennett W.L., Maruthur N.M., Singh S., Segal J.B., Wilson L.M., Chatterjee R. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann. Intern. Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodmer M., Meier C., Krahenbuhl S., Jick S.S., Meier C.R. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–2091. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode B.W., Steed R.D., Davidson P.C. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care. 1996;19:324–327. doi: 10.2337/diacare.19.4.324. [DOI] [PubMed] [Google Scholar]
- 15.D.C.C.T.R. Group, Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 17.Zunz E., Barre J.L. Contributions à l'étude des variations physiologiques de la sécrétion interne du pancréas. Archives Internationales de Physiologie. 1929 [Google Scholar]
- 18.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 19.Kreymann B., Williams G., Ghatei M.A., Bloom S.R. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 20.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Bullock B.P., Heller R.S., Habener J.F. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 22.Wei Y., Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 23.Erdogdu Ö., Eriksson L., Nyström T., Sjöholm A., Zhang Q. Exendin-4 restores glucolipotoxicity-induced gene expression in human coronary artery endothelial cells. Biochem. Biophys. Res. Commun. 2012;419:790–795. doi: 10.1016/j.bbrc.2012.02.106. [DOI] [PubMed] [Google Scholar]
- 24.Schirra J., Nicolaus M., Roggel R., Katschinski M., Storr M., Woerle H.J. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut. 2006;55 doi: 10.1136/gut.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willms B., Werner J., Holst J.J., Orskov C., Creutzfeldt W., Nauck M.A. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J. Clin. Endocrinol. Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 26.Buteau J., Foisy S., Joly E., Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 27.Ip W., Shao W., Chiang Y.T., Jin T. GLP-1-derived nonapeptide GLP-1(28–36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1348–58. doi: 10.1152/ajpendo.00376.2013. [DOI] [PubMed] [Google Scholar]
- 28.Burmeister M.A., Ayala J.E., Smouse H., Landivar-Rocha A., Brown J.D., Drucker D.J. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66:372–384. doi: 10.2337/db16-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sisley S., Gutierrez-Aguilar R., Scott M., D'Alessio D.A., Sandoval D.A., Seeley R.J. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J. Clin. Invest. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sisley S., Smith K., Sandoval D.A., Seeley R.J. Differences in acute anorectic effects of long-acting GLP-1 receptor agonists in rats. Peptides. 2014;58:1–6. doi: 10.1016/j.peptides.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H., Del Tredici K. Alzheimer's disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol. Aging. 2004;25:713–716. doi: 10.1016/j.neurobiolaging.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Aksoy D., Solmaz V., Cavusoglu T., Meral A., Ates U., Erbas O. Neuroprotective effects of eexenatide in a rotenone-Induced rat model of parkinson's disease. Am. J. Med. Sci. 2017;354:319–324. doi: 10.1016/j.amjms.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Li Y., Perry T., Kindy M.S., Harvey B.K., Tweedie D., Holloway H.W. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S., Moon M., Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson's disease. J. Endocrinol. 2009;202:431–439. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- 35.Teramoto S., Miyamoto N., Yatomi K., Tanaka Y., Oishi H., Arai H. Exendin-4 a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2011;31:1696–1705. doi: 10.1038/jcbfm.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Chigurupati S., Holloway H.W., Mughal M., Tweedie D., Bruestle D.A. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgmaier M., Liberman A., Möllmann J., Kahles F., Reith S., Lebherz C. Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9–37) and GLP-1(28–37) stabilize atherosclerotic lesions in apoe⁻/⁻ mice. Atherosclerosis. 2013;231:427–435. doi: 10.1016/j.atherosclerosis.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Ussher J.R., Drucker D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilsbøll T., Christensen M., Junker A.E., Knop F.K., Gluud L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyan-Ashraf M.H., Momen M.A., Ban K., Sadi A.M., Zhou Y.Q., Riazi A.M. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodera R., Shikata K., Kataoka H.U., Takatsuka T., Miyamoto S., Sasaki M. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 42.Muskiet M.H.A., Tonneijck L., Smits M.M., van Baar M.J.B., Kramer M.H.H., Hoorn E.J. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 2017;13:605–628. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 43.Mann J.F.E., Orsted D.D., Brown-Frandsen K., Marso S.P., Poulter N.R., Rasmussen S. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 44.Richter G., Goke R., Goke B., Arnold R. Characterization of receptors for glucagon-like peptide-1(7–36)amide on rat lung membranes. FEBS Lett. 1990;267:78–80. doi: 10.1016/0014-5793(90)80292-q. [DOI] [PubMed] [Google Scholar]
