Summary
Background
Retrospective studies provide conflicting interpretations of the effect of inherited genetic factors on the prognosis of patients with breast cancer. The primary aim of this study was to determine the effect of a germline BRCA1 or BRCA2 mutation on breast cancer outcomes in patients with young-onset breast cancer.
Methods
We did a prospective cohort study of female patients recruited from 127 hospitals in the UK aged 40 years or younger at first diagnosis (by histological confirmation) of invasive breast cancer. Patients with a previous invasive malignancy (except non-melanomatous skin cancer) were excluded. Patients were identified within 12 months of initial diagnosis. BRCA1 and BRCA2 mutations were identified using blood DNA collected at recruitment. Clinicopathological data, and data regarding treatment and long-term outcomes, including date and site of disease recurrence, were collected from routine medical records at 6 months, 12 months, and then annually until death or loss to follow-up. The primary outcome was overall survival for all BRCA1 or BRCA2 mutation carriers (BRCA-positive) versus all non-carriers (BRCA-negative) at 2 years, 5 years, and 10 years after diagnosis. A prespecified subgroup analysis of overall survival was done in patients with triple-negative breast cancer. Recruitment was completed in 2008, and long-term follow-up is continuing.
Findings
Between Jan 24, 2000, and Jan 24, 2008, we recruited 2733 women. Genotyping detected a pathogenic BRCA mutation in 338 (12%) patients (201 with BRCA1, 137 with BRCA2). After a median follow-up of 8·2 years (IQR 6·0–9·9), 651 (96%) of 678 deaths were due to breast cancer. There was no significant difference in overall survival between BRCA-positive and BRCA-negative patients in multivariable analyses at any timepoint (at 2 years: 97·0% [95% CI 94·5–98·4] vs 96·6% [95·8–97·3]; at 5 years: 83·8% [79·3–87·5] vs 85·0% [83·5–86·4]; at 10 years: 73·4% [67·4–78·5] vs 70·1% [67·7–72·3]; hazard ratio [HR] 0·96 [95% CI 0·76–1·22]; p=0·76). Of 558 patients with triple-negative breast cancer, BRCA mutation carriers had better overall survival than non-carriers at 2 years (95% [95% CI 89–97] vs 91% [88–94]; HR 0·59 [95% CI 0·35–0·99]; p=0·047) but not 5 years (81% [73–87] vs 74% [70–78]; HR 1·13 [0·70–1·84]; p=0·62) or 10 years (72% [62–80] vs 69% [63–74]; HR 2·12 [0·82–5·49]; p= 0·12).
Interpretation
Patients with young-onset breast cancer who carry a BRCA mutation have similar survival as non-carriers. However, BRCA mutation carriers with triple-negative breast cancer might have a survival advantage during the first few years after diagnosis compared with non-carriers. Decisions about timing of additional surgery aimed at reducing future second primary-cancer risks should take into account patient prognosis associated with the first malignancy and patient preferences.
Funding
Cancer Research UK, the UK National Cancer Research Network, the Wessex Cancer Trust, Breast Cancer Now, and the PPP Healthcare Medical Trust Grant.
Introduction
Although only 5% of breast cancers are diagnosed in women aged younger than 40 years, a high proportion of deaths from breast cancer occur in this age group, which includes a higher number of patients who carry a pathogenic BRCA1 or BRCA2 mutation compared with patients with onset of breast cancer at an older age.1, 2, 3 Second primary breast cancers are more frequent in high-risk gene carriers, and this higher frequency drives early genetic testing to inform surgical decision making; however, whether a germline BRCA1 or BRCA2 mutation has independent prognostic implications after an initial cancer diagnosis is unclear.
BRCA1 loss of function mutations are associated with high-histological-grade, oestrogen-receptor-negative, progesterone-receptor-negative, and HER2-negative (triple negative) breast cancer with a basal-like gene expression profile.4 BRCA2-associated breast tumours are usually high-grade, oestrogen-receptor positive, and HER2-negative.5, 6 BRCA1 mutation carriers have been reported to have enhanced sensitivity to neoadjuvant chemotherapy with cytotoxic drugs.7
Research in context.
Evidence before this study
At the initiation of this cohort study (Dec 3, 1999), we searched the PubMed database using the search terms [BRCA1 OR BRCA2] AND [breast cancer or breast neoplasm] AND [survival OR prognosis OR mortality] and identified a few published retrospective studies reporting prognosis in BRCA mutation carriers. On Dec 5, 2016, we did another PubMed search for studies of patients who carried a BRCA1 or BRCA2 mutation and their prognosis, using the following search terms: “(BRCA) AND (survival or prognosis or outcome or mortality) AND (breast neoplasms or breast neoplasm or breast cancer or breast tumour)”. Our search was not limited by date or language. We also hand-searched references cited in review papers for additional papers. Previous studies and meta-analyses have reported inconsistent effects of BRCA1 and BRCA2 mutations on the outcomes of early breast cancer with better, worse, and similar outcomes for patients with a BRCA1 or BRCA2 mutation compared with patients with sporadic breast cancer. These conflicting results might be explained by methodological issues with ascertainment biases introduced by retrospective and selective identification of cases, incomplete genetic testing, small numbers, an absence of adjustment for clinical variables, including treatment, and short follow-up.
Added value of this study
POSH is, to our knowledge, the largest prospective cohort study to compare breast cancer outcomes of patients with a BRCA1 or BRCA2 mutation with patients with sporadic cancer. Our findings showed that patients with young-onset breast cancer who have a BRCA mutation have a similar overall survival to non-carriers. However, in patients with triple-negative breast cancer, BRCA mutation carriers might have a survival advantage compared with non-carriers during the first few years after diagnosis. Our study was strengthened by unbiased recruitment, universal and central genetic testing at the end of the study, and comprehensive pathological, clinical, and follow-up data.
Implications of all the available evidence
Decisions about timing of risk-reducing surgery should take into account primary tumour prognosis and patient preference.
Published studies and meta-analyses have reported better, worse, and similar outcomes for patients with a BRCA1 or BRCA2 mutation compared with patients with sporadic breast cancer.8, 9, 10, 11, 12, 13, 14 A comprehensive meta-analysis of 66 studies of breast cancer survival in patients with a BRCA1 or BRCA2 mutation compared with non-carrier patients or the general breast cancer population, which assessed study quality as well as outcome data, concluded that “it is not yet possible to draw evidence based conclusions about the association between BRCA1 [or] BRCA2 mutation carriership and breast cancer prognosis”.12 We undertook the Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study, the primary aim of which was to determine the effect of inherited BRCA1 or BRCA2 mutations on outcomes in patients with young-onset breast cancer.15, 16
Methods
Study design and participants
We did a prospective cohort study at 127 hospitals in the UK (appendix pp 1–2). We recruited young women (aged 18–40 years) diagnosed with primary breast cancer in the UK. Patients were eligible if they were diagnosed with invasive breast cancer aged 40 years or younger. Potential recruits were identified by local breast cancer clinicians, nurses, or research clinical trial practitioners within 12 months of initial diagnosis of invasive breast cancer and the date of diagnosis was defined as the first histological confirmation of invasive breast cancer. All histological subtypes, disease stages (I–IV), comorbidities, and performance statuses were permitted. Patients with a previous invasive malignancy (with the exception of non-melanomatous skin cancer) were excluded.
