Summary
Antimicrobial resistance is an important threat to international health. Therapeutic guidelines for empirical treatment of common life-threatening infections depend on available information regarding microbial aetiology and antimicrobial susceptibility, but sub-Saharan Africa lacks diagnostic capacity and antimicrobial resistance surveillance. We systematically reviewed studies of antimicrobial resistance among children in sub-Saharan Africa since 2005. 18 of 1075 articles reviewed met inclusion criteria, providing data from 67 451 invasive bacterial isolates from inconsistently defined populations in predominantly urban tertiary settings. Among neonates, Gram-negative organisms were the predominant cause of early-onset neonatal sepsis, with a high prevalence of extended-spectrum β-lactamase-producing organisms. Gram-positive bacteria were responsible for a high proportion of infections among children beyond the neon atal period, with high reported prevalence of non-susceptibility to treatment advocated by the WHO therapeutic guidelines. There are few up-to-date or representative studies given the magnitude of the problem of antimicrobial resistance, especially regarding community-acquired infections. Research should focus on differentiating resistance in community-acquired versus hospital-acquired infections, implementation of standardised reporting systems, and pragmatic clinical trials to assess the efficacy of alternative treatment regimens.
Introduction
As a pressing threat to international health, antimicrobial resistance is of increasing importance. Resistance to antimicrobials threatens to undermine nearly a century of gains made since the discovery of antibiotics and the contribution of these drugs to improvements in childhood survival in the developing world, particularly among neonates.1, 2 Antimicrobial resistance has been reported in both community-acquired and health-care-associated infections worldwide.3 However, in low-income and middle-income countries, surveillance is often inconsistent because of insufficient integration and non-representativeness of local data, inconsistent laboratory quality, and scarce microbiological diagnostic facilities.3
Sub-Saharan Africa (as defined by the World Bank's World Development Indicators4) has the least comprehensive antimicrobial surveillance strategies of all world regions, alongside scarce infection prevention and control programmes. Only six (15%) of the 41 WHO Africa region member states carry out surveillance for bacterial antimicrobial resistance, and external quality assurance of laboratory procedures is infrequent.5, 6, 7
Sub-Saharan Africa has a high incidence of acute respiratory infections, diarrhoeal diseases, parasitic and invasive bacterial infections, and chronic conditions such as HIV, tuberculosis, and malnutrition.8, 9, 10, 11 These conditions increase demand for both preventive and therapeutic antimicrobials.12 Unregulated antibiotics are readily available in most communities through shops and drug stores, and are widely used in domestic and commercial animal husbandry.13 In clinics and hospitals, scarce diagnostic resources and consequent therapy based on clinical syndromes that are sensitive (rather than specific) for serious bacterial infections (therefore likely to capture viral, parasitic, and self-limiting illnesses) also drive antibiotic consumption, which is a key factor in promotion of resistance.14 Moreover, the spread of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBLs) and other multidrug-resistant (MDR) organisms in both community-based and hospital-based populations potentially limits the availability of suitable antimicrobials to treat such infections.15, 16 Escalation of resistance might also occur when therapies normally reserved for second-line, third-line, or fourth-line treatment in resource-rich settings (such as third-generation cephalosporins, carbapenems, and polymyxins) start to be used widely in sub-Saharan Africa without supportive microbiological facilities, expert advice, or adequate prescription controls.17, 18
Conversely, higher-level treatment is often unavailable or too expensive for most of the population of sub-Saharan Africa. Decreased susceptibility to antimicrobials is therefore important, not just because of the health-care implications of having few treatment options (especially in resource-poor settings such as sub-Saharan Africa) and potentially poorer clinical outcomes,3, 14, 19 but also because of the costs associated with use of more expensive therapies for a wide range of patients and prolonged stays in hospital.20
WHO recommends penicillin (or ampicillin) plus gentamicin as empirical therapy in suspected neonatal and paediatric sepsis in resource-limited settings (Table 1, Table 2), and advises tailoring therapy to local resistance patterns.21 However, in practice, tailoring of therapy is usually impossible because of a lack of data about local susceptibility due to insufficient reliable laboratory facilities with external quality assurance or collaborative surveillance.3 High prevalence of non-susceptibility to recommended empirical therapies has previously been reported among invasive bacterial isolates throughout sub-Saharan Africa;3, 6, 7, 43 however, most research has been limited to tertiary settings. Despite urgent calls for updated WHO guidelines to limit avoidable mortality due to antimicrobial resistance, these guidelines have remained unchanged for almost all causes of invasive paediatric bacterial infections.21, 44
Table 1.
