Summary
Background
Data on carcinogenicity of human papillomavirus (HPV) types in the anus are needed to inform anal cancer prevention through vaccination and screening. This is particularly the case for people infected with HIV, who are at an increased risk of anal cancer.
Methods
We did a systematic review of studies published from January, 1986, to July, 2017, in MEDLINE, Embase, and the Cochrane Library on anal HPV infection, without any language restrictions. Eligible studies reported type-specific HPV prevalence by strata of cytopathological or histopathological anal diagnosis, sex, and HIV status. Data requests were made to authors when necessary. We did a meta-analysis of type-specific HPV prevalence across the full spectrum of anal diagnoses, from normal cytology to anal cancer. We assessed the main outcome of type-specific HPV prevalence ratios [PR], calculated across strata of anal diagnoses, gender, or HIV status, by use of generalised linear models.
Findings
95 studies were identified from the search, published between 1992–2017, from which 18 646 individuals fulfilled the criteria for inclusion in the analyses: 8534 people with normal cytology, 5730 with low-grade lesions, 2024 with high-grade lesions, and 2358 with anal cancer. HPV prevalence varied in normal cytology from 42% in HIV-negative women to 76% in HIV-positive men and, for each diagnosis, was higher in individuals who were HIV positive than those who were HIV negative. HPV16 positivity increased with diagnosis severity, being the only HPV type accounting for more HPV infection in anal cancer than normal cytology, both in individuals who were HIV negative (PR 5·0, 95% CI 3·8–6·6, p<0·0001) and those who were HIV positive (2·3, 1·9–2·7, p<0·0001). HPV16 positivity increased even between high-grade lesions and anal cancer, whereas other high-risk HPV types accounted for high proportions of low-grade or high-grade lesions but their prevalence decreased in anal cancer. However, HPV16 was less frequent in HIV-positive than HIV-negative anal cancer, both in men (PR 0·8, 95% CI 0·7–0·9, p<0·0001) and women (0·8, 0·6–1·0, p=0·063), and in HIV-positive versus HIV-negative high-grade lesions in women (0·6, 0·5–0·9, p=0·0077). Type-specific attribution of the non-HPV16 fraction of HIV-positive anal cancer is hindered by a high prevalence of multiple HPV infections.
Interpretation
HPV16 is by far the most carcinogenic HPV type in the anus, with enrichment of HPV16 even from high-grade lesions to anal cancer, both in individuals who are HIV negative and those who are HIV positive. Nevertheless, the fraction of anal cancer attributable to HPV16 is smaller in the HIV-positive population.
Funding
International Agency for Research on Cancer.
Introduction
Anal cancer accounts for approximately 20 000 new cancer cases globally each year, distributed similarly by sex,1 of which nearly 90% have been estimated to be caused by human papillomavirus (HPV).2 Like other HPV-related cancers, anal cancer risk is increased by sexual exposure to HPV, most notably via anal intercourse and by HIV-related immunosuppression and, more weakly, by tobacco smoking.3 In high-income countries, incidence of anal cancer has greatly increased in people who are HIV-positive since the advent of combined antiretroviral therapy (ART) and subsequent increases in life expectancy.4, 5, 6 Consistent with very high prevalence of anal HPV infection,7 HIV-positive men who have sex with men (MSM) have the highest risk of developing anal cancer, which is higher even than in HIV-negative MSM.7, 8 HIV-positive women and HIV-positive men who have sex with women (MSW) also have a higher incidence of anal cancer than that seen in the general population.9
Research in context.
Evidence before this study
We searched MEDLINE, Embase, and the Cochrane Library for the terms “papillomaviridae”, “papillomavirus” or “HPV”, plus “anal canal”, “anus”, or “anal” for studies published between January, 1986, and July, 2017, without language restrictions. Large meta-analyses of type-specific prevalence across the full spectrum of human papillomavirus (HPV)-related disease have been used to characterise carcinogenicity of individual HPV types in the cervix, both in women who are HIV negative and those who are HIV positive. However, to the best of our knowledge, a similar comprehensive exercise has not yet been done for the anus.
Type-specific prevalence of HPV in anal cancer and anal intraepithelial neoplasia was the subject of a meta-analysis in 2009, including 955 invasive anal cancers (none of which were known to be HIV positive) and 1280 intraepithelial lesions, and contributed to a review of the evidence on the link between individual HPV types and cancer by an expert working group of the International Agency for Research on Cancer (IARC) in the same year. In 2012, another meta-analysis reported on HPV16 prevalence among men who have sex with men (MSM), of whom 2687 were HIV negative and 1371 were HIV positive, but did not report stratification by anal diagnosis.
Added value of this study
To our knowledge, this Article is the first to report the relative importance of different high-risk HPV types across the complete carcinogenic process from anal infection to cancer and stratified by HIV status. We have included data from 18 646 men and women, and benefitted from widespread sharing of data from the original study authors. This Article, therefore, informs on the natural history of individual types in populations at varying anal cancer risk, including, most notably, men who are HIV positive (predominantly MSM) and women who are at the highest risk of anal cancer.
Implications of all the available evidence
Although there are some differences between HIV-negative and HIV-positive populations, our findings establish HPV16 as a priority target for the prevention of anal cancer through vaccination and possibly screening in high-risk groups.
Although there are many similarities, the natural history of HPV infection in the anus is less well understood than in the cervix, on account of the rarity of anal cancer and the low availability of studies and screening for anal cancer.10 A stronger predominance of HPV16 in HPV-positive anal cancer (around 85%)2 than in cervical cancer (around 55%) is well established but, so far, no large meta-analyses exist on the distribution of HPV types in the anus in individuals with and without anal cancer. There is also little prospective information on the evolution of anal HPV infection to lesion by HPV type.11, 12 It is particularly unclear whether HIV-induced immunosuppression differentially affects individual HPV types and their potential to induce neoplastic changes in the anus, as it has been reported for the cervix uteri.13, 14
To improve our understanding of the complete carcinogenic process of high-risk HPV types in the anus, we have done a systematic review of the literature and a meta-analysis of HPV type distribution across the full spectrum of cytopathological and histopathological anal diagnoses from normal cytology to cancer in 18 646 individuals, of whom more than half were HIV positive.