Written informed consent was obtained from all participants. Ethical approval was granted in 2000 (MREC 00/6/69) and the study was approved for recruitment as part of the UK National Cancer Research Network (NCRN) portfolio in 2002, subsequently the NIHR portfolio. The protocol was published in 2007.15
Procedures
All patients received treatment according to local protocols. Details of personal characteristics, tumour pathology, disease stage, and surgical and cytotoxic treatment data were collected from medical records at study entry. Family history was collected by questionnaire. The BOADICEA algorithm, without adjustment for pathological subtype, was used to estimate the probability that an individual might carry a BRCA1 or BRCA2 pathogenic variant.17 Pathology and imaging data were verified with copies of the original reports from sites. For patients treated with neoadjuvant chemotherapy, the initial diameter of the tumour was derived from radiological reports.
The oestrogen-receptor, progesterone-receptor, and HER2-receptor status of the primary tumours was determined from reports of local routine pathology testing of diagnostic core biopsies or tumour resections for clinical use. Hormone-receptor concentrations equivalent to an Allred score of 3 or more were categorised as positive. Immunohistochemical staining of tissue microarrays in some cases enabled clinical source data for oestrogen-receptor, progesterone-receptor, and HER2-receptor statuses to be corroborated; tissue microarray scores were used to supplement missing datapoints for these receptors.16
DNA for genotyping was extracted from whole blood samples submitted at recruitment. A multiplex amplicon-based library preparation system, Fluidigm Access Array (Fluidigm UK, Cambridge, UK), targeted a panel of breast-cancer-susceptibility genes (including BRCA1, BRCA2, and TP53) for sequencing using an Illumina HiSeq2500 Next Generation Sequencing Platform (Illumina, Little Chesterford, UK; appendix pp 20–21). Targeted-sequence capture cannot reliably identify large exonic deletions or duplications, therefore multiplex ligation probe analysis was used for patients who met current UK guideline thresholds for clinical genetic testing.17, 18 Predicted protein truncating variants (frameshift, nonsense, and canonical-splice site and large rearrangements) plus other variants (mainly mis-sense) unequivocally defined as pathogenic on the basis of multiple lines of evidence and expert review were assigned to the BRCA-mutation carrier group (BRCA-positive). All pathogenic variants were confirmed by Sanger sequencing. All other patients, including those with BRCA1 or BRCA2 variants of uncertain significance or very low penetrance, were assigned to the same group as no mutation found (BRCA-negative) or excluded if they were found to carry a pathogenic variant of TP53. For the purposes of this analysis, mutations in other breast cancer genes were not curated.
The study protocol and patient information specified that patients would not be informed of the research genetic-testing results; however, patient information sheets gave information about seeking clinical genetic referral. Clinical referrals for genetic testing were made by the treating physician according to local protocols. Genetic test reports for the study patients generated by UK National Health Service (NHS) diagnostic laboratories were collected as part of the medical record.
Detailed clinical follow-up data, including date and site of disease recurrence, were obtained from medical records at 6 months, 12 months, and annually thereafter, until death or loss to follow-up. Patients were flagged in the NHS medical research information service for automatic notification of date and cause of death.
Outcomes
The primary outcome was overall survival, defined as the time from first diagnosis to death from any cause. The secondary outcomes were distant disease-free survival, defined as time from first diagnosis to first distant disease excluding local (in breast) recurrence.
Statistical analysis
The original study sample size of a minimum of 2000 patients was estimated based on a prevalence of BRCA1 or BRCA2 pathogenic mutations of 10%, and an absolute difference in event rate at 2 years between mutation carriers and non-carriers of 10% (20% in mutation carriers compared with 10% in sporadic cases).15 We also considered a prevalence of BRCA1 or BRCA2 mutations of 5% and 15%, and larger sample sizes. Good recruitment and data returns enabled us to continue study recruitment beyond 2000 participants providing sufficient power for multivariable analyses.
We did the statistical analyses according to a prespecified plan (appendix pp 22–31).19 The analysis population included all eligible patients recruited to the cohort who had available data for the primary tumour and genotyping, were aged 40 years or younger at the date of diagnosis, did not carry a TP53 gene, and who did not present with metastatic disease at presentation (M1 stage). A prespecified subgroup of the analysis population was patients with triple-negative breast cancer (ie, oestrogen-receptor-negative, HER2-negative, and progesterone-receptor-negative or unknown). All analyses were done for both the overall analysis population and the triple-negative breast cancer subgroup population, unless specified otherwise. Key patient data were described by BRCA mutation status, and formal comparisons by BRCA mutation status were done using Mann-Whitney tests (for continuous variables) and Pearson χ2 tests (for categorical variables) for patients with complete data. We used Kaplan-Meier plots to show survival data by BRCA status at 2, 5, and 10 years. The 2-year comparison was chosen because this timepoint was specified for the original sample size; the 5-year and 10-year comparisons were chosen because they are commonly used in such studies and are clinically relevant timepoints. Patients who did not have an event were censored at the date of their last follow-up. Hazard ratios (HRs) and 95% CIs for univariable analyses and multivariable analyses (for the primary and secondary outcomes) were calculated using Cox proportional-hazards models, or flexible parametric survival models for those that involved time-varying hazards.20 For each flexible parametric survival model, varying degrees of freedom for the baseline-hazard rate and time-dependent effect were explored to obtain the best-model fit. All missing data were assumed to be either missing at random or missing completely at random, and censoring was assumed to be non-informative. Prespecified sensitivity analyses included the generation of corresponding complete-case multivariable analysis model results.
Post-hoc sensitivity analyses were done to explore the possible reasons for some of the results in the triple-negative breast cancer group. Additionally, to investigate the degree of potential bias from time of diagnosis to blood draw for genetic testing at registration, a multivariable analysis model adjusting for the time from diagnosis to blood draw was generated accordingly for the analysis population only. We considered if the longer survival of BRCA mutation carriers with triple-negative breast cancer could be due to a beneficial effect of risk-reducing surgery in BRCA carriers, so we repeated the analysis in this subgroup excluding patients who underwent bilateral mastectomy within the first year after diagnosis. A further sensitivity analysis was done to compare the pattern of improved survival at an early timepoint with apparently worse survival in the long term by excluding patients who developed a new primary breast or ovarian cancer.
We did all analyses with Stata, version 14.2, and multiple imputation was incorporated in the multivariable analyses generated using the mi command.
Role of the funding source
The funders and their representatives had no role in study design, data collection, data analysis, data interpretation, or writing of the report or the decision to submit it for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
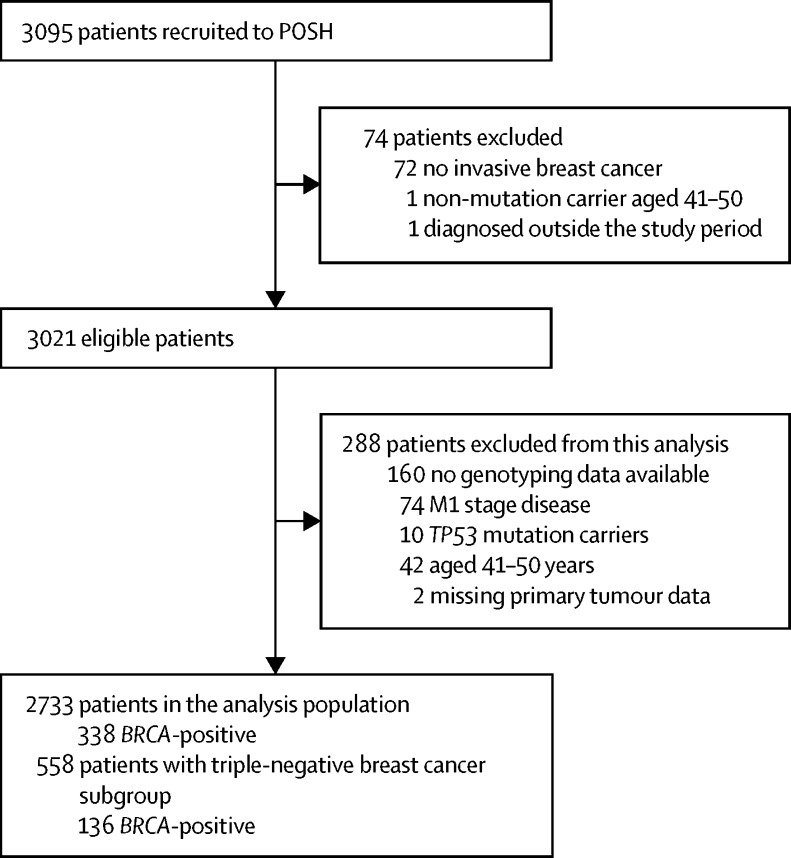
Between Jan 24, 2000, and Jan 24, 2008, we recruited 3021 eligible women, of whom 2733 (91%) were included in the analysis population, and 288 (9%) were excluded (figure 1; appendix p 11). We included all data received until July 26, 2016. Of 2721 patients for whom presentation was recorded, 45 (2%) were recorded as being enrolled in a surveillance programme, and 33 (1%) were recorded as having screen-detected breast cancer. Screening was offered according to local protocols; national guidelines were not formally established until after recruitment ended.