Choices of antibiotic recommendations for various diagnoses
| Dosage | ||
|---|---|---|
| Sepsis in a child aged <2 months | ||
| Ampicillin intravenous | 50 mg/kg QID for 7–10 days (21 days for meningitis) | |
| plus gentamicin intravenous | 5–7·5 mg/kg daily for 7–10 days (21 days for meningitis) | |
| Second line: ceftriaxone intravenous | 50–100 mg/kg once daily for 7–10 days | |
| Sepsis in a child aged <2 months where referral is not possible | ||
| Amoxicillin oral | 50 mg/kg BID for 7 days | |
| plus gentamicin intramuscular or intravenous | 5–7·5 mg/kg daily for 2–7 days | |
| Sepsis in a child aged <2 months if skin conditions suggest Staphylococcus aureus | ||
| Cloxacillin or flucloxacillin intravenous | 25–50 mg/kg BID or QID (age dependent) for 7–10 days | |
| plus gentamicin | 5–7·5 mg/kg daily for 7–10 days (21 days for meningitis) | |
| Sepsis in a child aged >2 months | ||
| Ampicillin intravenous | 50 mg/kg QID for 7–10 days | |
| plus gentamicin intravenous or intramuscular | 7·5 mg/kg daily for 7–10 days | |
| Second line: ceftriaxone intravenous or intramuscular | 50 mg/kg BID or 100 mg/kg daily for 7–10 days | |
| Sepsis in a child aged >2 months if skin conditions suggest S aureus | ||
| Flucloxacillin intravenous | 50 mg/kg QID for 7–10 days | |
| plus gentamicin | 7·5 mg/kg daily | |
| Typhoid fever | ||
| Ciprofloxacin oral | 15 mg/kg BID for 7–10 days | |
| Second line: intravenous ceftriaxone | 80–100 mg/kg daily for 5–7 days | |
| or azithromycin oral | 20 mg/kg daily for 5–7 days | |
| Pneumonia | ||
| Ampicillin intravenous | 50 mg/kg QID for 7–10 days | |
| plus gentamicin intravenous | 7·5 mg/kg daily for 7–10 days | |
| Second line: ceftriaxone intravenous | 80 mg/kg daily for 7–10 days | |
| Pneumonia (if S aureus is suspected) | ||
| Flucloxacillin or cloxacillin intravenous | 50 mg/kg QID for 7–10 days | |
| plus gentamicin | 7·5 mg/kg intramuscular or intravenous once a day | |
| Dysentery (presumed due to Shigella spp) | ||
| Ciprofloxacin oral | 15 mg/kg BID for 3 days | |
| Second line: ceftriaxone intravenous | 50–80 mg/kg daily for 3 days | |
| Osteomyelitis | ||
| Chloramphenicol | 25 mg/kg TID | |
| Second line: cloxacillin or flucloxacillin intravenous | 50 mg/kg QID for up to 5 weeks (step down to oral once clinically improving) | |
| or clindamycin or third-generation cephalosporins | No dosages specified; clear circumstances of when such therapy would be appropriate are not outlined | |
| Meningitis in neonates | ||
| Ampicillin | 50 mg/kg BID for 3 weeks | |
| plus gentamicin | 5–7·5 mg/kg daily for 3 weeks | |
| Ceftriaxone intravenous | 50–75 mg/kg daily for 3 weeks | |
| plus gentamicin | 5–7·5 mg/kg daily for 3 weeks | |
| Cefotaxime | 50 mg/kg BID or TID (age dependent) for 3 weeks | |
| plus gentamicin | 5–7·5 mg/kg daily for 3 weeks | |
| Meningitis in children older than 28 days | ||
| Ceftriaxone intravenous | 50 mg/kg intramuscular or intravenous BID for 7–10 days | |
| Second line: cefotaxime intravenous | 50 mg/kg intramuscular or intravenous QID for 7–10 days | |
| Meningitis in children older than 28 days with no known resistance to chloramphenicol or β-lactams locally | ||
| Chloramphenicol intravenous | 25 mg/kg QID for 10 days | |
| plus ampicillin intramuscular or intravenous | 50 mg/kg QID for 10 days | |
| or benzylpenicillin intravenous | 60 mg/kg QID for 10 days | |
| Urinary tract infection | ||
| Co-trimoxazole oral | 4 mg/kg plus 20 mg/kg BID for 5 days | |
| Second line: ampicillin | 50 mg/kg intramuscular or intravenous every 6 h | |
| plus gentamicin | 5–7·5 mg/kg daily | |
First-line and second-line treatment guidelines for common paediatric infective illnesses. Data are from WHO pocket book of hospital care for children21 and WHO guideline for managing possible serious bacterial infection in young infants when referral is not feasible.22 BID=twice daily. TID=three times daily. QID=four times daily.