Methods
Search strategy and criteria
We did a systematic literature review and meta-analysis of HPV type-specific prevalence, without language restrictions. We searched MEDLINE, Embase, and the Cochrane Library for studies published between January, 1986, and July, 2017, using the terms “papillomaviridae”, “papillomavirus”, or “HPV”, plus “anal canal”, “anus”, or “anal” (appendix p 3). References cited in retrieved articles and the abstracts from relevant conferences were also assessed. Studies were reviewed in parallel by authors CL and GMC. Eligible studies (of any study design) had to use broad-spectrum PCR-based assays to detect overall HPV DNA; report type-specific HPV prevalence for at least HPV16, by strata of cytopathological or histopathological anal diagnosis and sex; and stratify findings by HIV status (with the exception of anal cancer, for which we accepted HIV-unknown cases). Data on the source of HPV DNA (exfoliated cells vs biopsies or tissues) and PCR primers were also extracted. Whenever available, data in men were stratified by male sexual orientation. If a publication suggested that additional information was available on HPV types or more detailed stratification by anal diagnosis, HIV status, sex, or sexual orientation, data requests were made to the study authors.
Data analysis
Cases were first classified into nine grades of anal diagnosis, similar to previous meta-analyses in the cervix,14, 15 namely participants diagnosed by cytology as normal; atypical squamous cells of undetermined significance (ASCUS); low-grade squamous intraepithelial lesion (SIL); atypical squamous cells but that cannot exclude high-grade SIL (ASC-H); or high-grade SIL; and those diagnosed by histology as anal intraepithelial neoplasia (AIN) grade 1, AIN grade 2, AIN grade 3, or anal cancer, which refers to squamous cell carcinoma (SCC) or anal cancer of other or unspecified histology. Adeno-carcinomas are rare and their cause is not well understood.16 We excluded adeno-carcinomas whenever possible, to avoid misclassification with rectal carcinoma. To retain appropriate sample sizes in each grade, anal diagnoses were further collapsed into four categories: normal, including normal cytology only; low-grade cytology or histology diagnoses, encompassing ASCUS, low-grade SIL and AIN grade 1; high-grade cytology or histology diagnoses, including high-grade SIL, ASC-H, AIN grade 2, and AIN grade 3; and anal cancer.
Overall HPV DNA prevalence (any type) is reported as the percentage of all included patients tested by PCR as the denominator. Conversely, type-specific HPV positivity is presented as the proportion of HPV-positive cases in which a particular HPV type was detected, and only for the 13 HPV types judged to be carcinogenic or probably carcinogenic (ie, HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68),17 hereafter referred to as high-risk HPV types, and for HPV6 and 11. Type-specific positivity was estimated only in studies that both genotyped and reported the prevalence of the HPV type in question, and thus denominators vary by type. Each HPV type was assessed independently—ie, type-specific positivity includes that contributed by multiple HPV infections.
In subsets of studies with the availability of relevant individual-level data to separate out types in multiple infections, we also estimated the combined prevalence of HPV31/33/45/52/58 (ie, that of any of the five high-risk HPV types included in the nine-valent HPV vaccine in addition to HPV16 and 18) for all anal diagnoses; and compared the prevalence of HPV types in HPV-positive anal cancer by HIV status, distinguishing whether a type, or selected combinations of types, had been found in the absence or presence of any other HPV types.
We calculated prevalence ratios and corresponding 95% CI as in previous similar meta-analyses14, 15 using generalised linear models. We calculated prevalence ratios assuming the non-independence of cases within the same study using cluster-correlated robust variance estimates18 and we used them to compare, in individuals who were HPV positive only, HPV16, 18, and combined HPV31/33/45/52/58 positivity within each strata of anal diagnosis by HIV-status separately for men and women; HPV type-specific positivity in anal cancer versus normal cytology (cancer/normal PRs) separately for HIV-negative and HIV-positive anal cancer, for both men and women, adjusted for sex and region; and HPV type-specific positivity in high-grade lesions in men who are HIV positive by whether HPV was tested from cells or from biopsies or tissue samples. Publication bias and heterogeneity by study size was assessed by funnel plots (appendix pp 22–23) of HPV16 prevalence in individuals positive for HPV, in each of 16 strata of anal diagnosis, HIV status, and sex, using Beggs test for asymmetry by sample size. All statistical analyses were two-sided and were done in STATA version 14.