- 45.Richter G., Feddersen O., Wagner U., Barth P., Goke R., Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. 1993;265:L374–81. doi: 10.1152/ajplung.1993.265.4.L374. [DOI] [PubMed] [Google Scholar]
- 46.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 47.Deacon C.F., Johnsen A.H., Holst J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 48.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 49.Plamboeck A., Holst J.J., Carr R.D., Deacon C.F. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 50.Deacon C.F., Nauck M.A., Toft-Nielsen M., Pridal L., Willms B., Holst J.J. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 51.Lorenz M., Evers A., Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg. Med. Chem. Lett. 2013;23:4011–4018. doi: 10.1016/j.bmcl.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Cegla J., Troke R.C., Jones B., Tharakan G., Kenkre J., McCullough K.A. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711–3720. doi: 10.2337/db14-0242. [DOI] [PubMed] [Google Scholar]
- 53.Monami M., Marchionni N., Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur. J. Endocrinol. 2009;160:909–917. doi: 10.1530/EJE-09-0101. [DOI] [PubMed] [Google Scholar]
- 54.Amori R.E., Lau J., Pittas A.G. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 55.Fakhoury W.K., Lereun C., Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology. 2010;86:44–57. doi: 10.1159/000314690. [DOI] [PubMed] [Google Scholar]
- 56.Esposito K., Mosca C., Brancario C., Chiodini P., Ceriello A., Giugliano D. GLP-1 receptor agonists and HBA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2011;27:1519–1528. doi: 10.1185/03007995.2011.590127. [DOI] [PubMed] [Google Scholar]
- 57.Shyangdan D.S., Royle P.L., Clar C., Sharma P., Waugh N.R. Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Endocr. Disord. 2010;10:20. doi: 10.1186/1472-6823-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl. J. Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 59.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nauck M.A., Petrie J.R., Sesti G., Mannucci E., Courreges J.P., Lindegaard M.L. A phase 2, randomized dose-Finding study of the novel once-Weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. doi: 10.2337/dc15-0165. [DOI] [PubMed] [Google Scholar]
- 61.2017. Semaglutide Subcutaneous Once-weekly Treatment to Improve Glycemic Control in Adults with Type 2 Diabetes Mellitus. (Accessed November 28 2017) https://www.fda.gov/downloads/AdvisoryCommittees/%E2%80%A6/Drugs/%E2%80%A6/UCM580461.pdf. [Google Scholar]
- 62.Prasad-Reddy L., Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. doi: 10.7573/dic.212283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Troke R.C., Tan T.M., Bloom S.R. The future role of gut hormones in the treatment of obesity. Ther. Adv. Chronic Dis. 2014;5:4–14. doi: 10.1177/2040622313506730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inagaki N., Seino Y., Takeda J., Yano H., Yamada Y., Bell G.I. Gastric inhibitory polypeptide: structure and chromosomal localization of the human gene. Mol. Endocrinol. 1989;3:1014–1021. doi: 10.1210/mend-3-6-1014. [DOI] [PubMed] [Google Scholar]
- 65.Takeda J., Seino Y., Tanaka K., Fukumoto H., Kayano T., Takahashi H. Sequence of an intestinal cDNA encoding human gastric inhibitory polypeptide precursor. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7005–7008. doi: 10.1073/pnas.84.20.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lardinois C.K., Starich G.H., Mazzaferri E.L. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J. Am. Coll. Nutr. 1988;7:241–247. doi: 10.1080/07315724.1988.10720241. [DOI] [PubMed] [Google Scholar]
- 67.Nyberg J., Jacobsson C., Anderson M.F., Eriksson P.S. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. Res. 2007;85:2019–2099. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- 68.Fujita Y., Wideman R.D., Asadi A., Yang G.K., Baker R., Webber T. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology. 2010;138:1966–1975. doi: 10.1053/j.gastro.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 69.Ugleholdt R., Poulsen M.L., Holst P.J., Irminger J.C., Orskov C., Pedersen J. Prohormone convertase 1/3 is essen- tial for processing of the glucose-dependent insulinotropic poly- peptide precursor. J. Biol. Chem. 2006;281:11050–11057. doi: 10.1074/jbc.M601203200. [DOI] [PubMed] [Google Scholar]
- 70.Gault V.A., Porter D.W., Irwin N., Flatt P.R. Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1–30) and GIP(1- 42) in high-fat fed mice. J. Endocrinol. 2011;208:265–271. doi: 10.1530/JOE-10-0419. [DOI] [PubMed] [Google Scholar]