Table 2.
Reported non-susceptibility in most likely causative organisms to drugs documented in this Review
| Reported non-susceptibility | ||
|---|---|---|
| Sepsis in a child aged <2 months8, 23, 24, 25 | ||
| Klebsiella spp | ||
| Ampicillin | 100% (71–100)14, 26, 27, 28, 29 | |
| Gentamicin | 49% (48–58)14, 26, 27, 29, 30 | |
| Ceftriaxone | 43% (NA);26 50% (NA)27 | |
| Staphylococcus aureus | ||
| Ampicillin | 90% (85–100)14, 27, 28, 31, 32 | |
| Gentamicin | 29% (10–60)14, 32, 33, 34 | |
| Cloxacilllin | 20% (10–55)14, 27, 28 | |
| Streptococcus agalactiae | ||
| Ampicillin | 0% (NA)31, 35 | |
| Gentamicin | Not reported | |
| Ceftriaxone | 0% (NA)35 | |
| Escherichia coli | ||
| Ampicillin | 93% (78–96)14, 26, 27, 28, 29, 36 | |
| Gentamicin | 29% (20–46)14, 26, 27, 28, 29, 31 | |
| Ceftriaxone | 16% (12–34)26, 27, 28, 29 | |
| Sepsis in a child aged >2 months9, 23, 24, 31, 37 | ||
| Salmonella spp | ||
| Ampicillin | 66% (39–73)14, 29, 31, 37, 38, 39, 40 | |
| Gentamicin | 28% (23–32)14, 29, 38 | |
| Ceftriaxone | 0% (NA);32 0% (NA)38 | |
| E coli | ||
| Ampicillin | 93% (78–96)14, 26, 27, 28, 29, 36 | |
| Gentamicin | 29% (20–46)14, 26, 27, 28, 29, 31 | |
| Ceftriaxone | 16% (12–34)26, 27, 28, 29 | |
| Streptococcus pneumoniae | ||
| Ampicillin | 20% (NA)38 or 22% (NA)32 | |
| Gentamicin | 77% (NA)32 or 78% (NA)38 | |
| Ceftriaxone | 0% (NA)38 | |
| Klebsiella spp | ||
| Ampicillin | 100% (71–100)14, 26, 27, 28, 29 | |
| Gentamicin | 49% (48–58)14, 26, 27, 29, 34 | |
| Ceftriaxone | 43% (NA);26 50% (NA)27 | |
| S aureus | ||
| Ampicillin | 90% (85–100)14, 27, 28, 31, 32 | |
| Gentamicin | 29% (10–60)14, 32, 33, 34 | |
| Cloxacilllin | 20% (10–55)14, 27, 28 | |
| Typhoid fever | ||
| Salmonella enterica serotype Typhi | ||
| Ciprofloxacin | 0% (NA)32, 38 | |
| Ceftriaxone | 0% (NA)32, 38 | |
| Azithromycin | Not reported | |
| Pneumonia9, 24, 41 | ||
| S pneumoniae | ||
| Ampicillin | 20% (NA)38 or 22% (NA)32 | |
| Gentamicin | 77% (NA)32 or 78% (NA)38 | |
| Ceftriaxone | 0% (NA)38 | |
| S aureus | ||
| Ampicillin | 90% (85–100)14, 27, 28, 31, 32 | |
| Gentamicin | 29% (10–60)14, 32, 33, 34 | |
| Cloxacilllin | 20% (10–55)14, 27, 28 | |
| Dysentery (presumed due to Shigella spp) | ||
| Shigella spp | ||
| Ciprafloxacin | 0% (community-acquired) and 11% (hospital-acquired) when analysed in conjunction with other Enterobacteriaceae14 | |
