Abstract
Major progress is being made in vaccines against Respiratory Syncytial Virus (RSV), with multiple vaccine candidates currently in the clinical phase of development. Making an investment case for public sector financing of RSV vaccine will require estimation of burden, cost-effectiveness and impact. The aim of this study is to determine the proportion, age distribution and clinical spectrum of RSV associated hospitalizations in children in Karachi, Pakistan. A three years prospective study was conducted at the Aga Khan University hospital in Karachi, a city of 20 million in south Pakistan, from August 2009 to June 2012. Children less than five years old admitted with acute respiratory infections (ARI) were enrolled. Throat swabs were collected and tested for RSV using real-time PCR. Multivariable log binomial regression analysis was performed to identify the associated factors of RSV infection. Out of 1150 children enrolled, RSV was detected among 223 (19%). Highest rate of RSV detection was in young infants less than 3 months of age (48/168, 29%), which accounted for 22% of all RSV detected. Most common diagnosis in RSV positive infants (<12 months of age) was bronchiolitis followed by pneumonia, while in older children between the ages of one and five years of age, pneumonia and asthma were the most common diagnosis. Although identified year-round, RSV was most prevalent from August to October with peak in September, coinciding with the rainy season. This study identified RSV to be independently associated with younger age (p=0.036), rainy season (p<0.001), post-tussive emesis (p=0.008), intubation (p=0.003) and discharge diagnosis of bronchiolitis (p=0.004). Vaccines against RSV that target this age group are likely to yield remarkable benefit.
Keywords: Respiratory syncytial virus, RSV, pneumonia, ARI, bronchiolitis, asthma, Karachi, Log binomial regression, Pakistan
INTRODUCTION
RSV is the most important viral cause of severe ARI in children worldwide [Nair et al., 2010]. ARI account for approximately two million deaths per year in children globally, 70% of which are concentrated in Africa and South Asia [Bryce et al., 2005; Williams et al., 2002]. RSV associated deaths are estimated to be 66,000-199,000 in children under 5 years of age and 15-40% of pediatric hospitalizations with ARI in developing countries are attributable to RSV. This makes RSV one of the leading targets of public health interventions [Breiman et al., 2013; Hall et al., 2009; Nair et al., 2010].
The field of RSV vaccines is advancing at an unprecedented pace. While more than 50 RSV vaccine candidates are currently in development, around ten candidates are either entering, or are already in, clinical trials [Modjarrad et al., 2015]. Live attenuated approaches have been in development for decades and until recently were the only candidates in clinical testing. More recently there have been significant advances in RSV vaccine development using other technologies. While most of these are still at the preclinical stage, four of these newer candidates have now entered clinical development – Novavax (Ph2), GSK (Ph1), and MedImmune (Ph1) testing RSV F protein-based candidates and GSK (Ph1, 2013 acquisition of Okairos) testing an Adenovirus/MVA prime/boost candidate expressing RSV F, and a N and M2-1 fusion protein [PD-VAC, 2014]. The preliminary phase 2 results of RSV F nanoparticle vaccine made by Novavax show that immunizing mothers with this vaccine was safe for fetuses and could protect infants against RSV [NOVAVAX, 2015].
In order to make a case for vaccine introduction in resource-limited settings and guide the optimal vaccine delivery strategy, it is important to understand the morbidity and mortality risks of RSV associated hospitalizations in different age groups, particularly in settings with the highest burden of disease. With this goal in mind, we conducted a three year prospective surveillance study to determine the frequency, clinical features and severity of RSV in children of different age groups hospitalized with ARI in a tertiary care hospital of Karachi, Pakistan.
METHODS
The study was conducted from August 2009 to July 2012 at the Aga Khan University Hospital in Karachi, Pakistan. Pakistan, with the population of approximately 185 million [WorldBank, 2015], is one of the major countries in South Asia, which together with Africa account for 70% of the ARI associated mortality in children worldwide [Bryce et al., 2005]. Karachi is the major cosmopolitan city of Pakistan, located on the coast of Arabian Sea with a population of 20 million. Karachi has relatively mild climate with two main seasons of summer and winter, and receives the monsoon rains between July and September. Aga Khan University Hospital is a tertiary care, not-for profit, private sector hospital. It has general pediatric ward with 84 beds and a state of the art pediatric intensive care unit (ICU). It caters mainly to the population of Karachi but also receives referrals from the rest of the Sindh province. About three-quarters of the patients belong to lower and middle socioeconomic group. Financial assistance is available to those who cannot afford the services, and about 25% of the inpatients receive such assistance.