Figure 1.
Trial profile
BRCA-positive=patient with BRCA1 or BRCA2 pathogenic mutation. Patients were categorised as BRCA-negative if no BRCA pathogenic mutation was found or they had a BRCA1 or BRCA2 variant of uncertain significance or very low penetrance.
338 (12%) of 2733 patients included in the analysis population had either a BRCA1 or BRCA2 mutation, of whom 44 (13%) had large-copy-number variants (appendix pp 3–7). 75 (22%) of 338 patients did not meet current family history or pathology based genetic-testing guidelines.18 Referral for a clinical genetics consultation and BRCA testing occurred for 388 patients (14%), of whom 182 (47%) had a pathogenic mutation. Immunohistochemical staining of tissue microarrays in 1336 cases, during 2012 and 2016, enabled clinical source data for oestrogen-receptor, progesterone-receptor, and HER2-receptor statuses to be corroborated.
The median time from breast cancer diagnosis to study registration blood draw was 5·5 months (IQR 3·2–10·7). There were several significant clinicopathological differences between BRCA-positive and BRCA-negative patients, and between BRCA1 mutation carriers and BRCA2 mutation carriers (table 1). The most commonly used chemotherapy regimen was anthracycline with or without taxanes. Of the 2733 patients in the analysis population, 558 (20%) had triple-negative breast cancer. BRCA mutations were identified in 136 (24%) of patients with triple-negative breast cancer, of whom 123 (90%) had a BRCA1 mutation. Differences in tumour characteristics between BRCA1 and BRCA2 mutation carriers were also noted in patients with triple-negative breast cancer (table 2).
Table 1.
Baseline characteristics and clinicopathological information for all patients
| All patients (n=2733) | BRCA1-positive (n=201) | BRCA2-positive (n=137) | BRCA-positive (n=338) | BRCA-negative (n=2395) | p value* | ||
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 36 (34–38, 18–40) | 35 (32–38, 22–40) | 37 (33–38, 21–40) | 36 (32–38, 21–40) | 37 (34–39, 18–40) | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p=0·014 | |
| BMI (kg/m2) | BRCA-positive vs BRCA-negative p=0·48, BRCA1-positive vs BRCA2-positive p=0·40 | ||||||
| <25 | 1427/2632 (54%) | 114/192 (59%) | 70/133 (53%) | 184/325 (57%) | 1243/2307 (54%) | ||
| ≥25 to <30 | 714/2632 (27%) | 47/192 (25%) | 41/133 (31%) | 88/325 (27%) | 626/2307 (27%) | ||
| ≥30 | 491/2632 (19%) | 31/192 (16%) | 22/133 (17%) | 53/325 (16%) | 438/2307 (19%) | ||
| Missing | 101 (4%) | 9 (5%) | 4 (3%) | 13 (4%) | 88 (4%) | ||
| Ethnicity | BRCA-positive vs BRCA-negative p=0·28, BRCA1-positive vs BRCA2-positive p=0·99 | ||||||
| White | 2494/2698 (92%) | 178/196 (91%) | 122/134 (91%) | 300/330 (91%) | 2194/2368 (93%) | ||
| Black | 103/2698 (4%) | 10/196 (5%) | 6/134 (5%) | 16/330 (5%) | 87/2368 (4%) | ||
| Asian | 80/2698 (3%) | 5/196 (3%) | 4/134 (3%) | 9/330 (3%) | 71/2368 (3%) | ||
| Other | 21/2698 (<1%) | 3/196 (2%) | 2/134 (2%) | 5/330 (2%) | 16/2368 (<1%) | ||
| Missing | 35 (1%) | 5 (3%) | 3 (2%) | 8 (2%) | 27 (1%) | ||
| Histological grade | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p<0·0001 | ||||||
| 1 | 156/2658 (6%) | 2/197 (1%) | 0 | 2/326 (<1%) | 154/2332 (7%) | ||
| 2 | 904/2658 (34%) | 16/197 (8%) | 40/129 (31%) | 56/326 (17%) | 848/2332 (36%) | ||
| 3 | 1598/2658 (60%) | 179/197 (91%) | 89/129 (69%) | 268/326 (82%) | 1330/2332 (57%) | ||
| Missing or not graded | 75 (3%) | 4 (2%) | 8 (6%) | 12 (4%) | 63 (3%) | ||
| Oestrogen-receptor status | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p<0·0001 | ||||||
| Negative | 908/2719 (33%) | 151/200 (76%) | 21/136 (15%) | 172/336 (51%) | 736/2383 (31%) | ||
| Positive | 1811/2719 (67%) | 49/200 (25%) | 115/136 (85%) | 164/336 (49%) | 1647/2383 (69%) | ||
| Missing | 14 (<1%) | 1 (<1%) | 1 (<1%) | 2 (<1%) | 12 (<1%) | ||
| HER2 status | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p=0·18 | ||||||
| Negative | 1763/2412 (73%) | 164/176 (93%) | 111/125 (89%) | 275/301 (91%) | 1488/2111 (71%) | ||
| Positive | 649/2412 (27%) | 12/176 (7%) | 14/125 (11%) | 26/301 (9%) | 623/2111 (30%) | ||
| Missing | 321 (12%) | 25 (12%) | 12 (9%) | 37 (11%) | 284 (12%) | ||
| Progesterone-receptor status | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p<0·0001 | ||||||
| Negative | 951/2208 (43%) | 144/171 (84%) | 23/107 (22%) | 167/278 (60%) | 784/1930 (41%) | ||
| Positive | 1257/2208 (57%) | 27/171 (16%) | 84/107 (79%) | 111/278 (40%) | 1146/1930 (59%) | ||
| Missing | 525 (19%) | 30 (15%) | 30 (22%) | 60 (18%) | 465 (19%) | ||
| †Triple-negative breast cancer status | BRCA-positive vs BRCA-negative p<0·0001, BRCA1-positive vs BRCA2-positive p<0·0001 | ||||||
| No | 2175/2733 (80%) | 78/201 (39%) | 124/137 (91%) | 202/338 (60%) | 1973/2395 (82%) | ||
| Yes | 558/2733 (20%) | 123/201 (61%) | 13/137 (10%) | 136/338 (40%) | 422/2395 (18%) | ||
| Maximum invasive tumour size (mm) | 22 (15–33, 0–170) | 21 (15–30, 1–140) | 25 (16–32, 1–92) | 22 (15–31, 1–140) | 22 (15–34, 0–170) | BRCA-positive vs BRCA-negative p=0·97, BRCA1-positive vs BRCA2-positive p=0·060 | |