Role of the funding source
The sponsor had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
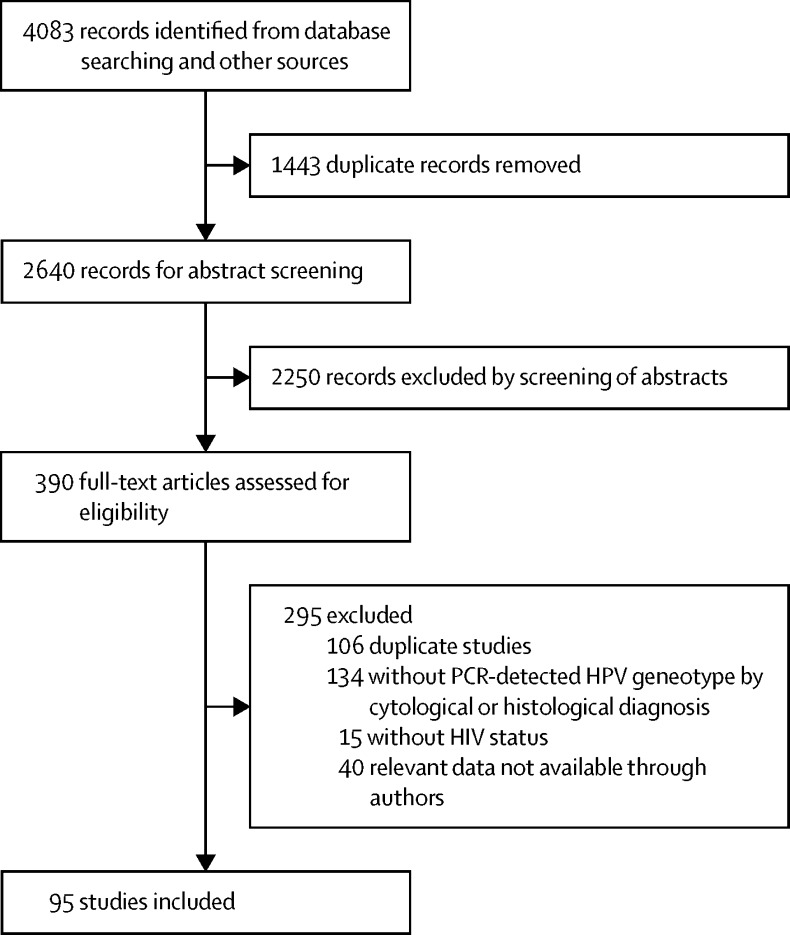
95 studies, including a total of 18 646 cases, met inclusion criteria (figure 1). Most cases came from North America (n=6985) and Europe (n=6267; table 1, appendix pp 6–11). 8534 (46%) of 18 646 cases had normal cytology, 5730 (31%) had low-grade lesions, 2024 (11%) had high-grade lesions, and 2358 (13%) had anal cancer (table 1). 12 850 (69%) of identified cases were in men, of whom most were known to be HIV positive, MSM, or both, except in anal cancer. Of note, for both sexes, most anal cancer cases (78%) were contributed by those of unknown HIV status, which are hereafter shown combined with HIV-negative anal cancer.
Figure 1.
Study selection
Table 1.
Included studies and participants by region, sex, HIV status, and anal diagnosis
| Number of studies (n=95)* | Normal diagnosis (n=8534) | Low grade diagnosis†(n=5730) | High grade diagnosis‡(n=2024) | Cancer (n=2358) | Total (n=18 646) | |||
|---|---|---|---|---|---|---|---|---|
| Region | ||||||||
| North America | 29 (31%) | 3242 (38%) | 2313 (40%) | 767 (38%) | 663 (28%) | 6985 (37%) | ||
| Europe | 44 (46%) | 2301 (27%) | 2056 (36%) | 586 (29%) | 1324 (56%) | 6267 (34%) | ||
| Latin America | 11 (12%) | 1790 (21%) | 674 (12%) | 320 (16%) | 225 (10%) | 3009 (16%) | ||
| Asia and Oceania | 13 (14%) | 1150 (13%) | 562 (10%) | 332 (16%) | 123 (5%) | 2167 (12%) | ||
| Africa | 3 (3%) | 51 (1%) | 125 (2%) | 19 (1%) | 23 (1%) | 218 (1%) | ||
| Sex and HIV status | ||||||||
| Men | 79 (83%) | 5446 (64%) | 4763 (83%) | 1599 (79%) | 1042 (44%) | 12 850 (69%) | ||
| HIV negative | 29 (31%) | 1695 (20%) | 902 (16%) | 339 (17%) | 144 (6%) | 3080 (17%) | ||
| Known MSM | 17 (18%) | 1565 (18%) | 843 (15%) | 310 (15%) | 1 (<1%) | 2719 (15%) | ||
| HIV unknown | 18 (19%) | ·· | ·· | ·· | 759 (32%) | 759 (4%) | ||
| Known MSM | 0 | ·· | ·· | ·· | 0 | 0 | ||
| HIV positive | 59 (62%) | 3751 (44%) | 3861 (67%) | 1260 (62%) | 139 (6%) | 9011 (48%) | ||
| Known MSM | 42 (44%) | 3202 (38%) | 3474 (61%) | 1114 (55%) | 17 (1%) | 7807 (42%) | ||
| Women | 55 (58%) | 3088 (36%) | 967 (17%) | 425 (21%) | 1316 (56%) | 5796 (31%) | ||
| HIV negative | 29 (31%) | 1764 (21%) | 323 (6%) | 194 (10%) | 226 (10%) | 2507 (13%) | ||
| HIV unknown | 19 (20%) | ·· | ·· | ·· | 1077 (46%) | 1077 (6%) | ||
| HIV positive | 23 (24%) | 1324 (16%) | 644 (11%) | 231 (11%) | 13 (1%) | 2212 (12%) | ||
MSM=men who have sex with men.
Individual categories sum to more than the total due to some studies including patients from more than one region, gender, or HIV status.
Low-grade diagnosis is defined as atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 1.
High-grade diagnosis is defined as high-grade squamous intraepithelial lesion, atypical squamous cells but cannot exclude high-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 2 or 3.