- 71.Christensen M., Vedtofte L., Holst J.J., Vilsbøll T., Knop F.K. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dupre J., Ross S.A., Watson D., Brown J.C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J. Clin. Endocrinol. Metab. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 73.Vilsboll T., Krarup T., Madsbad S., Holst J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 74.Gault V.A., Flatt P.R., O'Harte F.P. Glucose-dependent insulino- tropic polypeptide analogues and their therapeutic potential for the treatment of obesity-diabetes. Biochem. Biophys. Res. Commun. 2003;308:207–213. doi: 10.1016/s0006-291x(03)01361-5. [DOI] [PubMed] [Google Scholar]
- 75.Usdin T.B., Mezey E., Button D.C., Brownstein M.J., Bonner T.I. Gastric inhibitory polypeptide receptor a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 76.Yip R.G., Boylan M.O., Kieffer T.J., Wolfe M.M. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139:4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 77.Eckel R.H., Fujimoto W.Y., Brunzell J.D. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28:1141–1142. doi: 10.2337/diab.28.12.1141. [DOI] [PubMed] [Google Scholar]
- 78.Gögebakan Ö., Andres J., Biedasek K., Mai K., Kühnen P., Krude H. Glucose-dependent insulinotropic polypeptide reduces fat-specific expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and inhibits release of free fatty acids. Diabetes. 2012;61:292–300. doi: 10.2337/db10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oben J., Morgan L., Fletcher J., Marks V. Effect of the entero-pancreatic hormones gastric inhibitory polypeptide and glucagon-like polypeptide-1(7–36) amide, on fatty acid synthesis in explants of rat adipose tissue. J. Endocrinol. 1991;130:267–272. doi: 10.1677/joe.0.1300267. [DOI] [PubMed] [Google Scholar]
- 80.Gault V.A., McClean P.L., Cassidy R.S., Irwin N., Flatt P.R. Chemical gastric inhibitory polypeptide receptor antagonism protects againstobesity insulin resistance, glucose intolerance and associated distur-bances in mice fed high-fat and cafeteria diets. Diabetologia. 2007;50:1752–1762. doi: 10.1007/s00125-007-0710-4. [DOI] [PubMed] [Google Scholar]
- 81.McClean P.L., Irwin N., Cassidy R.S., Holst J.J., Gault V.A., Flatt P.R. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am. J. Physiol.- Endocrinol. Metab. 2007;293:E1746–1755. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- 82.McClean P.L., Irwin N., Hunter K., Gault V.A., Flatt P.R. (Pro(3))-GIP[mPEG]: novel long-acting, mPEGylated antagonist of gastricinhibitory polypeptide for obesity-diabetes (diabesity) therapy. Br. J. Pharmacol. 2008;155:690–701. doi: 10.1038/bjp.2008.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Althage M.C., Ford E.L., Wang S., Tso P., Polonsky K.S., Wice B.M. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resis-tance induced by a high fat diet. J. Biol. Chem. 2008;283:18365–18376. doi: 10.1074/jbc.M710466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bailey C.J., Flatt P.R., Kwasowski P., Powell C.J., Marks V. Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinol. (Copenh.) 1986;112:224–229. doi: 10.1530/acta.0.1120224. [DOI] [PubMed] [Google Scholar]
- 85.Flatt P.R., Bailey C.J., Kwasowski P., Swanston-Flatt S.K., Marks V. Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes. 1983;32:433–435. doi: 10.2337/diab.32.5.433. [DOI] [PubMed] [Google Scholar]
- 86.Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 87.Calanna S., Christensen M., Holst J.J., Laferrere B., Gluud L.L., Vilsboll T. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36:3346–3352. doi: 10.2337/dc13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Creutzfeldt W., Ebert R., Willms B., Frerichs H., Brown J.C. Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia. 1978;14:15–24. doi: 10.1007/BF00429703. [DOI] [PubMed] [Google Scholar]
- 89.Salera M., Giacomoni P., Pironi L., Cornia G., Capelli M., Marini A. Gastric inhibitory polypeptide release after oral glucose: relationship to glucose intolerance, diabetes mellitus, and obesity. J. Clin. Endocrinol. Metab. 1982;55:329–336. doi: 10.1210/jcem-55-2-329. [DOI] [PubMed] [Google Scholar]
- 90.Kim S.J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7:e40156. doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin C.M., Irwin N., Flatt P.R., Gault V.A. A novel acylated form of (d-Ala(2))GIP with improved antidiabetic potential, lacking effect on body fat stores. Biochim. Biophys. Acta. 2013;1830:3407–3413. doi: 10.1016/j.bbagen.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Renner S., Fehlings C., Herbach N., Hofmann A., von Waldthausen D.C., Kessler B. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bates H.E., Campbell J.E., Ussher J.R., Baggio L.L., Maida A., Seino Y. Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr(-/-) mice. Diabetes. 2012;61:40–48. doi: 10.2337/db11-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Speliotes E.K. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;41:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura T., Tanimoto H., Mizuno Y., Tsubamoto Y., Noda H. Biological and functional character- istics of a novel low-molecular weight antagonist of glucose- dependent insulinotropic polypeptide receptor SKL-14959, in vitro and in vivo. Diabetes Obes. Metab. 2012;14:511–517. doi: 10.1111/j.1463-1326.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- 96.Ravn P., Madhurantakam C., Kunze S., Matthews E., Priest C., O'Brien S. Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. J. Biol. Chem. 2013;288:19760–19772. doi: 10.1074/jbc.M112.426288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gault V.A., O'Harte F.P., Harriott P., Flatt P.R. Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochem. Biophys. Res. Commun. 2002;290:1420–1426. doi: 10.1006/bbrc.2002.6364. [DOI] [PubMed] [Google Scholar]