The inclusion criteria for enrollment in this study included age less than 5 years and admission to the hospital within 48 hours prior to enrollment with one or more of the following admission diagnosis as listed by the treating medical team: ARI, apnea, asthma exacerbation, bronchiolitis, croup, cystic fibrosis exacerbation, febrile neonate, febrile seizure, respiratory distress, otitis media, pharyngitis, pneumonia, sinusitis, and upper respiratory infection (URI). The admission diagnoses given by the primary team were taken as such, and were not verified or reclassified by the study physicians. Newborns who never left the hospital were excluded. The study was approved by the Ethical Review Committee of the Aga Khan University. After getting a written informed consent from parents/guardian, demographic and clinical information about the current illness was recorded. A throat swab was obtained using commercial flocked swabs (Diagnostics Hybrid Inc.) and was transported immediately to the research laboratory in the Universal Transport Media (Diagnostics Hybrid Inc.) for testing of RSV, HMPV and Influenza using monoplex real-time RT-PCR assay. RNA was extracted using QiaAmp Viral RNA mini Kit (Qiagen) according to the manufacturer's instructions. The primers and probes for RSV were according to the CDC protocol of real-time RT-PCR for RSV [Mentel et al., 2003]. After discharge from the hospital, the medical records of all enrolled participants were reviewed and data about the inpatient workup, management and clinical course were recorded.
STATISTICAL ANALYSIS
Descriptive analysis was performed to describe age of children and proportion of respiratory virus etiology. Associations between socio-demographic characteristics, seasonality, clinical manifestations, and RSV infection were assessed using univariable and multivariable log binomial regression analysis. All the variables found significant at p <0.30 at the univariate level were tested simultaneously in the multivariable regression model. The final model with variables significant at 5% level of significance was selected using the best subset method. Crude and adjusted risk ratios with 95% confidence intervals were calculated. Statistical analysis was performed using Stata software (Version 11).
RESULTS
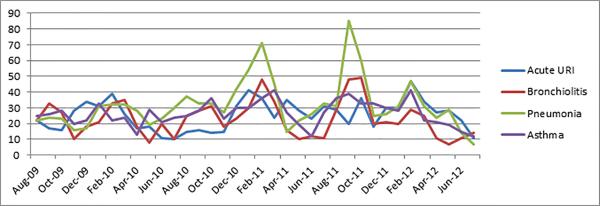
A total of 3830 children less than 5 years old were admitted at AKUH with ARI and associated diagnosis during the study period. Out of these, 30% had pneumonia, 25% had asthma, 24% had a URI and 22% had bronchiolitis. Acute URI admissions peaked in February and were relatively lower in the months of June and July (Figure 1). This was more apparent in the years 2010 and 2012. Cases of asthma did not show any specific trend. Patients with bronchiolitis did not have a particular peak, but fewer cases were observed from April to July each year. Pneumonia, on the other hand, showed a pattern of two peaks a year- in February and in the months of August to September.
Figure 1.