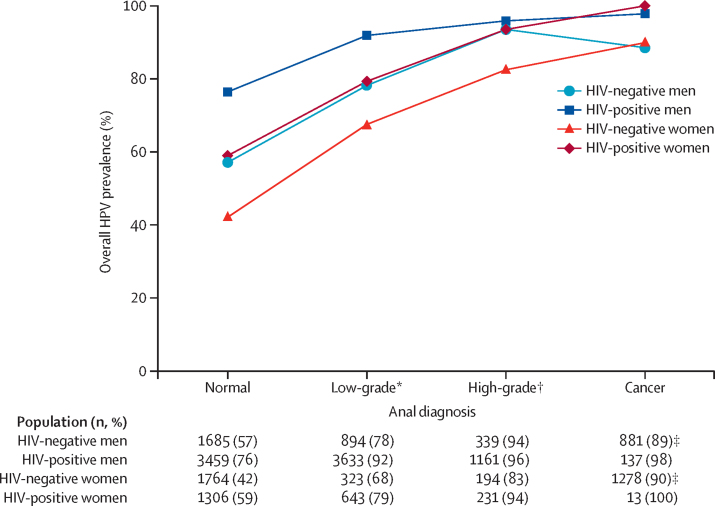
Overall HPV prevalence by sex and HIV status in four anal diagnoses are shown in figure 2. HPV prevalence varied widely in normal-cytology from 42% in HIV-negative women to 76% in HIV-positive men, but consistently increased with diagnosis severity. HPV prevalence in patients with anal cancer reached 80% or greater in all groups, although remained higher in HIV-positive (100%) than in HIV-negative (90%) anal cancer, irrespective of sex. Patterns were consistent in a sensitivity analysis using known HIV-negative cancer cases only instead of the combination of HIV-negative and HIV-unknown cases (appendix p 15). Of note, overall HPV prevalence in the 99 HIV-negative men that were known to have sex with women was 6%.
Figure 2.
Overall HPV prevalence by sex, anal diagnosis and HIV status
HPV=human papillomavirus. *Low-grade diagnosis is defined as atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 1. †High-grade diagnosis is defined as high-grade squamous intraepithelial lesion, atypical squamous cells but cannot exclude high-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 2 or 3. ‡Anal cancers of unknown HIV status are assumed to be HIV negative (see appendix p 15 for sensitivity analysis including known HIV-negative anal cancers only).
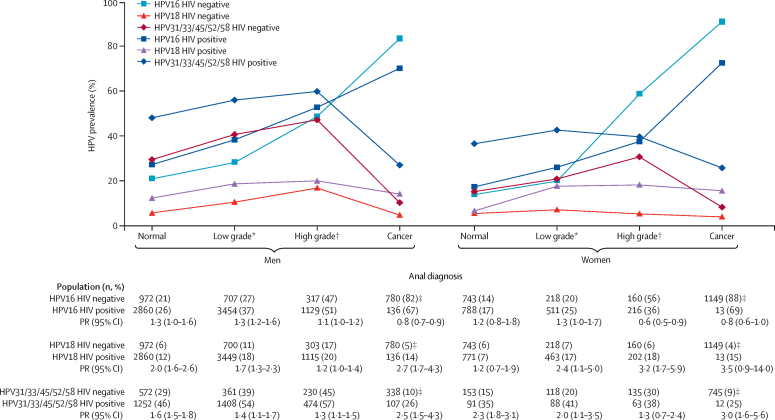
Figure 3 shows the positivity of HPV16, HPV18, and HPV31/33/45/52/58, for HIV-positive and HIV-negative and corresponding prevalence ratios by HIV status, for each of four anal diagnoses, separately for HPV-positive men and women. HPV16 positivity increased with the severity of anal diagnosis both in HIV-negative and HIV-positive men. HPV18 positivity was relatively stable, whereas HPV31/33/45/52/58 positivity was high in normal and intermediate diagnoses, but fell in anal cancer. For almost every combination of HPV type and anal diagnosis, HPV positivity was higher in HIV-positive than HIV-negative men, with the largest difference being for HPV18 (2·7, 95% CI 1·7–4·3, p<0·0001) and combined HPV31, 33, 45, 52, and 58 (2·5, 95% CI 1·5–4·3, p=0·0008) in anal cancer. The notable exception to this rule, however, was a significant under-representation of HPV16 in HIV-positive versus HIV-negative anal cancer (91 [67%] of 136 vs 638 [82%] of 780; prevalence ratio 0·8, 95% CI 0·7–0·9, p<0·0001). Consistent patterns of HPV16, HPV18, and HPV31/33/45/52/58 by anal diagnosis were also seen in sensitivity analyses for HPV-positive men in North America and Europe only (appendix pp 18–19).
Figure 3.
Prevalence of HPV16, HPV18, and HPV31/33/45/52/58 and prevalence ratios by anal diagnosis and HIV status, in HPV-positive men and women
HPV=human papillomavirus. PR=prevalence ratio. *Low-grade diagnosis is defined as atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 1. †High-grade diagnosis is defined as high-grade squamous intraepithelial lesion, atypical squamous cells but cannot exclude high-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 2 or 3. ‡Anal cancers of unknown HIV status are assumed to be HIV negative (see appendix p 16 for sensitivity analysis including known HIV-negative anal cancers only). §PR comparisons are HIV positive versus HIV negative.
In agreement with findings in men, HPV16 positivity increased with lesion severity in both HIV-negative and HIV-positive women, whereas HPV18 positivity was relatively stable and HPV31/33/45/52/58 positivity fell in anal cancer from intermediate diagnoses. HPV positivity was higher in HIV-positive than HIV-negative women for most combinations of HPV type and anal diagnosis, with the largest differences being for HPV18 (prevalence ratio 3·2, 95% CI 1·7–5·9, p=0·0003) in high-grade lesions and HPV31/33/45/52/58 (3·0, 95% CI 1·6–5·6, p=0·0009) in cancer. Again, an under-representation of HPV16 in nine (69%) of 13 HIV-positive women versus 1012 (88%) of 1149 HIV-negative women with anal cancer (0·8, 95% CI 0·6–1·0, p=0·063), which was an exception to this rule. By contrast with men, however, HPV16 was also significantly under-represented in high-grade lesions in women (0·6, 95% CI 0·5–0·9, p=0·0077).