- 98.Irwin N., Gault V.A., Green B.D., Greer B., McCluskey J.T., Harriott P. Effects of short-term chemical ablation of the GIP receptor on insulin secretion, islet morphology and glucose homeostasis in mice. Biol. Chem. 2004;385:845–852. doi: 10.1515/BC.2004.110. [DOI] [PubMed] [Google Scholar]
- 99.Gault V.A., Irwin N., Green B.D., McCluskey J.T., Greer B., Bailey C.J. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes. 2005;54:2436–2446. doi: 10.2337/diabetes.54.8.2436. [DOI] [PubMed] [Google Scholar]
- 100.Irwin N., McClean P.L., O'Harte F.P., Gault V.A., Harriott P., Flatt P.R. Early administration of the glucose-depen- dent insulinotropic polypeptide receptor antagonist (Pro3)GIP prevents the development of diabetes and related metabolic abnormalities associated with genetically inherited obesity in ob/ob mice. Diabetologia. 2007;50:1532–1540. doi: 10.1007/s00125-007-0692-2. [DOI] [PubMed] [Google Scholar]
- 101.Tseng C.C., Kieffer T.J., Jarboe L.A., Usdin T.B., Wolfe M.M. Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide (GIP). Effect of a specific glucose-dependent insulinotropic polypeptide receptor antagonist in the rat. J. Clin. Invest. 1996;98:2440–2445. doi: 10.1172/JCI119060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gault V.A., Parker J.C., Harriott P., Flatt P.R., O'Harte F.P. Evidence that the major degradation product of glucose-dependent insulinotropic polypeptide, GIP (3–42), is a GIP receptor antagonist in vivo. J. Endocrinol. 2002;175:525–533. doi: 10.1677/joe.0.1750525. [DOI] [PubMed] [Google Scholar]
- 103.Gelling R.W., Coy D.H., Pederson R.A., Wheeler M.B., Hinke S., Kwan T. GIP(6–30amide) contains the high affinity binding region of GIP and is a potent inhibitor of GIP 1–42 action in vitro. Regul. Pept. 1997;69:151–154. doi: 10.1016/s0167-0115(97)00009-8. [DOI] [PubMed] [Google Scholar]
- 104.Al-Sabah S., Al-Fulaij M., Ahmed H.A. Selectivity of peptide ligands for the human incretin receptors expressed in HEK-293 cells. Eur. J. Pharmacol. 2014;741:311–315. doi: 10.1016/j.ejphar.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Faivre E., Hamilton A., Hölscher C. Effects of acute and chronic administration of GIP analogues on cognition, synaptic plasticity and neurogenesis in mice. Eur. J. Pharmacol. 2012;674:294–306. doi: 10.1016/j.ejphar.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Sparre-Ulrich A.H., Hansen L.S., Svendsen B., Christensen M., Knop F.K., Hartmann B. Species-specific action of (Pro3) GIP −an efficacious agonist on human GIP receptor, but partial agonist and competitive antagonist on rat and mouse GIP receptors. Br. J. Pharmacol. 2016;173:27–38. doi: 10.1111/bph.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhong Q., Itokawa T., Sridhar S., Ding K.H., Xie D., Kang B. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. Endocrinol. Metab. 2007;292:E543–8. doi: 10.1152/ajpendo.00364.2006. [DOI] [PubMed] [Google Scholar]
- 108.Bollag R.J., Zhong Q., Ding K.H., Phillips P., Zhong L., Qin F. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol. Cell. Endocrinol. 2001;177:35–41. doi: 10.1016/s0303-7207(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 109.Bollag R.J., Zhong Q., Phillips P., Min L., Zhong L., Cameron R. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 110.Mabilleau G., Perrot R., Mieczkowska A., Boni S., Flatt P.R., Irwin N. Glucose-dependent insulinotropic polypeptide (GIP) dose-dependently reduces osteoclast differentiation and resorption. Bone. 2016;91:102–112. doi: 10.1016/j.bone.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 111.Mieczkowska A., Irwin N., Flatt P.R., Chappard D., Mabilleau G. Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone. 2013;56:337–342. doi: 10.1016/j.bone.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 112.Gaudin-Audrain C., Irwin N., Mansur S., Flatt P.R., Thorens B., Basle M. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to modifications of trabecular bone volume and quality in mice. Bone. 2013;53:221–230. doi: 10.1016/j.bone.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 113.Nyberg J., Anderson M.F., Meister B., Alborn A.M., Ström A.K., Brederlau A. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 2005;25:1816–1825. doi: 10.1523/JNEUROSCI.4920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Figueiredo C.P., Antunes V.L., Moreira E.L., de Mello N., Medeiros R., Di Giunta G. Glucose-dependent insulinotropic peptide receptor expression in the hippocampus and neocortex of mesial temporal lobe epilepsy patients and rats undergoing pilocarpine induced status epilepticus. Peptides. 2011;32:781–789. doi: 10.1016/j.peptides.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 115.Gault V.A., Holscher C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by Beta-amyloid. J. Neurophysiol. 2008;99:1590–1595. doi: 10.1152/jn.01161.2007. [DOI] [PubMed] [Google Scholar]