| Missing | 156 (6%) | 10 (5%) | 14 (10%) | 24 (7%) | 132 (6%) | ||
| Pathological N stage | BRCA-positive vs BRCA-negative p=0·013, BRCA1-positive vs BRCA2-positive p<0·0001 | ||||||
| 0 | 1304/2692 (48%) | 129/201 (64%) | 55/135 (41%) | 184/336 (55%) | 1120/2356 (48%) | ||
| 1 | 1388/2692 (52%) | 72/201 (36%) | 80/135 (59%) | 152/336 (45%) | 1236/2356 (53%) | ||
| Axillary nodal involvement | BRCA-positive vs BRCA-negative p=0·019, BRCA1-positive vs BRCA2-positive p=0·00017 | ||||||
| 1–3 | 899/2692 (33%) | 43/201 (21%) | 51/135 (38%) | 94/336 (28%) | 805/2356 (34%) | ||
| 4–9 | 330/2692 (12%) | 14/201 (7%) | 19/135 (14%) | 33/336 (10%) | 297/2356 (13%) | ||
| ≥10 | 159/2692 (6%) | 15/201 (8%) | 10/135 (7%) | 25/336 (7%) | 134/2356 (6%) | ||
| Missing | 41 (2%) | 0 | 2 (2%) | 2 (<1%) | 39 (2%) | ||
| Lymphovascular invasion | BRCA-positive vs BRCA-negative p=0·23, BRCA1-positive vs BRCA2-positive p=0·013 | ||||||
| Absent | 1327/2539 (52%) | 116/190 (61%) | 58/124 (47%) | 174/314 (55%) | 1153/2225 (52%) | ||
| Present | 1212/2539 (48%) | 74/190 (39%) | 66/124 (53%) | 140/314 (45%) | 1072/2225 (48%) | ||
| Missing | 194 (7%) | 11 (6%) | 13 (10%) | 24 (7%) | 170 (7%) | ||
| Chemotherapy | BRCA-positive vs BRCA-negative p=0·0058, BRCA1-positive vs BRCA2-positive p=0·016 | ||||||
| None | 294/2733 (11%) | 9/201 (5%) | 11/137 (8%) | 20/338 (6%) | 274/2395 (11%) | ||
| Adjuvant | 2027/2733 (74%) | 171/201 (85%) | 99/137 (72%) | 270/338 (80%) | 1757/2395 (73%) | ||
| Neoadjuvant | 412//2733 (15%) | 21/201 (10%) | 27/137 (20%) | 48/338 (14%) | 364/2395 (15%) | ||
| Type of surgery | BRCA-positive vs BRCA-negative p=0·30, BRCA1-positive vs BRCA2-positive p=0·00040 | ||||||
| Breast-conserving surgery | 1337/2733 (49%) | 106/201 (53%) | 43/137 (31%) | 149/338 (44%) | 1188 (50%) | ||
| Mastectomy | 1373/2733 (50%) | 94/201 (47%) | 92/137 (67%) | 186/338 (55%) | 1187/2395 (50%) | ||
| Nodal surgery only | 7/2733 (<1%) | 1/201 (<1%) | 0 | 1/338 (<1%) | 6/2395 (<1%) | ||
| None | 16/2733 (<1%) | 0 | 2/137 (2%) | 2/338 (<1%) | 14/2395 (<1%) | ||
| Chemotherapy regimen | BRCA-positive vs BRCA-negative p=0·015, BRCA1-positive vs BRCA2-positive p=0·38 | ||||||
| None | 294/2733 (11%) | 9/201 (5%) | 11/137 (8%) | 20/338 (6%) | 274/2395 (11%) | ||
| Anthracyclines | 1760/2733 (64%) | 145/201 (72%) | 89/137 (65%) | 234/338 (69%) | 1526/2395 (64%) | ||
| Taxanes | 24/2733 (<1%) | 0 | 1/137 (<1%) | 1/338 (<1%) | 23/2395 (1%) | ||
| Anthracyclines and taxanes | 635/2733 (23%) | 45/201 (22%) | 34/137 (25%) | 79/338 (23%) | 556/2395 (23%) | ||
| Other (including CMF) | 20/2733 (<1%) | 2/201 (1%) | 2/137 (2%) | 4/338 (1%) | 16/2395 (<1%) | ||
Data are median (IQR, range) or n (%). Patients with missing data were not included in the p value calculation. BMI=body-mass index. CMF=cyclophosphamide plus methotrexate plus fluorouracil.
Test excluded patients with both BRCA1 and BRCA2 mutations. Mann-Whitney tests used for continuous variables and Pearson χ2 tests for categorical variables, done on patients with complete data.
Defined as oestrogen-receptor-negative, HER2-negative, and progesterone-receptor-negative or unknown.
Table 2.
Baseline characteristics and clinicopathological information for patients with triple-negative breast cancer*
| All patients (n=558) | BRCA1-positive (n=123) | BRCA2-positive (n=13) | BRCA-positive (n=136) | BRCA-negative (n=422) | p value† | ||
|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 36 (33–38, 19–40) | 34 (32–37, 22–40) | 33 (32–38, 30–40) | 34 (32–37, 22–40) | 36 (33–38, 19–40) | BRCA-positive vs BRCA-negative p=0·00056, BRCA1-positive vs BRCA2-positive p=0·79 | |
| BMI (kg/m2) | BRCA-positive vs BRCA-negative p=0·26, BRCA1-positive vs BRCA2-positive p=0·47 | ||||||
| <25 | 274/546 (50%) | 67/119 (56%) | 5/13 (39%) | 72/132 (55%) | 202/414 (49%) | ||
| ≥25 to <30 | 149/546 (27%) | 32/119 (27%) | 5/13 (39%) | 37/132 (28%) | 112/414 (27%) | ||
| ≥30 | 123/546 (23%) | 20/119 (17%) | 3/13 (23%) | 23/132 (18%) | 100/414 (24%) | ||
| Missing | 12 (2%) | 4 (3%) | 0 | 4 (3%) | 8 (2%) | ||
| Ethnicity | BRCA-positive vs BRCA-negative p=0·52, BRCA1-positive vs BRCA2-positive p=0·052 | ||||||
| White | 500/550 (91%) | 110/122 (90%) | 9/13 (69%) | 119/135 (88%) | 381/415 (92%) | ||
| Black | 26/550 (5%) | 7/122 (6%) | 2/13 (15%) | 9/135 (7%) | 17/415 (4%) | ||
| Asian | 19/550 (4%) | 3/122 (3%) | 2/13 (15%) | 5/135 (4%) | 14/415 (3%) | ||
| Other | 5/550 (<1%) | 2/122 (2%) | 0 | 2/135 (2%) | 3/415 (<1%) | ||
| Missing | 8 (1%) | 1 (<1%) | 0 | 1 (<1%) | 7 (2%) | ||
| Histological grade | BRCA-positive vs BRCA-negative p=0·49, BRCA1-positive vs BRCA2-positive p=0·41 | ||||||
| 1 | 3/541 (<1%) | 0 | 0 | 0 | 3/406 (<1%) | ||
| 2 | 30/541 (6%) | 6/122 (5%) | 0 | 6/135 (4%) | 24/406 (6%) | ||
| 3 | 508/541 (94%) | 116/122 (95%) | 13/13 (100%) | 129/135 (96%) | 379/406 (93%) | ||
| Missing or not graded | 17 (3%) | 1 (<1%) | 0 | 1 (<1%) | 16 (4%) | ||