For both men and women, consistent patterns of prevalence of HPV16, HPV18, and HPV31/33/45/52/58 by anal diagnosis were seen in sensitivity analyses in which HIV negative or unknown HIV status cancer cases were replaced by known HIV-negative cases only (appendix p 16). Patterns of HPV16, HPV18, and HPV31/33/45/52/58 positivity according to nine cytohistological diagnoses (appendix pp 20–21) as well as individual HPV types by four anal diagnoses are also shown in appendix (pp 13–14), for men and women separately. Finally, there was no evidence of heterogeneity in HPV16 prevalence by study sample size in any strata of anal diagnosis, sex, and HIV status (appendix pp 22–23).
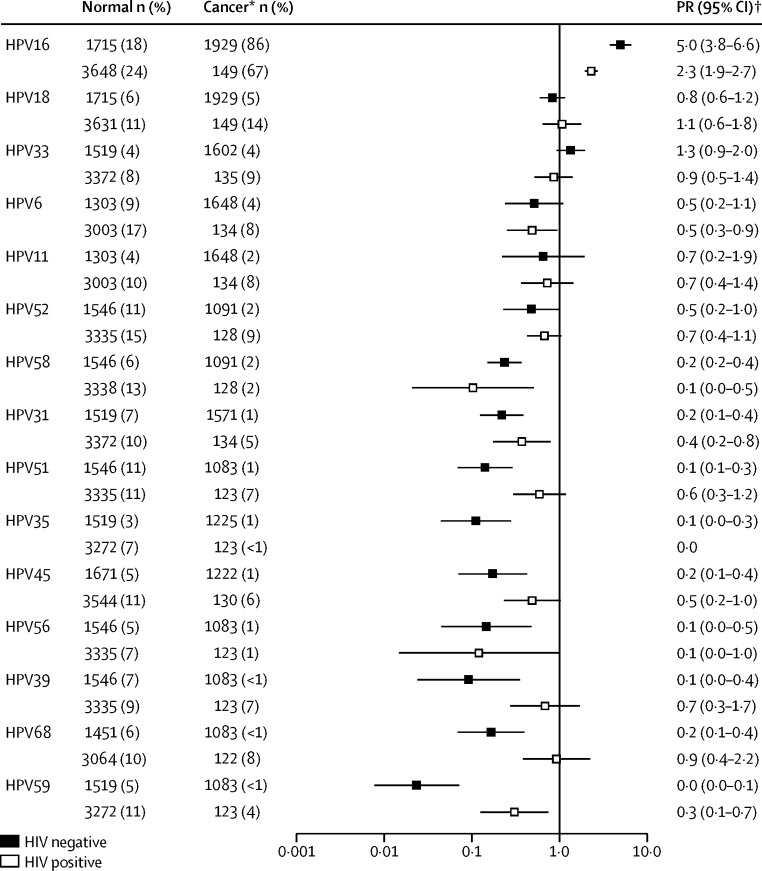
Figure 4 shows region and sex-adjusted cancer/normal PRs in HPV-positive men and women by HIV status. HPV types are shown in descending order of positivity in HIV-negative anal cancer. In the HIV-negative population, HPV16 was the only HPV type that was significantly more frequent in cancer than normal cytology (prevalence ratio 5·0, 95% CI 3·8–6·6, p<0·0001). The next most common HPV types in HIV-negative anal cancer were HPV18 (prevalence ratio 0·8, 0·6–1·2) and HPV33 (prevalence ratio 1·3, 0·9–2·0). Prevalence ratios for other types of HPV in HIV-negative anal cancer ranged from 0·7 for HPV11 to 0·1 or lower for HPV types 35, 39, 51, 56, and 59. HPV16 was also the only type of HPV more frequent in cancer than normal cytology in the HIV-positive population (2·3, 1·9–2·7, p<0·0001). Prevalence ratios tended to be similar between HIV-positive individuals and HIV-negative individuals.
Figure 4.
Cancer/normal prevalence ratios in HPV-positive samples by HPV type and HIV status
HPV=human papillomavirus. PR=prevalence ratio. *Anal cancers of unknown HIV status are assumed to be HIV negative (see appendix p 17 for sensitivity analysis including known HIV-negative anal cancers only). †Adjusted by sex and region.
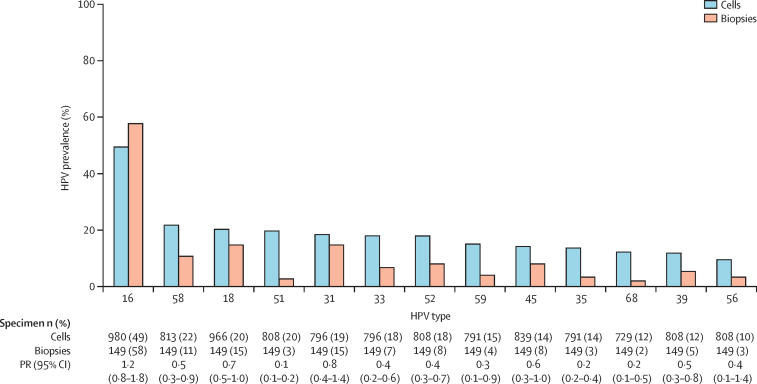
Comparisons of HPV prevalence in HIV-positive high-grade SIL tested from cells (37 studies) or biopsies (six studies) are shown in figure 5 for 13 high-risk HPV types, in decreasing order of detection in cells. HPV16 prevalence was not significantly different between biopsies and cells, whereas the prevalence of most other high-risk HPV types (types 51, 35, and 68) was five times lower in biopsies than in cells. However, even in biopsies, the sum of the 13 type-specific estimates was about 140%, highlighting a remaining issue of multiple infections.
Figure 5.
Prevalence of high-risk HPV types by type of sample in HIV-positive men with HPV-positive high-grade anal lesions
High-grade diagnosis is defined as high-grade squamous intraepithelial lesion, atypical squamous cells but cannot exclude high-grade squamous intraepithelial lesion, or anal intraepithelial neoplasia grade 2 or 3. HPV=human papillomavirus. PR=prevalence ratio.