- 116.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 117.Mentlein R. Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23:443–452. doi: 10.1016/j.beem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Gault V.A., Flatt P.R., Harriott P., Mooney M.H., Bailey C.J., O'Harte F.P. Improved biological activity of Gly2- and Ser2-substituted analogues of glucose-dependent insulinotro- phic polypeptide. J. Endocrinol. 2003;176:133–141. doi: 10.1677/joe.0.1760133. [DOI] [PubMed] [Google Scholar]
- 119.Irwin N., Green B.D., Gault V.A., Greer B., Harriott P., Bailey C.J. Degradation, insulin secretion, and anti- hyperglycemic actions of two palmitate-derivitized N-terminal pyroglutamyl analogues of glucose-dependent insulinotropic polypeptide. J. Med. Chem. 2005;48:1244–1250. doi: 10.1021/jm049262s. [DOI] [PubMed] [Google Scholar]
- 120.Irwin N., Green B.D., Mooney M.H., Greer B., Harriott P., Bailey C.J. A novel, long-acting agonist of glucose- dependent insulinotropic polypeptide suitable for once-daily administration in type 2 diabetes. J. Pharmacol. Exp. Ther. 2005;314:1187–1194. doi: 10.1124/jpet.105.086082. [DOI] [PubMed] [Google Scholar]
- 121.Irwin N., McClean P.L., Flatt P.R. Comparison of the subchronic antidiabetic effects of DPP IV-resistant GIP and GLP-1 analogues in obese diabetic (ob/ob) mice. J. Pept. Sci. 2007;13:400–405. doi: 10.1002/psc.861. [DOI] [PubMed] [Google Scholar]
- 122.Kerr B.D., Irwin N., O'Harte F.P.M., Bailey C.J., Flatt P.R., Gault V.A. Fatty acid derivatised analogues of glucose- dependent insulinotropic polypeptide with improved antihypergly- caemic and insulinotropic propertie. Biochem. Pharmacol. 2009;78:1008–1016. doi: 10.1016/j.bcp.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 123.O'Harte F.P., Mooney M.H., Flatt P.R. NH2-terminally modified gastric inhibi- tory polypeptide exhibits amino-peptidase resistance and enhanced antihyperglycemic activity. Diabetes. 1999;48:758–765. doi: 10.2337/diabetes.48.4.758. [DOI] [PubMed] [Google Scholar]
- 124.Gault V.A., Flatt P.R., Bailey C.J., Harriott P., Greer B., Mooney M.H. Enhanced cAMP generation and insulin- releasing potency of two novel Tyr1-modified enzyme-resistant forms of glucose-dependent insulinotropic polypeptide is asso- ciated with significant antihyperglycaemic activity in spontaneous obesity-diabetes. Biochem. J. 2002;367 doi: 10.1042/BJ20020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O'Harte F.P., Gault V.A., Parker J.C., Harriott P., Mooney M.H., Bailey C.J. Improved stability, insulin-releasing activity and antidiabetic potential of two novel N-terminal ana- logues of gastric inhibitory polypeptide: N-acetyl-GIP and pGlu- GIP. Diabetologia. 2002;45:1281–1291. doi: 10.1007/s00125-002-0894-6. [DOI] [PubMed] [Google Scholar]
- 126.Pederson R.A., Satkunarajah M., McIntosh C.H., Scrocchi L.A., Flamez D., Schuit F. Enhanced glucose-dependent insu- linotropic polypeptide secretion and insulinotropic action in Glu- cagon-like peptide 1 receptor–/–mice. Diabetes. 1998;47:1046–1052. doi: 10.2337/diabetes.47.7.1046. [DOI] [PubMed] [Google Scholar]
- 127.Gault V.A., O'Harte F.P., Harriott P., Flatt P.R. Degradation, cyclic adenosine mono- phosphate production, insulin secretion, and glycemic effects of two novel N-terminal Ala2-substituted analogs of glucose-depen- dent insulinotropic polypeptide with preserved biological activity in vivo. Metabolism. 2003;52:679–687. doi: 10.1016/s0026-0495(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 128.Rouille Y., Bianchi M., Irminger J., Halban P. Role of the prohormone convertase PC2 in the processing of proglucagon to glucagon. FEBS Lett. 1997;413:119–123. doi: 10.1016/s0014-5793(97)00892-2. [DOI] [PubMed] [Google Scholar]
- 129.Rouille Y., Westermark G., Martin S., Steiner D. Proglucagon is processed to glucagon by prohormone convertase PC2 in alphaTC1–6 cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3242–3246. doi: 10.1073/pnas.91.8.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Furuta M., Zhou A., Webb G., Carroll R., Ravazzola M., Orci L. Severe defect in proglucagon processing in islet A-cells of prohormone convertase2 null mice. J. Biol. Chem. 2001;276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 131.Muller T.D., Finan B., Clemmensen C., DiMarchi R.D., Tschop M.H. The new biology and pharmacology of glucagon. Physiol. Rev. 2017;97:721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- 132.Jiang G., Zhang B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003;284:E671–8. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 133.Jelinek L.J., Lok S., Rosenberg G.B., Smith R.A., Grant F.J., Biggs S. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993;259:1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- 134.Burcelin R., Katz E.B., Charron M.J. Molecular and cellular aspects of the glucagon receptor: role in diabetes and metab- olism. Diabetes Metab. 1996;22:373–396. [PubMed] [Google Scholar]