| Ceftriaxone | Not documented | |
| Osteomyelitis | ||
| S aureus | ||
| Chloramphenicol | 47% (21–81)14, 32, 34 | |
| Cloxacillin | 20% (9–68)15, 27, 28 | |
| Clindamycin | 21% (NA)33 or 44% (NA)27 | |
| Third-generation cephalosporins | Not reported | |
| Flucloxacillin | 17% (NA)32 | |
| Meningitis in a child aged <28 days | ||
| Klebsiella spp | ||
| Ampicillin | 100% (71–100)14, 26, 27, 28, 29 | |
| Gentamicin | 49% (48–58)14, 26, 27, 29, 30 | |
| Ceftriaxone | 43% (NA);26 50% (NA)27 | |
| S agalactiae | ||
| Ampicillin | 0% (NA)31, 35 | |
| Gentamicin | Not reported | |
| Ceftriaxone | 0% (NA)35 | |
| E coli | ||
| Ampicillin | 93% (78–96)14, 26, 27, 28, 29, 36 | |
| Gentamicin | 29% (20–46)14, 26, 27, 28, 29, 31 | |
| Ceftriaxone | 16% (12–34)26, 27, 28, 29 | |
| Haemophilus influenzae type b | ||
| Penicillin and ampicillin | 5–100%* | |
| S pneumoniae | ||
| Ampicillin | 20% (NA)38 or 22% (NA)32 | |
| Gentamicin | 77% (NA)32 or 78% (NA)38 | |
| Ceftriaxone | 0% (NA)38 | |
| Urinary tract infection19, 24, 42 | ||
| E coli | ||
| Co-trimoxazole | 87–90%* (community-acquired)14, 31 or 77% (NA) (hospital-acquired)14 | |
| Ampicillin | 93% (78–96)14, 26, 27, 28, 29, 36 | |
| Gentamicin | 29% (20–46)14, 26, 27, 28, 29, 31 | |
| Klebsiella spp | ||
| Co-trimoxazole | 63% (community-acquired) or 94% (hospital-acquired)14 | |
| Ampicillin | 100% (71–100)14, 26, 27, 28, 29 | |
| Gentamicin | 49% (48–58)14, 26, 27, 29, 34 | |
Data are presented as median (IQR) where available. NA=not available.
Data are presented as the range where indicated.
The 2014 Global Report on Antimicrobial Surveillance3 highlighted the pressing need to strengthen knowledge and surveillance mechanisms for antimicrobial resistance, reiterating a theme which has resonated in the literature for more than a decade.28, 45 Therefore, we aimed to systematically review data published since 2005 on antimicrobial susceptibility for the commonest bacteria that cause serious infections in children in sub-Saharan Africa, with a focus on WHO recommendations for empirical treatment among children without specific risk factors (HIV or tuberculosis) to increase the knowledge and evidence base regarding local non-susceptibility patterns among a generalisable paediatric population.