In a subset of 20 studies of HIV-positive anal cancer and 27 studies of HIV-negative anal cancer, it was possible to distinguish HPV types by the absence or presence of another HPV type (table 2). Multiple HPV infections were much more frequent in HIV-positive than HIV-negative anal cancer (42% vs 10%), and for each individual HPV type (table 2). In HIV-negative or HIV-unknown anal cancer, HPV16 was found ten times more frequently in single infections (78%) than in multiple infections (8%). Conversely, in HIV-positive anal cancer, HPV16 was only twice as likely to be found in single infections (44%) than in multiple (23%) infections, and the total HPV16 prevalence in HIV-positive anal cancer was lower than the prevalence of single HPV16 infection in HIV-negative anal cancer (67% vs 78%; p=0·0037). Most other HPV types were substantially more frequent in HIV-positive than in HIV-negative anal cancer, mainly on account of multiple infections.
Table 2.
Number and prevalence of single and multiple infections of HPV types in HPV-positive anal cancer by HIV status
|
HIV-negative/unknown* |
HIV-positive |
|||||
|---|---|---|---|---|---|---|
| Overall prevalence | Single | Multiple | Overall prevalence | Single | Multiple | |
| HPV16 | 1333/1554 (86%) | 1216 (78%) | 117 (8%) | 96/144 (67%) | 63 (44%) | 33 (23%) |
| HPV18 | 66/1554 (4%) | 37 (2%) | 29 (2%) | 21/144 (15%) | 3 (2%) | 18 (13%) |
| HPV33 | 44/1369 (3%) | 32 (2%) | 12 (1%) | 12/130 (9%) | 3 (2%) | 9 (7%) |
| HPV6 | 54/1415 (4%) | 29 (2%) | 25 (2%) | 8/124 (6%) | 3 (2%) | 5 (4%) |
| HPV58 | 23/1198 (2%) | 18 (2%) | 5 (<1%) | 1/123 (1%) | 0 | 1 (1%) |
| HPV35 | 12/1332 (1%) | 11 (1%) | 1 (<1%) | 0/123 | 0 | 0 |
| HPV31 | 19/1338 (1%) | 11 (1%) | 8 (1%) | 6/129 (5%) | 2 (2%) | 4 (3%) |
| HPV52 | 21/1198 (2%) | 9 (1%) | 12 (1%) | 12/123 (10%) | 2 (2%) | 10 (8%) |
| HPV11 | 37/1415 (3%) | 10 (1%) | 27 (2%) | 10/124 (8%) | 1 (1%) | 9 (7%) |
| HPV45 | 10/1329 (1%) | 6 (1%) | 4 (<1%) | 8/125 (6%) | 3 (2%) | 5 (4%) |
| HPV56 | 6/1190 (1%) | 4 (<1%) | 2 (<1%) | 1/123 (1%) | 0 | 1 (1%) |
| HPV39 | 7/1190 (1%) | 2 (<1%) | 5 (<1%) | 8/123 (7%) | 1 (1%) | 7 (6%) |
| HPV68 | 4/1190 (<1%) | 2 (<1%) | 2 (<1%) | 10/123 (8%) | 0 | 10 (8%) |
| HPV59 | 2/1190 (<1%) | 1 (<1%) | 1 (<1%) | 5/123 (4%) | 0 | 5 (4%) |
| HPV51 | 11/1190 (1%) | 0 | 11 (1%) | 8/123 (7%) | 1 (1%) | 7 (6%) |
| Any HPV | 1424/1430 (>99%) | 1279 (89%) | 145 (10%) | 128/130 (98%) | 74 (57%) | 54 (42%) |
| HPV16/18 | 552/629 (88%) | 499 (79%)† | 53 (8%)‡ | 87/118 (74%) | 58 (49%)† | 29 (25%)‡ |
| HPV6/11/16/18 | 579/629 (92%) | 541 (86%)† | 38 (6%)‡ | 91/118 (77%) | 66 (56%)† | 25 (21%)‡ |
| HPV6/11/16/18/31/33/45/52/58 | 618/629 (98%) | 599 (95%)† | 19 (3%)‡ | 109/118 (92%) | 89 (75%)† | 20 (17%)‡ |
Data are n/N (%) for overall prevalence. HPV=human papillomavirus.
See appendix p 12 for sensitivity analyses showing known HIV-negative and HIV unknown anal cancers separately.
Single infections were those in the absence of other HPV types.
Multiple infections were in the presence of other known HPV types.
The proportion of anal cancer that was directly preventable by HPV vaccines of different valencies (assuming lack of cross-protection against non-vaccine types; table 2). In HIV-negative anal cancer, attributable fraction ranges were 79–88% for a HPV16/18 vaccine, 86–92% for an HPV6/11/16/18 vaccine and 95–98% for a HPV6/11/16/18/31/33/45/52/58 vaccine. In HIV-positive anal cancer, corresponding preventable fractions were substantially lower (ie, 49–74% for the bivalent vaccine, 56–77% for the quadrivalent vaccine, and 75–92% for the nine-valent vaccine).
Prevalence of single and multiple infections by individual HPV types and their combinations were similar between known HIV-negative and HIV-unknown anal cancer cases (appendix p 12).
Discussion
Our large meta-analysis that includes 18 646 men and women with and without anal cancer provides, for the first time, a comprehensive picture of the contribution of different HPV types according to the severity of anal diagnosis. A steady rise in HPV16 positivity through increasing severity of diagnoses from normal anal cytology, through high-grade lesions, to anal cancer, irrespective of sex and HIV status, confirms HPV16 to be far more carcinogenic than any other HPV type in the anus. This builds on prospective evidence of a higher AIN grade 2 or grade 3 risk for HPV16 versus other high-risk HPV types seen in HIV-positive MSM who are positive for HIV infection,11 and extends evidence to female and HIV-negative populations, and to anal cancer. Indeed, HPV16 positivity increased (and that of other high-risk HPV types fell) even between high-grade lesions and anal cancer, suggesting that HPV16-related high-grade SIL are more likely to progress to cancer than non-HPV16 high-grade SIL.