Monthly number of cases for each diagnosis among the total admissions at Aga Khan University Karachi
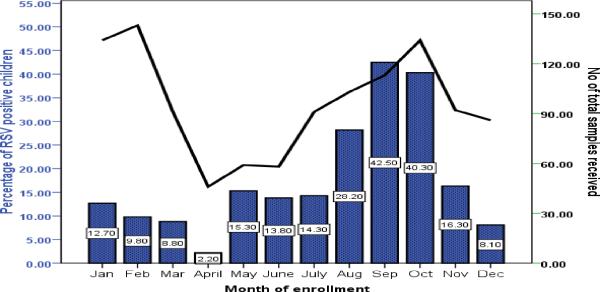
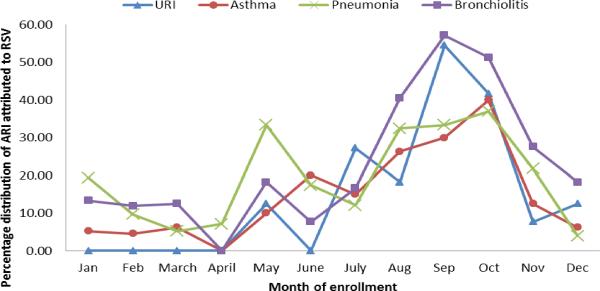
A total of 1150 (33%) out of 3830 children were enrolled in the study. The proportion of RSV cases in various months varied from 2% to 43%. The cases started increasing in the month of July (14%) reaching the peak (43%) in September and then slowly declining reaching to 8% in the month of December. The rate of RSV positivity is lowest during Jan –Feb and highest during September-October (Figure 2). Figure 3 shows the percentage distribution of RSV associated ARI cases with various discharge diagnoses across different months of enrollments. Cases of Pneumonia showed higher RSV positivity between the months of April and June While on the other hand cases of bronchiolitis and upper respiratory infections during the month of August to October showed the similar trend of higher cases attributed to RSV.
Figure 2.
Percentage of RSV infection among children less than 5 years of age across months of enrollment during Aug 2009 to July 2012 (Bar chart)
Figure 3.
Percentage distribution of ARI attributed to RSV infection among children less than 5 years of age across the months of enrollment during Aug 2009 to July 2012. (Bar chart)
The most common admission diagnosis among those enrolled was pneumonia (38%) followed by bronchiolitis (28%), asthma (17%) and URI (13%). RSV was detected in 223(19%) of the enrolled children (Table 1). Overall, 65% of the enrolled children were male with a slightly higher proportion among RSV negative children (66%) than RSV positive (62%). Children with RSV were relatively younger (median age 7; IQR 3-13 months) than RSV negative children (median 10; IQR 4.9-21 months). About 30% of the enrolled children had a smoker within the household with almost similar proportion between those with RSV positive and negative status. More than 85% of the enrolled children were presented with cough, followed by fever (73%), nasal congestion (69%), and shortness of breath (68%). About 2% of the children required intubation with slightly higher proportion among RSV positive cases than RSV negative (3.6% vs. 2% respectively). In addition, 41(3.6%) children needed admission/transferred to the intensive care unit (ICU) and 22 children (1.9%) died during hospitalization (2 among RSV positive and 20 among RSV negative).
Table 1.
Comparison of enrolled RSV-positive and negative children less than 5 years old, admitted with acute respiratory illness at Aga Khan University Hospital Karachi, Pakistan (Aug 2009-Jul 2012)
| Baseline characteristics | RSV positive n=223 (%) | RSV negative n=927 (%) | Total N (%) |
|---|---|---|---|
| Age in months (median, IQR) | 7 (3 - 13.4) | 10 (4.9 - 21) | 9(4.1-18.8) |
| Age groups (months): | |||
| 0-2 | 48 (21.5) | 120 (12.9) | 168 (14.6) |
| 3-5 | 47 (21.1) | 157 (16.9) | 204 (17.7) |
| 6-11 | 53 (23.8) | 242 (26.1) | 295 (25.7) |
| 12-60 | 75 (33.6) | 408 (44.0) | 483 (42.0) |
| Male | 139 (62.3) | 611 (65.9) | 750 (65.2) |
| Birth weight (kg) (median, IQR) | 2.8 (2.5 - 3.2) | 2.9 (2.5 - 3.3) | 2.9 (2.5-3.3) |
| Months of breast feeding (median, IQR) | 6 (3 - 12) | 6 (2 - 12) | 6 (2-12) |
| Day care attendance | 11 (5.0) | 50 (5.5) | 61 (5.4) |
| Smokers in the house | 62 (28.1) | 281 (30.7) | 343 (30.2) |
| Number of household members (median, IQR) | 7 (5 - 10) | 7 (5 - 10) | 7 (5-10) |
| Clinical features | |||
| fever | 152 (68.2) | 688 (74.2) | 840 (73.0) |
| Cough | 200 (89.7) | 805 (86.8) | 1005 (87.4) |
| wheezing/noisy breathing | 120 (53.8) | 454 (49.0) | 574 (49.9) |
| poor appetite | 137 (61.4) | 559 (60.3) | 696 (60.5) |
| shortness of breath | 164 (73.5) | 619 (66.8) | 783 (68.1) |
| ear ache | 23 (10.3) | 119 (12.8) | 142 (12.3) |
| sore throat | 110 (49.3) | 456 (49.2) | 566 (49.2) |
| Post-tussive emesis | 89 (39.9) | 294 (31.7) | 383 (33.3) |
| nasal congestion | 168 (75.3) | 620 (66.9) | 788 (68.5) |
| Hospital course | |||
| Intubated | 8 (3.6) | 19 (2.0) | 27 (2.3) |
| ICU admission or transfer | 9 (4.0) | 32 (3.5) | 41 (3.6) |
| Died during hospitalization | 2 (0.9) | 20 (2.2) | 22 (1.9) |
Units are number (%) unless indicated otherwise in the left column.