Methods
Search strategy and selection criteria
After conferring on the search terms, the primary investigator (PCMW) reviewed published and grey literature on Dec 12, 2015 and later on Dec 3, 2016. JAB reviewed the included reports. Data for this Review were identified by searches of MEDLINE, PubMed, Embase, and Cochrane, and by identifying references from relevant articles using the search terms “child*”, “pediat*”, “paediat*”, “Africa*”, “sub-Sahara*”, “antimicrobial” or “antibiotic”, “resistance”, and “susceptibility” or “sensitivity”. Only reports of studies done in human beings published between Dec 12, 2005, and Dec 12, 2015, were included. There were no language restrictions on the search. To ensure current susceptibility patterns were investigated, articles were restricted to those reporting data collated since 2005 to make sure emerging threats to susceptibility—such as the spread of ESBL—were captured.
Inclusion criteria were predefined as research providing information on bacterial infections (including either aetiology, disease burden, or incidence), specified paediatric data (or clearly delineated from adult data), and information on antimicrobial testing methods documented. Predefined exclusion criteria were data aggregated with regions beyond sub-Saharan Africa, literature focused on solely analysing subpopulations with potentially confounding comorbidities (such as HIV or tuberculosis), poor methodological study design (such as retrospective observational studies with small patient numbers or those in which laboratory procedures were poorly defined), data collection that occurred substantially before the search period, and data pertaining to carriage only (rather than invasive isolates).
After abstracts were screened for these criteria, information was extracted from selected articles and converted into tabulated form (appendix), including study year, location, setting, population age group, study design, microbiological methods (bacterial isolation methods, and antibiotic susceptibility testing), and level of evidence, as per the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE) method. The GRADE method was used to summarise the quality of evidence for each study by assessment of study type, quality, limitations, inconsistency, or possibility of bias.46 Grading was performed by both PCMW and JAB, and any disagreements were resolved by consensus.
Results
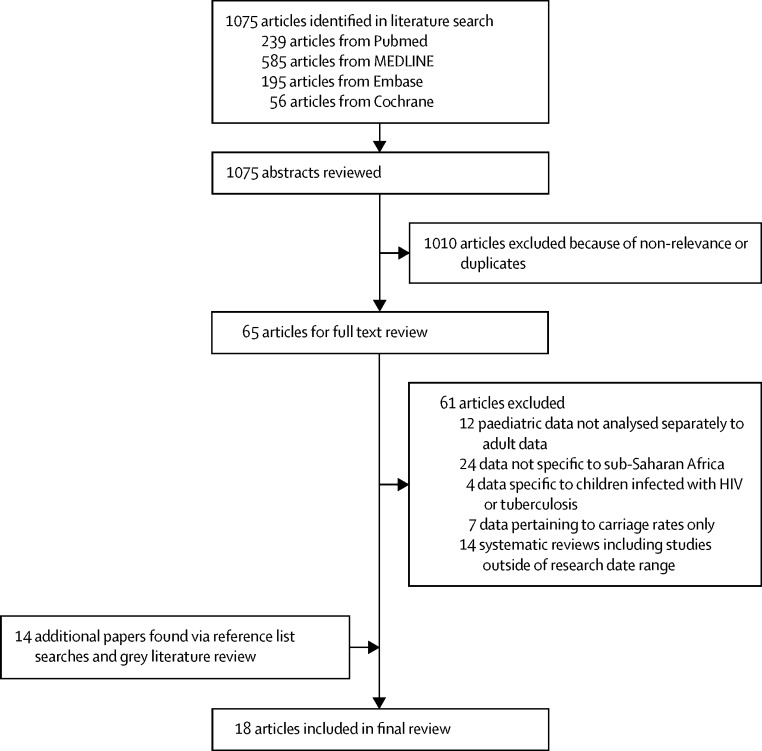
The initial search identified 1075 potentially relevant papers. Abstract review excluded 1010 papers that did not meet inclusion criteria or were identified as duplicate studies. Of the 65 papers that underwent full text review, four met the inclusion criteria. 14 further studies were identified from reference lists, resulting in a total of 18 studies for inclusion (figure 1).
Figure 1.