Nevertheless, HIV was shown to influence the natural history of HPV types, with the result that the fraction of anal cancer attributable to HPV16 was lower in HIV-positive than HIV-negative anal cancer. In women, this shift towards non-HPV16 was also seen in HIV-positive high-grade anal lesions (as shown previously by de Vuyst and colleagues19). This mirrors a shift towards non-HPV16 seen in HIV-positive high-grade cervical lesions20 and cervical cancer,21 a phenomenon thought to be explained by the lower influence of immunological deficiency on HPV16 than on other high-risk HPV types, on account of HPV16's better intrinsic ability to evade immunological control, even in immunocompetent individuals.13 A similar mechanism might well apply to HIV-positive anal cancer, but with a greater background predominance of HPV16 than for cervical cancer. The fraction of HPV16 in HIV-negative anal cancer in our meta-analysis was confirmed to be over 85%,2 and nearly entirely due to single HPV16 infections. However, this was not the case for HIV-positive anal cancer patients among whom only about 70% of HPV-positive cases harboured HPV16 infection, a third of which were in combination with other types. Under-representation in HIV-positive anal cancer was seen uniquely for HPV16. All other high-risk HPV types were significantly over-represented. Nevertheless, attribution of the non-HPV16-positive fraction of HIV-positive anal cancer (33%) to individual types is difficult because only a small proportion of this fraction (12%) could be explained by single high-risk HPV type infection.
We addressed the causality issue through different approaches. First by calculating type-specific prevalence ratios for HPV-positive cancers versus HPV-positive normal cytology as a proxy of relative carcinogenicity,14 for which only HPV16 stood out from other HPV types, irrespective of HIV status. Second by comparing whether individual HPV types in anal cancer were found in the absence or presence of other HPV infections, for which there was no type other than HPV16 for which a sizeable proportion of anal cancer could be attributed to single infection (the highest in HIV-negative anal cancer were 2% for HPV18 and for HPV33). Lastly, in HIV-positive high-grade anal lesions, we showed that non-HPV16 types were substantially less frequent when testing from tissue biopsies as opposed to exfoliated cells, as seen in the cervix of HIV-infected women.22
Taken together, our findings suggest a strong causal link between HPV16 detection and anal cancer, but warn against attempting to attribute causality of non-HPV16 types in lesions infected with more than one HPV type. Establishing any hierarchy in the attribution to a specific type in multiple infection is rather arbitrary, especially in HIV-positive anal cancer, requiring studies of laser capture microdissection with PCR genotyping of HPV types, like those done in the cervix.23 Although HPV18 was arguably the second most carcinogenic HPV type in the anus (based on its prevalence in anal cancer and cancer/normal PR) and is the only type other than HPV16 to have been shown to confer a higher prospective risk for AIN2/3 versus other high-risk HPV strains (in HIV-positive MSM),11 it did not stand out from other high-risk HPV types, most notably not from HPV33, to the extent that is seen in the cervix.15 Even HPV6 was not distinguishable from HPV18 in terms of single infection in HIV-negative anal cancer, suggesting that low-risk types might be occasionally associated with anal cancer, as observed in laser capture microdissection studies.24, 25
Our present meta-analysis builds on previous meta-analyses19, 26 and large case series27 on account of the large number of both HIV-negative and HIV-positive men and women, and the inclusion of the whole spectrum of cytohistological anal diagnoses. The mix of included individuals reflects the epidemiology of HPV infection and anal neoplasia with a large representation of high-risk groups (ie, a predominance of HIV-positive populations and, in both HIV-positive and HIV-negative men, a predominance of MSM, in whom HPV exposure is higher than in the general male population). Thus, in our meta-analysis, although HPV prevalence in HIV-negative men with normal cytology was high (53%), prevalence was only 6% in the small subset (n=99) of these HIV-negative men that were known to have sex with women. Indeed, in 1305 men who have sex with women who were not included in the meta-analysis because of an absence of anal diagnosis, population-based HPV prevalence was 12%.28 The 3584 women who were HIV-negative or HIV-unknown in the meta-analysis can be considered to more closely represent a population-based sample.
We recognise that our findings on HPV and HIV come mostly from high-income settings, and there is insufficient data from sub-Saharan Africa. However, findings were consistent in region-specific sensitivity analyses and after adjustment for region. Data on HIV-positive cancer in women were also limited, which is in agreement with HIV accounting for relatively little of anal cancer in women at a population level.29 Nevertheless, HPV type-specific findings in women were consistent with those for men, hence the decision to combine sexes in some cancer-related analyses.
For 85% of included individuals, published data were supplemented with more details from the authors (appendix pp 4–5). Nevertheless, we did not have information on HIV status (nor on sexual preference in men) for a substantial fraction of anal cancer cases. This is because (unlike data for diagnoses of lower severity which arise predominantly through screening or research studies of targeted populations), anal cancer data arise predominantly from retrospective pathology series, (ie, from anal cancer cases for whom HIV status and sexual preference were either not recorded, or not investigated). Consideration of HIV-unknown anal cancer as HIV-negative was, in our view, justifiable because study location, period, or population-based study designs ruled out the possibility of a sizeable proportion of HIV-positive anal cancer. Indeed, type-specific HPV prevalence was similar in HIV-unknown and known HIV-negative cases, and findings were consistent in sensitivity analyses restricted to the subset of known HIV-negative cases only.