- 135.Christophe J. Glucagon and its receptor in various tissues. Ann. N. Y. Acad. Sci. 1996;805:31–43. doi: 10.1111/j.1749-6632.1996.tb17471.x. [DOI] [PubMed] [Google Scholar]
- 136.Esquibel A.J., Kurland A.A., Mendelsohn D. The use of glucagon in terminating insulin coma. Dis. Nerv. Syst. 1958;19:485–486. [PubMed] [Google Scholar]
- 137.Gratzer W.B., Creeth J.M., Beaven G.H. Presence ot trimers in glucagon solution. E. Eur. J. Biochem. 1972;31:505–509. doi: 10.1111/j.1432-1033.1972.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 138.Chabenne J.R., DiMarchi M.A., Gelfanov V.M., DiMarchi R.D. Optimization of the native glucagon sequence for medicinal purposes. J. Diabetes Sci. Technol. 2010;4:1322–1331. doi: 10.1177/193229681000400605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Honda K., Kamisoyama H., Uemura T., Yanagi T., Saito N., Kurose Y. The mechanism underlying the central glucagon-induced hyperglycemia and anorexia in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012;163:260–264. doi: 10.1016/j.cbpa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Amatuzio D.S., Grande F., Wada S. Effect of glucagon on the serum lipids in essential hyperlipemia and in hypercholesterolemia. Metabolism. 1962;11:1240–1249. [PubMed] [Google Scholar]
- 141.Billington C.J., Briggs J.E., Link J.G., Levine A.S. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am. J. Physiol. 1991;261:R501–507. doi: 10.1152/ajpregu.1991.261.2.R501. [DOI] [PubMed] [Google Scholar]
- 142.Kuroshima A., Yahata T. Thermogenic responses of brown adipocytes to noradrenaline and glucagon in heat-acclimated and cold-acclimated rats. Jpn. J. Physiol. 1979;29:683–690. doi: 10.2170/jjphysiol.29.683. [DOI] [PubMed] [Google Scholar]
- 143.Glick G., Parmley W.W., Wechsler A.S., Sonnenblick E.H. Glucagon Its enhancement of cardiac performance in the cat and dog and persistence of its inotropic action despite beta-receptor blockade with propranolol. Circ. Res. 1968;22:789–799. doi: 10.1161/01.res.22.6.789. [DOI] [PubMed] [Google Scholar]
- 144.Mochiki E., Suzuki H., Takenoshita S., Nagamachi Y., Kuwano H., Mizumoto A. Mechanism of inhibitory effect of glucagon on gastrointestinal motility and cause of side effects of glucagon. J. Gastroenterol. 1998;33:835–841. doi: 10.1007/s005350050184. [DOI] [PubMed] [Google Scholar]
- 145.Salter J.M., Ezrin C., Laidlaw J.C., Gornall A.G. Metabolic effects of glucagon in human subjects. Metabolism. 1960;9:753–768. [PubMed] [Google Scholar]
- 146.Cohen P.A., Goday A., Swann J.P. The return of rainbow diet pills. Am. J. Public Health. 2012;7:288–298. doi: 10.2105/AJPH.2012.300655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weintraub M., Hasday J.D., Mushlin A.I., Lockwood D.H. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch. Intern. Med. 1984;144 [PubMed] [Google Scholar]
- 148.Weintraub M., Sundaresan P.R., Madan M., Schuster B., Balder A., Lasagna L. Long-term weight control study. I (weeks 0–34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clin. Pharmacol. Ther. 1992;51:586–594. doi: 10.1038/clpt.1992.69. [DOI] [PubMed] [Google Scholar]
- 149.Weintraub M., Sundaresan P.R., Schuster B., Averbuch M., Stein E.C., Byrne L. Long-term weight control study. V (weeks 190–210). Follow-up of participants after cessation of medication. Clin. Pharmacol. Ther. 1992;51:615–618. doi: 10.1038/clpt.1992.73. [DOI] [PubMed] [Google Scholar]
- 150.Weintraub M., Sundaresan P.R., Schuster B., Averbuch M., Stein E.C., Cox C. Long-term weight control study. IV (weeks 156–190). The second double-blind phase. Clin. Pharmacol. Ther. 1992;51:608–614. doi: 10.1038/clpt.1992.72. [DOI] [PubMed] [Google Scholar]
- 151.Weintraub M., Sundaresan P.R., Schuster B., Moscucci M., Stein E.C. Long-term weight control study. III (weeks 104–156). An open-label study of dose adjustment of fenfluramine and phentermine. Clin. Pharmacol. Ther. 1992;51:602–607. doi: 10.1038/clpt.1992.71. [DOI] [PubMed] [Google Scholar]
- 152.Brauer L.H., Johanson C.E., Schuster C.R., Rothman R.B., de Wit H. Evaluation of phentermine and fenfluramine alone and in combination, in normal healthy volunteers. Neuropsychopharmacology. 1996;14:233–241. doi: 10.1016/0893-133X(95)00113-R. [DOI] [PubMed] [Google Scholar]
- 153.Li Z., Hong K., Yip I., Huerta S., Bowerman S., Walker J. Body weight loss with phentermine alone versus phentermine and fenfluramine with very-low-calorie diet in an outpatient obesity management program: a retrospective study. Curr. Ther. Res. Clin. Exp. 2003;64:447–460. doi: 10.1016/S0011-393X(03)00126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aronne L.J., Wadden T.A., Peterson C., Winslow D., Odeh S., Gadde K.M. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171. doi: 10.1002/oby.20584. [DOI] [PubMed] [Google Scholar]
- 155.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garvey W.T., Ryan D.H., Bohannon N.J., Kushner R.F., Rueger M., Dvorak R.V. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care. 2014;37:3309–3316. doi: 10.2337/dc14-0930. [DOI] [PubMed] [Google Scholar]