Search strategy
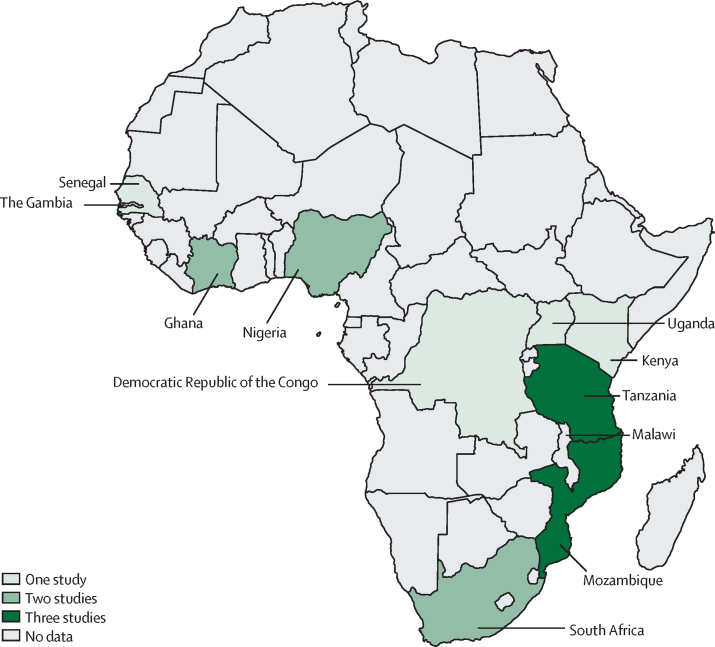
The 18 reports included in this Review were from 11 nations throughout sub-Saharan Africa (figure 2). Seven studies26, 31, 32, 38, 39, 47, 48 were done in rural settings, ten in urban settings,14, 19, 28, 29, 34, 35, 36, 37, 38, 49 and one was a laboratory-based study collating data across both urban and rural settings.33 The hospital-based studies were almost exclusively done in tertiary health facilities, whereas one study also included patients presenting at a secondary health facility.37 There was one cross-sectional study,27 one case-control study,36 and six case series;19, 26, 28, 29, 33, 35 the remaining ten studies14, 31, 32, 34, 37, 38, 39, 40, 48, 49 were cohort designs. Six studies33, 35, 38, 39, 40, 50 examined only one genus of pathogen, whereas the remaining studies14, 19, 25, 26, 28, 29, 31, 32, 34, 36, 37, 48 examined invasive disease. Because of the heterogeneity of the studies (in terms of settings, inclusion criteria, laboratory methods, reported outcomes, and quality of evidence) a formal meta-analysis was not possible; however, where possible, we calculated IQRs for specific pathogen susceptibilities.
Figure 2.
Study locations
Six of the studies14, 31, 32, 37, 38, 49 were moderate quality (GRADE level B), seven19, 26, 34, 35, 36, 39, 40 were low quality (GRADE level C), and the remaining five25, 28, 29, 33, 48 were classified as very low quality (GRADE level D). All studies described the microbiological techniques used (an inclusion criterion), although culture media, methods for identification of organisms, and definitions of non-susceptibility varied between studies. 12 studies14, 19, 26, 31, 32, 35, 37, 38, 39, 47, 48, 51 used automated culture techniques, whereas the remainder used manual methods. Only three studies14, 30, 38 (17%) ascertained recent antimicrobial exposure (and took this into account in the data analysis). Five papers14, 26, 35, 37, 38 (28%) reported external quality control of the laboratory. Most isolates were identified from blood cultures, although one study34 included induced sputum samples, and four studies26, 35, 48, 50 investigated both blood and CSF samples in patients with meningitis. Across the studies, a total of 67 451 cultures were collected, of which 5607 (8·3%) were positive for a bacterial pathogen. Further information on non-susceptibility prevalence was obtained from 236 laboratory-stored isolates33 and 149 diarrhoeal isolates of children infected with Shigella spp or Salmonella spp.39 Non-susceptibility medians, ranges, and IQRs (including comparison to international treatment guidelines) are documented in Table 3, Table 4.
Table 3.