The age-wise distribution of RSV and its detection rate is summarized in Table 2. Of the 223 children testing positive for RSV, 148 were less than 12 months old (22% detection rate for RSV), 47 were between 12 and 24 months (18% detection rate) and 27 were between 24 and 60 months old (12% detection rate). During the first year, the highest detection rate was in young infants less than 3 months old (29%), followed by infants between 3 and 6 months of age (23%). Cough and nasal congestion were the predominant clinical features while fever was reported by parents in two third of the RSV positive cases across all age groups. Bronchiolitis was the leading discharge diagnosis in RSV positive children less than 12 months old, followed by pneumonia. Pneumonia was the most common diagnosis in RSV positive children between the ages of 1 and 5 years, followed by asthma exacerbation.
Table 2.
Age-wise distribution of RSV associated ARI admissions at the Aga Khan University Hospital (Aug 2009-Jul 2012)
| 0-2 months n=168(%) | 3-5 months n=204(%) | 6-11 months n=295(%) | 12-60 months n=483(%) | |
|---|---|---|---|---|
| % RSV positive | 48 (28.6) | 47 (23.0) | 53 (18.0) | 75 (15.5) |
| Clinical features of RSV positive patients | ||||
| Fever | 26 (54.2) | 29 (61.7) | 39 (73.6) | 58 (77.3) |
| Cough | 44 (91.7) | 43 (91.5) | 46 (86.8) | 67 (89.3) |
| Wheezing/Noisy breathing | 28 (58.3) | 23 (48.9) | 31 (58.5) | 38 (50.7) |
| Poor appetite | 31 (64.6) | 30 (63.8) | 42 (79.2) | 34 (45.3) |
| Shortness of breath | 35 (72.9) | 37 (78.7) | 40 (75.5) | 52 (69.3) |
| Ear ache | 3 (6.3) | 8 (17.0) | 7 (13.2) | 5 (6.7) |
| Sore throat | 22 (45.8) | 23 (48.9) | 32 (60.4) | 33 (44.0) |
| Post-tussive emesis | 17 (35.4) | 17 (36.2) | 20 (37.7) | 35 (46.7) |
| Nasal congestion | 40 (83.3) | 32 (68.1) | 42 (79.2) | 54 (72.0) |
| Discharge diagnosis of RSV positive patients* | ||||
| Pneumonia | 17 (35.4) | 17 (36.2) | 20 (37.7) | 33 (44.0) |
| Bronchiolitis | 26 (54.2) | 25 (53.2) | 23 (43.4) | 15 (20.5) |
| Asthma | 4 (8.3) | 4 (8.5) | 7 (13.2) | 16 (21.3) |
| Upper respiratory infection | 3 (6.3) | 0 | 8 (15.1) | 9 (12.0) |
| Others | 4 (8.3) | 6 (12.8) | 3 (5.7) | 7 (9.3) |
| Hospital course of RSV positive patients | ||||
| Length of stay(days), mean (SD) | 2.7 (2.6) | 3.2 (2.6) | 3.6 (2.8) | 3.5 (4.4) |
| Intubated | 2 (4.2) | 1 (2.1) | 1 (1.9) | 4 (5.3) |
| ICU admission or transfer | 2 (4.2) | 1 (2.1) | 2 (3.8) | 4 (5.3) |
| Death during hospitalization | 0 | 0 | 0 | 2 (2.7) |
Multiple diagnoses possible,
Units are number (%) unless indicated otherwise in the left column
The multivariable regression analysis showed that the risk of RSV linearly decreases with increasing age (adjusted RR 1.81; 95% CI 1.17-2.81 among <3 months old children, RR 1.51; 95%CI 0.99-2.32 among 3-5 months and RR 1.15; 95%CI 0.77-1.72 among 6-12 months considering ≥12 months old children as the reference category). Also, the risk of RSV is highest during July-Sep (Adjusted RR 3.56; 95%CI 2.33-5.43), followed by Oct-Dec (Adjusted RR 2.89; 95%CI 1.88-4.44) as compared to the first quarter of the year. We also found that RSV positive children were almost four times more likely to be intubated as compared to RSV negative children (Adjusted RR 3.97; 95% CI 1.59-9.91). Post tussive emesis, and diagnosis of bronchiolitis were also significantly associated with the RSV infection (Adjusted RR 1.54; 95%CI 1.12-2.11 and RR 1.63;95%CI 1.16-2.27 respectively). (Table 3)