In conclusion, HPV16 is a clear priority target for anal cancer prevention, even in people who are HIV positive. With respect to primary prevention, HPV16-containing vaccines have the potential to prevent most anal cancer, with the gain in protection offered by the nine-valent vaccine versus the bivalent and quadrivalent vaccines being smaller for anal cancer than for cervical cancer.2 Assuming, however, lack of cross-protection, nine-valent vaccines would provide a larger gain against anal cancer in HIV-positive than in HIV-negative individuals. With respect to risk stratification of high-risk populations,30, 31 although our meta-analysis cannot be used to calculate screening test specificity, it does clarify a very strong HPV16 versus non-HPV16 risk differential. Indeed, the fact that this risk differential increases further between high-grade lesions and anal cancer sends a warning that screening studies with high-grade lesions as a principal outcome measure30, 31, 32 might be underestimating the relative importance of HPV16 and overestimating that of non-HPV16 types, to the prevention of anal cancer. This is consistent with recent prospective findings in HIV-positive MSM that HPV16-positive high-grade lesions are significantly less likely to regress than those infected by other types.33
Acknowledgments
Acknowledgments
The work of CL was undertaken during the tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions/People/Co-founding of regional, national and international programmes (COFUND).
Contributors
GMC initiated and coordinated the study. CL was responsible for the data collection and data analysis. Studies were reviewed by CL and GMC. CL and SF wrote the first draft of the manuscript. All the authors were involved in the interpretation of the analyses and gave input to the final manuscript.
Declaration of interests
All authors report grants from the European Commission during the conduct of the study.
Supplementary Material
References
- 1.GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr (accessed May 9, 2017).
- 2.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Martel C, Shiels MS, Franceschi S. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29:2173–2181. doi: 10.1097/QAD.0000000000000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS. 2016;30:1663–1668. doi: 10.1097/QAD.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg MJ, Lau B, Achenbach CJ. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163:507–518. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford GM, Polesel J, Rickenbach M. Cancer risk in the Swiss HIV cohort study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 7.Machalek DA, Poynten M, Jin F. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza G, Wiley DJ, Li X. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverberg MJ, Lau B, Justice AC. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009;21:433–438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Pokomandy A, Rouleau D, Ghattas G. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011;52:1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 12.Phanuphak N, Teeratakulpisarn N, Triratanachat S. High prevalence and incidence of high-grade anal intraepithelial neoplasia among young Thai men who have sex with men with and without HIV. AIDS. 2013;27:1753–1762. doi: 10.1097/QAD.0b013e328360a509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strickler HD, Burk RD, Fazzari M. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 14.Clifford GM, Tully S, Franceschi S. Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis. 2017;64:1228–1235. doi: 10.1093/cid/cix135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan P, Howell-Jones R, Li N. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 16.Shiels MS, Kreimer AR, Coghill AE, Darragh TM, Devesa SS. Anal cancer incidence in the United States, 1977–2011: distinct patterns by histology and behavior. Cancer Epidemiol Biomarkers Prev. 2015;24:1548–1556. doi: 10.1158/1055-9965.EPI-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IARC Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:1–475. [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 19.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 20.Clifford GM, Gonçalves MA, Franceschi S, HPV and HIV Study Group Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20:2337–2344. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]
- 21.Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr. 2016;73:332–339. doi: 10.1097/QAI.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vuyst H, Chung MH, Baussano I. Comparison of HPV DNA testing in cervical exfoliated cells and tissue biopsies among HIV-positive women in Kenya. Int J Cancer. 2013;133:1441–1446. doi: 10.1002/ijc.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quint W, Jenkins D, Molijn A. One virus, one lesion—individual components of CIN lesions contain a specific HPV type. J Pathol. 2012;227:62–71. doi: 10.1002/path.3970. [DOI] [PubMed] [Google Scholar]
- 24.Guimera N, Lloveras B, Lindeman J. The occasional role of low-risk human papillomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: results from a global study. Am J Surg Pathol. 2013;37:1299–1310. doi: 10.1097/PAS.0b013e31828b6be4. [DOI] [PubMed] [Google Scholar]
- 25.Cornall AM, Roberts JM, Garland SM, Hillman RJ, Grulich AE, Tabrizi SN. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int J Cancer. 2013;133:2253–2258. doi: 10.1002/ijc.28228. [DOI] [PubMed] [Google Scholar]
- 26.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 27.Alemany L, Saunier M, Alvarado-Cabrero I. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyitray AG, Carvalho da Silva RJ, Baggio ML. Six-month incidence, persistence, and factors associated with persistence of anal human papillomavirus in men: the HPV in men study. J Infect Dis. 2011;204:1711–1722. doi: 10.1093/infdis/jir637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104:1591–1598. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castle PE, Follansbee S, Borgonovo S. A comparison of human papillomavirus genotype-specific DNA and E6/E7 mRNA detection to identify anal precancer among HIV-infected men who have sex with men. Cancer Epidemiol Biomarkers Prev. 2013;22:42–49. doi: 10.1158/1055-9965.EPI-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin F, Roberts JM, Grulich AE. The performance of human papillomavirus biomarkers in predicting anal high-grade squamous intraepithelial lesions in gay and bisexual men. AIDS. 2017;31:1303–1311. doi: 10.1097/QAD.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 32.Phanuphak N, Teeratakulpisarn N, Keelawat S. Use of human papillomavirus DNA, E6/E7 mRNA, and p16 immunocytochemistry to detect and predict anal high-grade squamous intraepithelial lesions in HIV-positive and HIV-negative men who have sex with men. PLoS One. 2013;8:e78291. doi: 10.1371/journal.pone.0078291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grulich AE, Jin F, Poynten IM, et al. Predictors of 12-month persistent high-grade squamous intraepithelial lesions (HSIL) in gay and bisexual men (GBM): data from the SPANIC study. 31st International Papillomavirus Conference; Cape Town; Feb 28–March 4, 2017. HPV17-0567.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.