Non-susceptibility patterns of key Gram-negative pathogens
| Number of isolates not susceptible (n)/number tested (N) | Median non-susceptibility rate (IQR) | |
|---|---|---|
| Klebsiella spp | ||
| Penicillin and ampicillin | 45/100 (45%);29 55/57 (96%);26 17/17 (100%; CA and HA);28 53/53 (100%; CA and HA);14 50/50 (100%; CA)27 | 100% (71–100) |
| Gentamicin | 49/100 (49%);29 28/57 (49%);26 25/53 (47%; CA and HA);14 33/50 (66%)27 | 49% (48–58) |
| Ceftriaxone | 25/57 (43%);26 25/50 (50%);27 1/3 (33%)34 | ·· |
| Cefotaxime | 24/50 (48%);26 12/53 (22%; CA);14 8/53 (15%; HA)14 | ·· |
| Ceftazidime | 28/57 (49%);26 11/53 (21%; CA);14 8/53 (15%; HA)14 | ·· |
| Ciprofloxacin | 4/50 (8%);27 0/3 (0%)34 | ·· |
| Chloramphenicol | 10/19 (53%; CA);14 15/34 (44%; HA)14 | ·· |
| Co-trimoxazole | 12/19 (63%; CA);14 32/34 (94%; HA)14 | ·· |
| ESBL-producing proportion | 27/35 (76%; CA);19 93/119 (78%; HA);19 33/40 (83%; HA)36 | ·· |
| Escherichia coli | ||
| Penicillin and ampicillin | 155/310 (50%); 29 32/41 (78%);26 11/13 (85%; HA);14 13/14 (93%);28 148/154 (96%; CA);31 23/24 (96%; CA);14 22/22 (100%)27 |
93% (78–96) |
| Amoxicillin-clavulanate | 6/24 (25%; CA);14 9/13 (69%; HA)14 | ·· |
| Gentamicin | 4/41 (10%);26 62/310 (20%);29 40/142 (28%);31 7/24 (29%; CA);14 6/14 (43%);28 6/13 (46%; HA);14 15/22 (68%)27 |
29% (20–46) |
| Ceftriaxone | 31/310 (10%);29 2/14 (14%);28 7/41 (17%);26 11/22 (50%)27 | 16% (12–34) |
| Cefotaxime | 11/22 (50%)27 | ·· |
| Ceftazidime | 11/22 (50%)27 | ·· |
| Chloramphenicol | 120/155 (78%; CA)31 | ·· |
| Co-trimoxazole | 21/24 (87%; CA);14 10/13 (77%; HA);14 128/142 (90%; CA)31 | ·· |
| ESBL-producing proportion | 9/76 (12%; CA);19 4/19 (22%; HA);19 11/22 (50%);27 23/40 (58%; HA)36 | 36% (17–54) |
| Salmonella spp | ||
| Penicillin and ampicillin | 10/40 (25%);39 10/30 (30%);29 13/27 (48%; CA);14 60/92 (65%);37 8/12 (67%; HA);14 74/103 (72%);40 296/401 (74%);31 107/128 (84%; CA)38 |
66% (39–73) |
| Amoxicillin-clavulanate | 6/30 (20%);29 152/401 (38%);31 95/128 (74%; CA)38 | ·· |
| Gentamicin | 6/30 (20%);29 7/27 (26%; CA);14 38/128 (30%; CA);38 4/12 (33%; HA)14 | 28% (23–32) |
| Co-trimoxazole | 7/40 (18%);39 17/30 (55%);29 13/27 (48%; CA);14 8/12 (67%; HA);14 55/92 (60%);37 264/401 (66%);31 98/128 (77%; CA)38 |
60% (48–67) |
| Tetracycline | 14/128 (11%; CA);38 6/40 (15%)39 | ·· |
| Chloramphenicol | 6/40 (15%);39 6/30 (20%);29 4/27 (15%; CA);14 4/12 (33%; HA);14 216/401 (54%);31 105/128 (82%; CA)38 | 27% (15–54) |
| Ciprofloxacin | 0/128 (0%; CA);38 0/129 (0%)32 | ·· |
| Ceftriaxone | 0/128 (0%; CA);38 0/129 (0%)32 | ·· |
| Multidrug-resistant proportion | Salmonella enterica serotype Typhi: 34/133 (33%);40 84/129 (65%);32 non-typhoidal salmonella: 99/129 (77%);32 97/128 (76%);38 101/103 (98%)40 | ·· |