| Maximum invasive tumour size (mm) | 22 (15–31, 1–160) | 21 (15–30, 4–140) | 23 (16–30, 15–30) | 21 (15–30, 4–140) | 23 (15–32, 1–160) | BRCA-positive vs BRCA-negative p=0·17, BRCA1-positive vs BRCA2-positive p=0·72 | |
| Missing | 35 (6%) | 5 (4%) | 3 (23%) | 8 (6%) | 27 (6%) | ·· | |
| Pathological N stage | BRCA-positive vs BRCA-negative p=0·46, BRCA1-positive vs BRCA2-positive p=0·64 | ||||||
| 0 | 341/552 (62%) | 80/123 (65%) | 7/12 (58%) | 87/135 (64%) | 254/417 (61%) | ||
| 1 | 211/552 (38%) | 43/123 (35%) | 5/12 (42%) | 48/135 (36%) | 163/417 (39%) | ||
| Axillary nodal involvement | BRCA-positive vs BRCA-negative p=0·044, BRCA1-positive vs BRCA2-positive p=0·68 | ||||||
| 1 to 3 | 141/552 (26%) | 26/123 (21%) | 4/12 (33%) | 30/135 (22%) | 111/417 (27%) | ||
| 4 to 9 | 45/552 (8%) | 7/123 (6%) | 0 | 7/135 (5%) | 38/417 (9%) | ||
| ≥10 | 25/552 (5%) | 10/123 (8%) | 1/12 (8%) | 11/135 (8%) | 14/417 (3%) | ||
| Missing | 6 (1%) | 0 | 1 (8%) | 1 (<1%) | 5 (1%) | ||
| Lymphovascular invasion | BRCA-positive vs BRCA-negative p=0·83, BRCA1-positive vs BRCA2-positive p=0·19 | ||||||
| Absent | 312/517 (60%) | 71/116 (61%) | 4/10 (40%) | 75/126 (60%) | 237/391 (61%) | ||
| Present | 205/517 (40%) | 45/116 (39%) | 6/10 (60%) | 51/126 (41%) | 154/391 (39%) | ||
| Missing | 41 (7%) | 7 (6%) | 3 (23%) | 10 (7%) | 31 (7%) | ||
| Chemotherapy | BRCA-positive vs BRCA-negativep=0·17, BRCA1-positive vs BRCA2-positive, p=0·074 | ||||||
| None | 13/558 (2%) | 3/123 (2%) | 0 | 3/136 (2%) | 10/422 (2%) | ||
| Adjuvant | 450/558 (81%) | 108/123 (88%) | 9/13 (69%) | 117/136 (86%) | 333/422 (79%) | ||
| Neoadjuvant | 95/558 (17%) | 12/123 (10%) | 4/13 (31%) | 16/136 (12%) | 79/422 (19%) | ||
| Type of surgery | BRCA-positive vs BRCA-negative p=0·19, BRCA1-positive vs BRCA2-positive p=0·014 | ||||||
| Breast-conserving surgery | 331/558 (59%) | 69/123 (56%) | 5/13 (39%) | 74/136 (54%) | 257/422 (61%) | ||
| Mastectomy | 223/558 (40%) | 53/123 (43%) | 7/13 (54%) | 60/136 (44%) | 163/422 (39%) | ||
| Nodal surgery only | 1/558 (<1%) | 1/123 (<1%) | 0 | 1/136 (<1%) | 0 | ||
| None | 3/558 (<1%) | 0 | 1/13 (8%) | 1/136 (<1%) | 2/422 (<1%) | ||
| Chemotherapy regimen | BRCA-positive vs BRCA-negative p=0·097, BRCA1-positive vs BRCA2-positive p=0·086 | ||||||
| None | 13 (2%) | 3 (2%) | 0 | 3 (2%) | 10 (2%) | ||
| Anthracyclines | 382/558 (69%) | 91/123 (74%) | 6/13 (46%) | 97/136 (71%) | 285/422 (68%) | ||
| Taxanes | 2/558 (<1%) | 0 | 0 | 0 | 2/422 (<1%) | ||
| Anthracyclines and taxanes | 159/558 (29%) | 27/123 (22%) | 7/13 (54%) | 34/136 (25%) | 125/422 (30%) | ||
| Other (includes CMF) | 2/558 (<1%) | 2/123 (2%) | 0 | 2/136 (2%) | 0 | ||
Data are median (IQR, range) or n (%). Patients with missing data were not included in the p value calculation. BMI=body-mass index. CMF=cyclophosphamide plus methotrexate plus fluorouracil.
Defined as oestrogen-receptor-negative, HER2-negative, and progesterone-receptor-negative or unknown.
Test excluded patients with both BRCA1 and BRCA2 mutations. Mann-Whitney tests used for continuous variables and Pearson χ2-tests for categorical variables, done on patients with complete data.
Median follow-up was 8·2 years (IQR 6·0–9·9); 91 (3%) patients were lost to follow-up. Contralateral breast tumours occurred in 151 (6%) patients: in 37 (18%) of 201 BRCA1 mutation carriers, 17 (12%) of 137 BRCA2 mutation carriers, and 97 (4%) of 2395 BRCA-negative patients. Median time to contralateral breast cancer was 3·0 years (IQR 1·5–4·8) in BRCA-positive patients and 2·7 years (1·2–5·3) in BRCA-negative patients. 752 (28%) women developed a distant recurrence. Of 678 deaths, 651 (96%) were due to breast cancer. Deaths due to non-breast malignancies included six (3%) of 201 new primary cancers in BRCA1 mutation carriers (three ovarian, one primary peritoneal, one oesophageal, and one pancreatic) and 12 (<1%) of 2395 malignancies in BRCA-negative patients (four haematological, three lung, and one each of brain, colorectal, gastric, pancreatic, and sarcoma; appendix p 8). There were no deaths attributed to second primary cancers among BRCA2 mutation carriers.
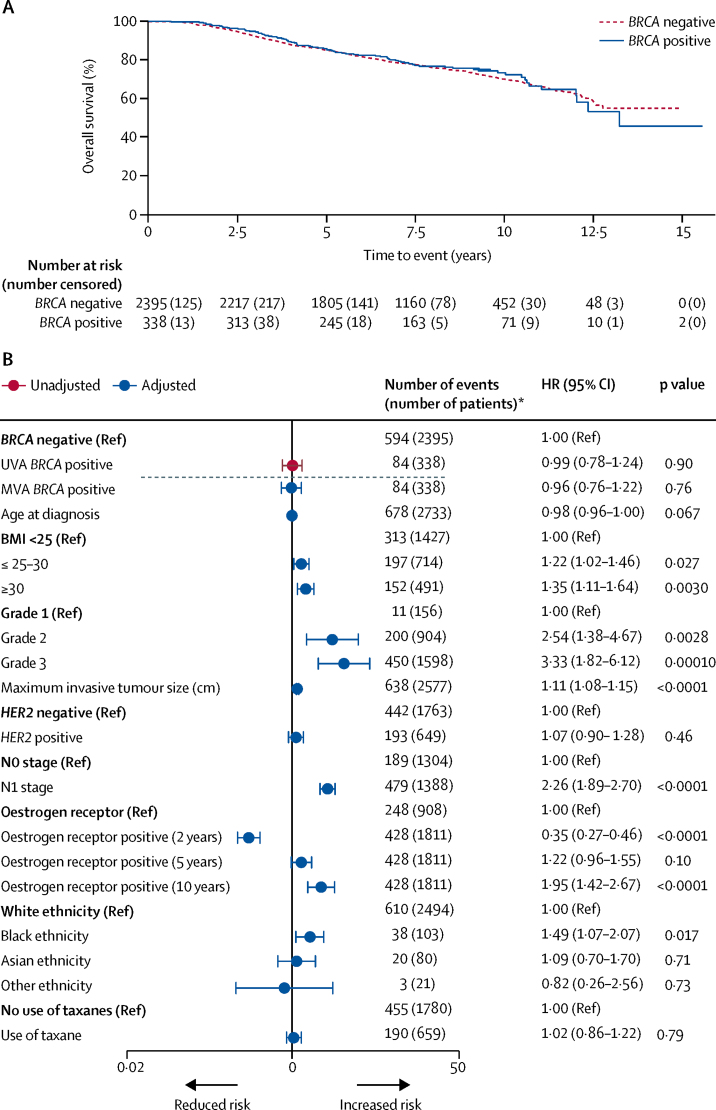
Overall survival was 97·0% (95% CI 94·5–98·4) in BRCA-positive patients versus 96·6% (95·8–97·3) in BRCA-negative patients at 2 years; 83·8% (79·3–87·5) versus 85·0% (83·5–86·4) at 5 years; and 73·4% (67·4–78·5) versus 70·1% (67·7–72·3) at 10 years (figure 2). There was no difference in overall survival between groups either before or after adjusting for known prognostic factors, including adjustments for ethnicity and body-mass index (BMI; univariable analysis negative vs positive HR 0·99 [95% CI 0·78–1·24], p=0·90; multivariable analysis HR 0·96 [0·76–1·22], p=0·76). Similar results were noted when comparing distant disease-free survival between BRCA-positive and BRCA-negative groups (appendix p 12). Additionally, comparison of overall survival in BRCA-negative patients versus BRCA1 or BRCA2 carriers separately showed similar results (appendix pp 13–14).