Table 3.
Correlates of RSV infection among children less than five years of age admitted with acute respiratory illness at Aga Khan University Hospital Karachi, Pakistan (Aug 2009-Jul 2012)
| Baseline characteristics | Crude RR (95% CI) | P value for Crude RR | Adj RR (95% CI) | P value for Adj RR |
|---|---|---|---|---|
| Age in months | 0.97 (0.96-0.99) | <0.001 | - | - |
| Age groups (months) | ||||
| 0-2 | 1.84 (1.28-2.64) | 1.81 (1.17-2.81) | ||
| 3-5 | 1.48 (1.03-2.14) | 0.006 | 1.51 (0.99-2.32) | 0.036 |
| 6-11 | 1.16 (0.81-1.65) | 1.15 (0.77-1.72) | ||
| 12-60 | 1 | 1 | ||
| Male | 0.88 (0.67-1.16) | 0.366 | - | - |
| Birth weight (kg) | 0.89 (0.73-1.09) | 0.264 | - | - |
| Born early | 0.99 (0.72-1.35) | 0.952 | - | - |
| Enrollment period (Seasonality): | ||||
| Jan-March | 1 | 1 | ||
| April-June | 1.04 (0.59-1.82) | <0.001 | 1.03 (0.56-1.89) | <0.001 |
| July-Sep | 2.77 (1.90-4.03) | 3.56 (2.33-5.43) | ||
| Oct-Dec | 2.29 (1.56-3.38) | 2.89 (1.88-4.44) | ||
| Duration of breast feeding (months) | 0.99 (0.96-1.02) | 0.566 | - | - |
| Day care attendance | 1.09 (0.59-2.00) | 0.777 | - | - |
| Smokers in the house | 1.10 (0.83-1.49) | 0.489 | - | - |
| Number of household members | 1.01 (0.98-1.03) | 0.647 | - | - |
| Crowding index (household members/# bedrooms) | 0.94 (0.84-1.06) | 0.312 | - | - |
|
Clinical features | ||||
| fever | 0.79 (0.60-1.05) | 0.101 | - | - |
| Cough | 1.26 (0.81-1.93) | 0.303 | - | - |
| wheezing/noisy breathing | 1.02 (0.99-1.05) | 0.245 | - | - |
| poor appetite | 0.98 (0.86-1.12) | 0.780 | - | - |
| shortness of breath | 1.14 (0.98-1.33) | 0.081 | - | - |
| ear ache | 0.93 (0.81-1.08) | 0.357 | - | - |
| sore throat | 1.01 (0.94-1.07) | 0.974 | - | - |
| Post-tussive emesis | 1.05 (1.01-1.09) | 0.037 | 1.54 (1.12-2.11) | 0.008 |
| nasal congestion | 1.05 (1.01-1.10) | 0.029 | - | - |
|
Hospital course | ||||
| Intubated | 1.55 (0.76-3.13) | 0.225 | 3.97 (1.59-9.91) | 0.003 |
| ICU admission or transfer | 1.14 (0.58-2.22) | 0.705 | - | - |
| Length of hospital stay (days) | 1.01 (0.97-1.04) | 0.667 | - | - |
| Died during hospitalization | 0.46 (0.12-1.87) | 0.280 | - | - |
|
Discharge diagnosis | ||||
| URI | 0.67 (0.42-1.06) | 0.09 | - | - |
| Asthma | 0.78 (0.53-1.14) | 0.20 | - | - |
| Pneumonia | 1.06 (0.81-1.38) | 0.69 | - | - |
| Bronchiolitis | 1.70 (1.30-2.22) | <0.001 | 1.63 (1.16-2.27) | 0.004 |
Risk ratio based on log binomial regression analysis. Outcome variable: RT-PCR status for RSV=1 for positive and 0 for negative. Variables with p values less than 0.30 at the univariate level were taken forward to the multivariables regression model.