- 157.Garvey W.T., Ryan D.H., Henry R., Bohannon N.J., Toplak H., Schwiers M. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37:912–921. doi: 10.2337/dc13-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Winslow D.H., Bowden C.H., DiDonato K.P., McCullough P.A. A randomized double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35:1529–1539. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Day J.W., Ottaway N., Patterson J.T., Gelfanov V., Smiley D., Gidda J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009;5:749–759. doi: 10.1038/nchembio.209. [DOI] [PubMed] [Google Scholar]
- 160.Day J.W., Gelfanov V., Smiley D., Carrington P.E., Eiermann G., Chicchi G. Optimization of Co-Agonism at GLP-1 and glucagon receptors to safely maximize weight reduction in DIO- rodents. Pept. Sci. 2012;98:443–450. doi: 10.1002/bip.22072. [DOI] [PubMed] [Google Scholar]
- 161.Clemmensen C., Chabenne J., Finan B., Sullivan L., Fischer K., Kuchler D. GLP-1/glucagon coagonism restores leptin responsiveness in obese mice chronically maintained on an obesogenic diet. Diabetes. 2014;63:1422–1427. doi: 10.2337/db13-1609. [DOI] [PubMed] [Google Scholar]
- 162.Müller T.D., Sullivan L.M., Habegger K., Yi C.X., Kabra D., Grant E. Restoration of leptin re- sponsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J. Pept. Sci. 2012;18:383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- 163.Vasselli J.R., Scarpace P.J., Harris R.B., Banks W.A. Dietary components in the development of leptin resistance. Adv. Nutr. 2013;4:164–175. doi: 10.3945/an.112.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baldissera F.G., Holst J.J., Knuhtsen S., Hilsted L., Nielsen O.V. Oxyntomodulin (glicentin-(33–69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul. Pept. 1988;21:151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- 165.Dakin C.L., Gunn I., Small C.J., Edwards C.M., Hay D.L., Smith D.M. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 166.Dakin C.L., Small C.J., Batterham R.L., Neary N.M., Cohen M.A., Patterson M. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 167.Dakin C.L., Small C.J., Park A.J., Seth A., Ghatei M.A., Bloom S.R. Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats. Am. J. Physiol.- Endocrinol. Metab. 2002;283:E1173–1177. doi: 10.1152/ajpendo.00233.2002. [DOI] [PubMed] [Google Scholar]
- 168.Wynne K., Park A.J., Small C.J., Meeran K., Ghatei M.A., Frost G.S. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int. J. Obes. 2006;30:1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 169.Wynne K., Park A.J., Small C.J., Patterson M., Ellis S.M., Murphy K.G. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind randomized, controlled trial. Diabetes. 2005;54:2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 170.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Henderson S.J., Konkar A., Hornigold D.C., Trevaskis J.L., Jackson R., Fritsch Fredin M. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016;18:1176–1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Evers A., Haack T., Lorenz M., Bossart M., Elvert R., Henkel B. Design of novel exendin-Based dual glucagon-like peptide 1 (GLP- 1)/Glucagon receptor agonists. J. Med. Chem. 2017;60:4293–4303. doi: 10.1021/acs.jmedchem.7b00174. [DOI] [PubMed] [Google Scholar]
- 173.Zhou J., Cai X., Huang X., Dai Y., Sun L., Zhang B. A novel glucagon-like peptide-1/glucagon receptor dual agonist exhibits weight-lowering and diabetes-protective effects. Eur. J. Med. Chem. 2017;138:1158–1169. doi: 10.1016/j.ejmech.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 174.Tan T.M., Field B.C., McCullough K.A., Troke R.C., Chambers E.S., Salem V. Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62:1131–1138. doi: 10.2337/db12-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Finan B., Ma T., Ottaway N., Müller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007218. (209ra151) [DOI] [PubMed] [Google Scholar]
- 176.Portron A., Jadidi S., Sarkar N., DiMarchi R., Schmitt C. Pharmacodynamics pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects. Diabetes Obes. Metab. 2017;19:1446–1453. doi: 10.1111/dom.13025. [DOI] [PubMed] [Google Scholar]
- 177.Schmitt C P.A., Jadidi S., Sarkar N., DiMarchi R. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes Obes. Metab. 2017;19:1436–1445. doi: 10.1111/dom.13024. [DOI] [PubMed] [Google Scholar]