Figure 2.
Overall survival for all patients (analysis population) by BRCA mutation status
(A) Kaplan-Meier plot and (B) forest plot of corresponding univariable and multivariable hazard ratios. In (B), multivariable analysis was adjusted for age, body-mass index (BMI; kg/m2), grade, tumour size, HER2 status, oestrogen-receptor status, ethnicity, and use of taxane chemotherapy. Groups without a reference were assessed as a continuous variable. The dashed line separates the univariable analysis (UVA) from the multivariable analysis (MVA). Oestrogen-receptor-positive group assessed at 2, 5, and 10 years because the hazard ratio associated with oestrogen-positive status varies with time.16 HR=hazard ratio. *Number of events (number of patients) from complete data obtained before multiple imputation.
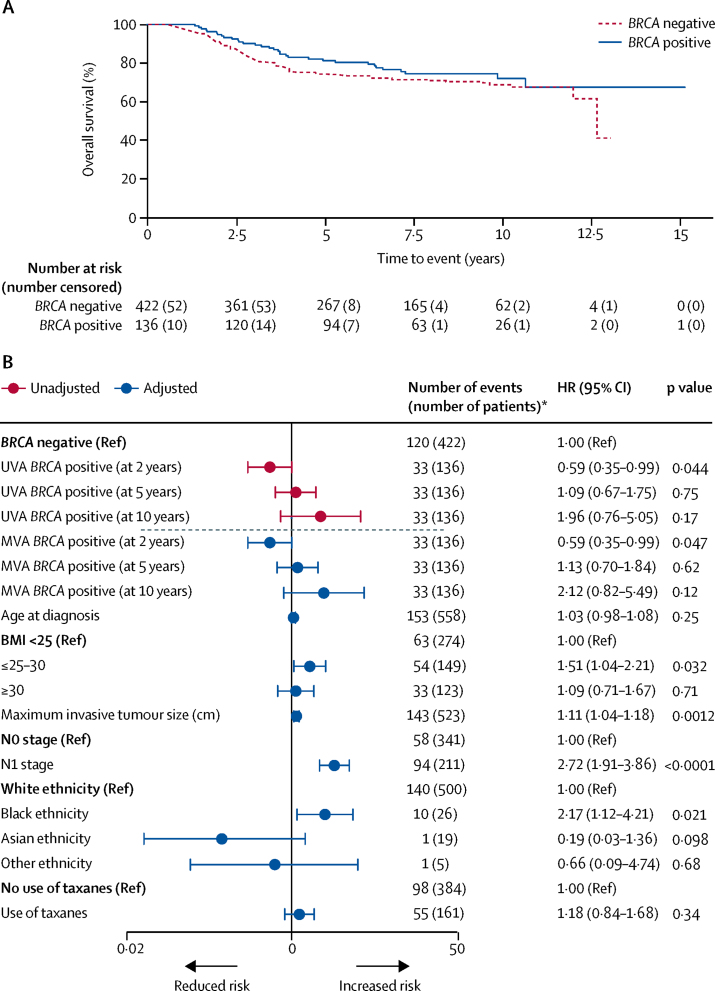
In the subgroup of 558 patients with triple-negative breast cancer, 159 (28%) women developed a distant recurrence, 153 (27%) died, and all deaths were due to breast cancer. The estimated hazard for death after diagnosis of triple-negative breast cancer varied over time (appendix p 32). In the triple-negative breast cancer subgroup, overall survival was significantly better at 2 years for BRCA-positive patients than for BRCA-negative patients (95% [95% CI 89–97]) vs 91% [88–94]; multivariable analysis flexible parametric survival model HR 0·59 [95% CI 0·35–0·99], p=0·047). Overall survival at 5 years was 81% (95% CI 73–87) versus 74% (70–78; multivariable analysis flexible parametric survival model HR 1·13 [95% CI 0·70–1·84], p=0·62); and at 10 years was 72% (62–80) versus 69% (63–74; multivariable analysis flexible parametric survival model HR 2·12 [95% CI 0·82–5·49], p=0·12; figure 3). For distant disease-free survival, however, the difference between BRCA-positive and BRCA-negative patients was not significant (appendix p 15). Inclusion of time from diagnosis to registration blood draw in multivariable analyses did not affect the results (appendix p 16). For analyses of both the overall population and the subgroup of patients with triple-negative breast cancer, results with imputation were almost identical to complete case results (appendix pp 9–10). Results from tests of proportional hazards are also in the appendix (p 17).
Figure 3.
Overall survival for all patients with triple-negative breast cancer* by BRCA mutation status
(A) Kaplan-Meier plot and (B) forest plot of corresponding univariable and multivariable hazard ratios. In (B), multivariable analysis was adjusted for age, body-mass index (BMI; kg/m2), grade, tumour size, HER2 status, oestrogen-receptor status, ethnicity, and use of taxane chemotherapy. Groups without a reference were assessed as a continuous variable. The dashed line separates the univariable analyses (UVA) from the multivariable analyses (MVA). HR=hazard ratio. *Number of events (number of patients) from complete data obtained before multiple imputation.
A post-hoc, multivariable sensitivity analysis of overall survival in patients with triple-negative breast cancer excluding 31 (6%) patients (21 BRCA-positive and ten BRCA-negative) who underwent bilateral mastectomy within the first year after diagnosis showed a significant difference in overall survival at 2 years for BRCA-positive versus BRCA-negative patients (95% [95% CI 89–98] vs 91% [88–94]; HR 0·52 [95% CI 0·29–0·91], p=0·023). However, there was no significant difference for 5-year overall survival (83% [95% CI 74–89] vs 74% [69–78]; HR 0·98 [95% CI 0·58–1·65], p=0·94; appendix p 18). We also repeated the primary analysis in patients with triple-negative breast cancer excluding 37 (7%) patients who developed a new primary breast or ovarian cancer. Overall survival at 10 years for BRCA-positive versus BRCA-negative patients was 78% (95% CI 69–85) versus 69% (64–74; HR 1·24 [95% CI 0·39–3·96], p=0·73; appendix p 19).