DISCUSSION
Our study showed that RSV circulates throughout the year in Karachi, Pakistan, but the peak season is during the monsoon rains between July and September each year. These findings are consistent with studies conducted in coastal areas of India, Thailand as well as Malaysia where monsoon season follows a similar pattern [John et al., 1991; Khor et al., 2012; Naorat et al., 2013]. Our findings are also consistent with a previous study conducted in a large public sector tertiary care hospital of Karachi, Pakistan [Ali et al., 2013]. On the other hand, a study from Islamabad and Rawalpindi, Pakistan, showed that RSV was frequently detected from December through February [Tariq et al., 2005]. This is likely due to climatic differences between different cities, with Islamabad and Rawalpindi having temperate climate while Karachi having milder, coastal climate. In areas with temperate and Mediterranean climates, RSV outbreaks occur during winter months while in tropical settings, RSV infections typically peak after seasonal rainfalls [Weber et al., 1998].
RSV accounted for almost a third of the ARI associated hospitalizations in young infants less than 3 months old in our study. Risk of RSV among children less than three months of age hospitalized with ARI was almost 80% higher compared to those above one year of age. These findings are consistent with a study conducted in the USA by Hall et al, showing the highest risk of RSV among children less than five months of age [Hall et al., 2009]. The higher burden of disease in young infants has implications for the anticipated vaccine program. A future RSV vaccine which aims to protect infants in this age group, either through maternal immunization or immunization to the baby immediately after birth will likely have most public health impact.
We found that children with RSV infection were more likely to have post-tussive emesis and be diagnosed as bronchiolitis compared to children admitted for ARI but testing negative for RSV. Interestingly, children with RSV associated ARI were almost 4 times more likely to be intubated compared to children who had a non-RSV associated ARI. This increased severity of disease associated with RSV has been infrequently reported in the literature [Weber et al., 1998]. Two children enrolled in our study who had RSV associated ARI died, but the overall mortality rate was not different in RSV positive and RSV negative children. Of the two children who had RSV and died during hospitalization, one was 3.5 years old boy with Downs's syndrome, ventricular septum defect and pulmonary hypertension, who died after 7 days of hospitalization in the ICU. Besides RSV, he was also found to have influenza and pseudomonas bacteremia. The second child was a 2 years 4 months old girl, born after 31 weeks gestation but otherwise previously healthy, who was hospitalized with pneumonia for 3 days, which included 1 day in the ICU requiring mechanical ventilation before her death.