- 178.Frias J.P., Bastyr E.J., III, Vignati L., Tschöp M.H., Schmitt C., Owen K. The sustained effects of a dual GIP/GLP-1 receptor agonist NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26:343–352. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 179.Tamargo I.A., Bader M., Li Y., Yu S.-J., Wang Y., Talbot K. Novel GLP-1R/GIPR co-agonist twincretin is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp. Neurol. 2017;288:176–186. doi: 10.1016/j.expneurol.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yuan Z., Li D., Feng P., Xue G., Ji C., Li G. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson's disease. Eur. J. Pharmacol. 2017;812:82–90. doi: 10.1016/j.ejphar.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 181.Jalewa J., Sharma M.K., Gengler S., Hölscher C. A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson's disease. Neuropharmacology. 2017;117:238–248. doi: 10.1016/j.neuropharm.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 182.Shi L., Zhang Z., Li L., Hölscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signaling in the Alzheimer icv. STZ rat model. Behav. Brain Res. 2017;327:65–74. doi: 10.1016/j.bbr.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 183.Tschöp M.H., Finan B., Clemmensen C., Gelfanov V., Perez-Tilve D., Müller T.D. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metab. 2016;24:51–62. doi: 10.1016/j.cmet.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 184.Finan B., Yang B., Ottaway N., Smiley D.L., Ma T., Clemmensen C. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015;21:27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- 185.Kim J.A., Lee S.H., Choi I.Y., Kim Y.H., Lee Y.M., Kwon S.C. Neuroprotective effects of HM15211, a novel long-acting GLP-1/glucagon/GIP triple agonist in the MPTP Parkinson's disease mouse model. American Diabetes Association's 77th Scientific Sessions; San Diego, CA USA; 2017. [Google Scholar]
- 186.Choi I.Y., Lee J.S., Kim J.K., Park Y.J., Jung S.Y., Kim Y.H. Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP triple-agonist (HM15211). American Diabetes Association's 77th Scientific Session; San Diego, CA USA; 2017. [Google Scholar]
- 187.Bhat V.K., Kerr B.D., Vasu S., Flatt P.R., Gault V.A. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56:1417–1424. doi: 10.1007/s00125-013-2892-2. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y., Du J., Zou H., Liu Y., Zhang Y., Gonzalez J. Multifunctional antibody agonists targeting glucagon-like peptide-1, glucagon, and glucose-dependent insulinotropic polypeptide receptors. Angew. Chem. Int. Ed. Engl. 2016;55:12475–12478. doi: 10.1002/anie.201606321. [DOI] [PubMed] [Google Scholar]
- 189.Schacke H., Docke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 190.Le May C., Chu K., Hu M., Ortega C.S., Simpson E.R., Korach K.S. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 192.Vital P., Larrieta E., Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J. Endocrinol. 2006;190:425–432. doi: 10.1677/joe.1.06596. [DOI] [PubMed] [Google Scholar]
- 193.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Finan B., Yang B., Ottaway N., Stemmer K., Muller T.D., Yi C.X. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tiano J.P., Tate C.R., Yang B.S., DiMarchi R., Mauvais-Jarvis F. Effect of targeted estrogen delivery using glucagon-like peptide-1 on insulin secretion, insulin sensitivity and glucose homeostasis. Sci. Rep. 2015;5:10211. doi: 10.1038/srep10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vogel H., Wolf S., Rabasa C., Rodriguez-Pacheco F., Babaei C.S., Stober F. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016;110:396–406. doi: 10.1016/j.neuropharm.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 197.Quarta C., Clemmensen C., Zhu Z., Yang B., Joseph S.S., Lutter D. Molecular integration of incretin and glucocorticoid action reverses immunometabolic dysfunction and obesity. Cell Metab. 2017;26:620–632. doi: 10.1016/j.cmet.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 198.Sayre N.L., Lechleiter J.D. Fatty acid metabolism and thyroid hormones. Curr. Trends Endocinol. 2012;6:65–76. [PMC free article] [PubMed] [Google Scholar]
- 199.Pucci E., Chiovato L., Pinchera A. Thyroid and lipid metabolism. Int. J. Obes. Relat. Metab. Disord. 2000;24(Suppl. 2):S109–12. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 200.Mullur R., Liu Y.Y., Brent G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ochs N., Auer R., Bauer D.C., Nanchen D., Gussekloo J., Cornuz J. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann. Intern. Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 202.Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail. Rev. 2010;15:125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mundy G.R., Shapiro J.L., Bandelin J.G., Canalis E.M., Raisz L.G. Direct stimulation of bone resorption by thyroid hormones. J. Clin. Invest. 1976;58:529–534. doi: 10.1172/JCI108497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Williams G.R. Thyroid hormone actions in cartilage and bone. Eur. Thyroid. J. 2013;2:3–13. doi: 10.1159/000345548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Salvatore D., Simonides W.S., Dentice M., Zavacki A.M., Larsen P.R. Thyroid hormones and skeletal muscle–new insights and potential implications. Nat. Rev. Endocrinol. 2014;10:206–214. doi: 10.1038/nrendo.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Finan B., Clemmensen C., Zhu Z., Stemmer K., Gauthier K., Muller L. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell. 2016;167:843–857. doi: 10.1016/j.cell.2016.09.014. (e14) [DOI] [PubMed] [Google Scholar]