Discussion
The POSH prospective cohort study showed no significant difference in overall survival or distant disease-free survival between patients carrying a BRCA1 or BRCA2 mutation and patients without these mutations after a diagnosis of breast cancer. These results did not vary between unadjusted or adjusted analyses, including adjustments for ethnicity and BMI.21, 22 Following a diagnosis of early breast cancer, BRCA mutation carriers are frequently offered additional management options including bilateral mastectomy. Any prognostic implication of carrying a BRCA mutation for primary treatment is important to clarify to facilitate clinician and patient decisions around the optimum timing of additional surgery. Furthermore, clinical trials of treatments that are specifically targeted toward BRCA mutation carriers might need to take into account any effect of BRCA mutational status on primary treatment outcomes.
To our knowledge, this is the largest prospective study to report the prognostic implication of germline BRCA mutations and the only one with a preplanned analysis of patients presenting with triple-negative tumours. Our results are in broad agreement with more recent studies,8, 9, 10, 23 but others have reported conflicting results.24, 25, 26 Ascertainment biases introduced by retrospective and selective identification of cases, incomplete genetic testing, small numbers, absence of adjustments for clinical variables including treatment, and short follow-up probably explain many discrepancies, although some studies have generally used stronger methods.11, 12, 13, 14
The percentage of BRCA-positive patients in POSH (12%) was higher than anticipated from historical studies of patients diagnosed aged 40 years and younger, perhaps because of more sensitive mutation-testing options.1 However, only 14% of all patients had clinical genetic testing. The ratio of patients with BRCA1 to BRCA2 mutations was 1·5 to 1, which is similar to that reported in other large western population-based cohorts.2, 23 Deaths due to other malignancies were low in frequency in all groups reflecting the young age group; however, causes of deaths in patients who were BRCA1-positive included potentially preventable ovarian cancers at age 41–46 years. Bilateral risk-reducing mastectomy is not a necessary part of treating a unilateral breast cancer but unilateral mastectomy might enable breast radiotherapy to be omitted. Discussion about future primary cancer prevention during primary breast cancer treatment should take into account individual circumstances, including the likely tumour prognosis and the physical and psychological implications of more extensive surgery. In the POSH cohort, immediate bilateral mastectomy was not associated with improved survival, although the reported use of risk-reducing surgery was low; bilateral salpingo-oophorectomy was recorded in 32 patients and bilateral mastectomies in 107 patients.27 This probably reflects the low level of clinical testing at the time of the study. Although risk-reducing bilateral salpingo-oophorectomy is highly effective at reducing ovarian cancer incidence, the risk of primary peritoneal cancer is not reduced and studies indicate that the previously reported effect of this procedure on future breast cancer risk in BRCA1 and BRCA2 mutation carriers might have been overestimated because of uncorrected bias.28
Our analysis of the 558 patients with triple-negative breast cancer in our cohort showed an intriguing difference in overall survival over the first few years after diagnosis. BRCA mutation carriers were less likely to die from early breast cancer than non-carriers. This early survival advantage has also been observed among patients with ovarian cancer who are BRCA mutation carriers.29, 30 If real, this advantage might reflect greater sensitivity of BRCA-mutant breast cancers to chemotherapy or the greater visibility of BRCA-mutant cancers to host immune attack.31 One theory that could explain the slight survival advantage for BRCA mutation carriers not undergoing immediate bilateral mastectomy is that a major surgical intervention might compromise host immunity at a time when this is particularly important for eradicating micrometastases. This hypothesis would need further exploration due to the small number of patients in this subgroup.
Results from several published studies have suggested that the DNA repair deficiency associated with BRCA mutations results in enhanced sensitivity to many chemotherapy agents, particularly higher response rates to platinum-based drugs, have occurred in both metastatic and neoadjuvant settings.4, 7 Only 13 patients in our cohort were treated with platinum-based adjuvant regimens for early breast cancer, including one patient with a BRCA1 mutation and one with BRCA2.
Our study illustrates the high breast cancer mortality in this unscreened young population and the effect of known tumour and patient-prognostic characteristics on mortality. Inevitably, there have been substantial changes in the management of BRCA1 and BRCA2 mutation carriers since the recruitment period of this study, including the exploration in trials of systemic therapies that exploit BRCA-null tumours, including platinum-based drugs and PARP inhibitors. The association of BRCA mutations with improved early outcomes related to breast cancer in patients with triple-negative breast cancer has the potential to affect early results from clinical trials. As advanced genomic investigations increasingly become a part of routine oncological care, many patients with breast cancer now learn their BRCA mutation status close to the time of diagnosis. In many cancer centres, immediate or post-chemotherapy bilateral mastectomy has become an almost routine recommendation for BRCA1 and BRCA2 mutation carriers regardless of the size or focality of the presenting tumour. In the longer term, risk-reducing surgery, particularly for BRCA1 gene carriers is an appropriate management; in our analysis, the rising hazard for death in BRCA carriers over time was negated by removing from the analysis all patients who developed a second new primary breast or ovarian cancer during the follow-up period.
Clinicians need to consider short-term and long-term risks and benefits in discussing risk-reducing bilateral mastectomy with patients. The number of patients with triple-negative breast cancer who had immediate bilateral mastectomy in our cohort was small but our analysis suggests it is unlikely that the early bilateral mastectomy accounted for the early survival advantage in the BRCA mutation carriers with triple-negative breast cancer. With modern MRI-based breast screening, we conclude that patients who choose to delay additional surgery for 1 or 2 years until they are psychologically and physically recovered from their cancer treatment can be reassured that this choice is unlikely to lead to any substantial survival disadvantage. The importance of appropriately timed risk-reducing bilateral salpingo-oophorectomy, for BRCA1 mutation carriers in particular, is clear, but should take plans for further pregnancy into account. Furthermore, risk-reducing bilateral salpingo-oophorectomy in very young women will have negative health consequences as a result of oestrogen deprivation from an early age.
The strengths of the POSH study include the large cohort size, few missing data, and inclusion of patients with young-onset breast cancer, which led to a large number of BRCA1 and BRCA2 mutation carriers and a high number of events, ensuring that the study was well powered for the main outcome analysis. Our study minimised many of the biases present in other studies by recruiting patients within the first year after diagnosis from oncology clinics nationally to minimise survival and selection bias and by establishing BRCA mutation status for all patients included in the analysis. POSH participants recruited from England represented 23% of the available population during the recruitment period and comparison with cancer registry data confirmed that the POSH cohort is representative of the wider population.16 Comprehensive details of pathology enabled us to do a separate analysis of outcome in patients with triple-negative breast tumours; a unique contribution to this field. We have previously reported the significant and independent prognostic effects of obesity and ethnicity on long-term outcomes in this young patient group, and this study is the only prospective study to date to include these host factors in multivariable analyses.21, 22
Limitations of this study included the non-universal use of multiplex ligation probe analysis; we therefore cannot exclude the possibility that some structural BRCA variants were not identified. However, even clinical diagnostic mutation testing is not 100% sensitive because of occult mutations not amenable to current methods (eg, deep intronic splice variants); the investigation of BRCA1 and BRCA2 gene sequences in this cohort was more comprehensive than in most other publications. All participants were tested for TP53 mutations and carriers were excluded from this analysis because of the high risk of non-breast malignancies. We acknowledge that other breast cancer susceptibility gene variants were not excluded; however, these were expected to be very low in frequency or low penetrance, and there is no evidence that they specifically affect prognosis. We had national outcome data up to a median 8·2 years. The treatments given reflected modern oncological practice with almost 90% of patients receiving neoadjuvant or adjuvant chemotherapy; in more than 95% of cases this was an anthracycline or anthracycline plus taxane combination regimen.