Strengths of this study include PCR based testing for RSV, which is the gold standard and often not available outside research settings in the third world settings. Data was collected prospectively over three years, so seasonal inferences are likely valid. The study also had multiple limitations. Although our study has a relatively large sample size of 1150 infants, a significant proportion of the possibly eligible children were not enrolled. Major reasons for non-enrollment included unclear diagnosis at the time of admission, lack of surveillance during weekends, early discharge before the family could be approached for enrollment, delay of greater than 48 hours in approaching family for enrollment (particularly when the patients were admitted over the weekend), and unavailability or refusal of parents to give consent. Nonetheless, as the figures 1-3 show, the enrollment pattern followed the pattern of ARI admissions, so the seasonality and the relative distribution of different syndromes is likely valid. Study was performed at a single tertiary care hospital which may not represent the entire socioeconomic spectrum of Pakistani population and likely does not represent patterns from other cities located in different lattitudes. With majority of pediatric deaths occurring at home because of low rates of care seeking at tertiary care by the population, the etiological spectrum in the community based studies may be different than what has been identified in this hospital based study. For more accurate estimation of RSV burden in the country, community based studies should ideally be conducted.
Acknowledgments
Funding
This study was supported by grant 1R01TW008126 from FIC/NIH, titled ‘Burden of Influenza and RSV in Children in Pakistan’ (PI Asad Ali). Dr. Ali's training was partially supported by Fogarty training grants D43TW007585 and 2D43TW001035. Data was stored and managed using the REDCap electronic data capture tool, funded by an NIH grant UL1 TR000445 from NCATS/NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
REFERENCES
- Ali SA, Khowaja AR, Bashir MZ, Aziz F, Mustafa S, Zaidi A. Role of Human Metapneumovirus, Influenza A Virus and Respiratory Syncytial Virus in Causing WHO-Defined Severe Pneumonia in Children in a Developing Country. Plos One. 2013;8(9):e74756. doi: 10.1371/journal.pone.0074756. Doi:10.1371/journal.pone.0074756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman RF, Van Beneden CA, Farnon EC. Surveillance for respiratory infections in low- and middle-income countries: experience from the Centers for Disease Control and Prevention's Global Disease Detection International Emerging Infections Program. J Infect Dis. 2013;208(Suppl 3):S167–172. doi: 10.1093/infdis/jit462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John TJ, Cherian T, Steinhoff MC, Simoes EA, John M. Etiology of acute respiratory infections in children in tropical southern India. Rev Infect Dis. 1991;13(Suppl 6):S463–469. doi: 10.1093/clinids/13.supplement_6.s463. [DOI] [PubMed] [Google Scholar]
- Khor CS, Sam IC, Hooi PS, Quek KF, Chan YF. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of 27 years. BMC Pediatr. 2012;12:32. doi: 10.1186/1471-2431-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentel R, Wegner U, Bruns R, Gurtler L. Real-time PCR to improve the diagnosis of respiratory syncytial virus infection. J Med Microbiol. 2003;52(Pt 10):893–896. doi: 10.1099/jmm.0.05290-0. [DOI] [PubMed] [Google Scholar]
- Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23-24 March 2015. Vaccine. 2016;34(2):190–197. doi: 10.1016/j.vaccine.2015.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naorat S, Chittaganpitch M, Thamthitiwat S, Henchaichon S, Sawatwong P, Srisaengchai P, Lu Y, Chuananon S, Amornintapichet T, Chantra S, Erdman DD, Maloney SA, Akarasewi P, Baggett HC. Hospitalizations for acute lower respiratory tract infection due to respiratory syncytial virus in Thailand, 2008-2011. J Infect Dis. 2013;208(Suppl 3):S238–245. doi: 10.1093/infdis/jit456. [DOI] [PubMed] [Google Scholar]
- NOVAVAX Novavax Announces Positive Top-Line Data from Phase 2 Clinical Trial of RSV F Vaccine to Protect Infants via Maternal Immunization. 2015 http://www.prnewswire.com/news-releases/novavax-announces-positive-top-line-data-from-phase-2-clinical-trial-of-rsv-f-vaccine-to-protect-infants-via-maternal-immunization-300149808.html.
- PD-VAC W. Status of Vaccine Research and Development of Vaccines for RSV. World Health Organization; 2014. [Google Scholar]
- Tariq WU, Waqar T, Ali S, Ghani E. Winter peak of respiratory syncytial virus in Islamabad. Trop Doct. 2005;35(1):28–29. doi: 10.1258/0049475053001958. [DOI] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3(4):268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2(1):25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- WorldBank . Pakistan: 2015. [Sep 15, 2015]. from: http://www.worldbank.org/en/country/pakistan. [Google Scholar]