Other limitations of this study included restricting the main cohort to patients aged 40 years or younger at the time of diagnosis to enrich for BRCA mutation carriers. It is possible that observations in young-onset breast cancer patients might not translate to older ages at diagnosis. Progesterone-receptor testing was not done routinely in many UK centres during the period of recruitment and supplementary data were derived from tissue microarrays rather than full tumour sections. The relevance of triple-negative breast cancer in terms of biology and treatment has only become apparent since the POSH study was designed, so the study was not powered for this as the primary outcome; notably, the only difference in overall survival in this study was seen between mutation carriers and non-carriers in this subgroup. Recommendations for adjuvant treatment in the UK changed over the course of recruitment, with taxanes being recommended for node-positive disease from 2006 and adjuvant trastuzumab for HER2-positive breast cancer routinely available only from 2006. Although we specifically collected information at 5 years about risk-reducing surgery, we cannot exclude the possibility that risk-reducing mastectomy and oophorectomy might have been done at different hospitals from the recruiting cancer centre (eg, at specialist plastic surgery or gynaecological units).
This study confirmed that patients diagnosed with invasive breast cancer aged 18–40 years have a high breast-cancer-specific mortality, and a high proportion are BRCA1 and BRCA2 mutation carriers. We found no clear evidence that either BRCA1 or BRCA2 germline mutations significantly affect overall survival with breast cancer after adjusting for known prognostic factors. Decisions about timing of risk-reducing surgery should take into account primary tumour prognosis and patient preference. BRCA mutation carriers presenting with triple-negative breast cancer might have an improved survival during the first few years after diagnosis compared with non-carriers, although immediate bilateral mastectomy did not account for this advantage. Finally, analysis of early outcome data from trials exploring BRCA-deficient tumour treatment in patients with triple-negative breast cancer should be interpreted with caution in view of the possible early survival advantage for BRCA mutation carriers.
For more about the POSH study see http://www.southampton.ac.uk/medicine/research/posh.page
For the BOADICEA algorithm see http://ccge.medschl.cam.ac.uk/boadicea/
Acknowledgments
Acknowledgments
We are grateful to all the patients, clinicians, research staff at the National Cancer Research Institute Clinical Research Network, and the POSH research team who made this study possible. Funding over 18 years has been provided by the Wessex Cancer Trust, Cancer Research UK (C1275/A7572, C22524, A11699, A19187), and Breast Cancer Now (2005Nov53). Sample handling was facilitated by Southampton CRUK Centre tissue bank and Southampton University Faculty of Medicine DNA bank (Southampton, UK) and the Barts Cancer Research Centre (London, UK). DNA sequencing for the whole cohort took place in the Strangeways research laboratories (Cambridge, UK), and validation Sanger sequencing and multiplex ligation-dependent probe amplification was done in the Wessex Regional Genetics Laboratories (Wessex, UK). IT support, histopathology image storage, and reporting software were developed and supported by the University of Southampton Clinical Informatics Support team.
Contributors
The study was conceived and designed by DME, PS, and DGA, and planned and executed by DME, DGA, PS, DGE, AMT, PP, LJ, HH, SL, RE, AH, FJG, and SH. Data acquisition, management and curation was done by SG, LTD, ERC, TCM, WJT, RIC, SG-H, BE, LS, and DME. LJ was responsible for central pathology review, and AMD and DFE supervised the final research DNA sequencing. The statistical analysis plan was prepared by TCM, DGA, DME, ERC, and RIC. TCM did the statistical analysis and prepared the figures. DME, ERC, TCM, DGA, and RIC interpreted the data and ERC, TCM, and DME wrote the manuscript. All authors critically reviewed iterations of the manuscript and approved the final draft for submission.
Declaration of interests
ERC declares honoraria from Roche. RIC declares honoraria from GSK and Pfizer. DME declares honoraria from AstraZeneca and Pierre Fabre. All other authors declare no competing interests.
Supplementary Material
References
- 1.Anglian Breast Cancer Study Group Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;83:1301–1308. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malone KE, Daling JR, Doody DR. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 3.Anders CK, Hsu DS, Broadwater G. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 4.Turner NC, Tutt AN. Platinum chemotherapy for BRCA1-related breast cancer: do we need more evidence? Breast Cancer Res. 2012;14:115. doi: 10.1186/bcr3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakhani SR, Jacquemier J, Sloane JP. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90:1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 6.Mavaddat N, Barrowdale D, Andrulis IL. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomarkers Prev. 2012;21:134–147. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrski T, Gronwald J, Huzarski T. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;14:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 8.Rennert G, Bisland-Naggan S, Barnett-Griness O. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 9.Verhoog LC, Brekelmans CT, Seynaeve C. Survival and tumour characteristics of breast cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–321. doi: 10.1016/s0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 10.Huzarski T, Byrski T, Gronwald J. Ten year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013;31:3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 11.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119:13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 12.van den Broek AJ, Schmidt MK, van 't Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PLoS One. 2015;10:e0120189. doi: 10.1371/journal.pone.0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt MK, Van den Broek AJ, Tollenaar RA. Breast cancer survival of BRCA1/2 mutation carriers in a hospital based cohort of young women. JNCI. 2017;109:djw329. doi: 10.1093/jnci/djw329. [DOI] [PubMed] [Google Scholar]
- 15.Eccles D, Gerty S, Simmonds P, Hammond V, Ennis S, Altman DG. Prospective study of outcomes in sporadic versus hereditary breast cancer (POSH): study protocol. BMC Cancer. 2007;7:160. doi: 10.1186/1471-2407-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copson E, Eccles B, Maishman T. Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. JNCI. 2013;105:978–988. doi: 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou AC, Cunningham AP, Peto J ED. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence . Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. 2013. https://www.nice.org.uk/guidance/cg164 (accessed July 22, 2016) [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observationalstudies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. [Google Scholar]
- 21.Copson E, Maishman T, Gerty S. Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer. 2014;110:230–241. doi: 10.1038/bjc.2013.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copson ER, Cutress RI, Maishman T. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. Ann Oncol. 2015;26:101–112. doi: 10.1093/annonc/mdu509. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin PJ, Phillips KA, West DW. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012;30:19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 24.Robson M, Chappuis PO, Satagopan J. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6:R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoppa-Lyonnet D, Ansquer Y, Dreyfus H. Familial invasive breast cancers: worse outcome related to BRCA1 mutations. J Clin Oncol. 2000;18:4053–4059. doi: 10.1200/JCO.2000.18.24.4053. [DOI] [PubMed] [Google Scholar]
- 26.Moller P, Evans DG, Reis MM. Surveillance for familial breast cancer: differences in outcome according to BRCA mutation status. Int J Cancer. 2007;121:1017–1020. doi: 10.1002/ijc.22789. [DOI] [PubMed] [Google Scholar]
- 27.Maishman T, Cutress RI, Hernandez A. Local recurrence and breast oncological surgery in young women with breast cancer: the POSH observational cohort study. Ann Surg. 2017;266:165–172. doi: 10.1097/SLA.0000000000001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heemskerk-Gerritsen BA, Seynaeve C, Van Asperen CJ. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. JNCI. 2015;107:djv033. doi: 10.1093/jnci/djv033. [DOI] [PubMed] [Google Scholar]
- 29.Bolton KL, Chenevix-Trench G, Goh C. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–390. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candido Dos Reis FJ, Song H, Goode EL. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–657. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang T, Shi W, Wali VB. Predictors of chemosensitivity in triple negative breast cancer: an integrated genomic analysis. PLoS Med. 2016;13:e1002193. doi: 10.1371/journal.pmed.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.