| Shigella spp | ||
| Penicillin and ampicillin | 61/109 (56%)39 | ·· |
| Co-trimoxazole | 92/109 (84%)39 | ·· |
| Tetracycline | 72/109 (66%)39 | ·· |
| Chloramphenicol | 57/109 (52%)39 | ·· |
| Multidrug-resistant proportion | 71/109 (65%)39 | ·· |
| Haemophilus influenzae type b | ||
| Penicillin and ampicillin | 7/14 (50%);48 61/113 (54%);31 6/6 (100%; CA)34 | ·· |
| Chloramphenicol | 56/113 (50%);31 9/10 (90%);48 6/6 (100%; CA)34 | ·· |
| Co-trimoxazole | 26/113 (23%)31 | ·· |
| Acinetobacter spp | ||
| Penicillin and ampicillin | 37/66 (56%);26 0/3 (0%; CA);14 0/9 (0%; HA)14 | ·· |
| Gentamicin | 18/66 (27%);26 2/3 (67%; CA);14 4/9 (44%; HA)14 | ·· |
| Ceftriaxone | 23/66 (35%)26 | ·· |
| Ceftazidime | 2/9 (22%; HA);14 1/3 (33%; CA)14 | ·· |
| Multidrug-resistant proportion | 4/16 (25%; CA);19 49/68 (72%; HA)19 | ·· |
Median and IQR could not be calculated if there were fewer than three papers assessing non-susceptibility rates. CA=community-acquired, where specified in the literature (blank=not specified). HA=hospital-acquired, where specified in the literature (blank=not specified). ESBL=extended-spectrum β-lactamases.
Table 4.
Non-susceptibility patterns of key Gram-positive pathogens
| Number of isolates not susceptible (n)/number tested (N) | Median non-susceptibility rate (IQR) | |
|---|---|---|
| Streptococcus pneumoniae | ||
| Penicillin and ampicillin | 4/20 (20%; CA);38 5/22 (23%)32 | ·· |
| Amoxicillin-clavulanate | 2/18 (11%; CA)38 | ·· |
| Gentamicin | 17/22 (77%);32 16/20 (78%; CA)38 | ·· |
| Chloramphenicol | 2/18 (11%; CA);38 5/20 (25%)32 | ·· |
| Co-trimoxazole | 20/116 (17%; CA);37 19/20 (95%; CA);32 17/17 (100%; CA);38 11/11 (100%);50 29//29 (100%)31 | 100% (56–100) |
| Ciprofloxacin | 9/21 (43%)32 | ·· |
| Tetracycline | 14/19 (74%);32 15/20 (75%; CA)38 | ·· |
| Ceftriaxone | 0/20 (0%; CA)38 | ·· |
| Streptococcus agalactae | ||
| Penicillin and ampicillin | 0/35 (0%);31 0/57 (0%)35 | ·· |
| Chloramphenicol | 10/35 (29%);31 11/57 (19%)31 | ·· |
| Erythromycin | 12/57 (21%)35 | ·· |
| Co-trimoxazole | 5/34 (15%)31 | ·· |
| Ceftriaxone | 0/57 (0%)35 | ·· |
| Staphylococcus aureus | ||
| Penicillin and ampicillin | 17/32 (52%);31 23/27 (85%);28 170/189 (90%);31 29/32 (90%);27 13/13 (100%; CA);14 17/17 (100%; HA)14 | 90% (85–100) |
| Flucloxacillin | 5/30 (17%)32 | ·· |
| Oxacillin | 17/189 (9%)31 | ·· |
| Cloxacillin | 1/13 (8%; CA);14 2/17 (12%; HA);14 9/32 (28%);27 22/27 (81%)28 | 20% (10–55) |
| Co-trimoxazole | 11/24 (54%);32 19/32 (60%)27 | ·· |
| Gentamicin | 0/13 (0%; CA);14 3/17 (19%; HA);14 9/32 (29%);32 3/9 (33%; CA);34 210/248 (85%)33 | 29% (10–60) |
| Nitrofurantoin | 94/248 (38%)33 | ·· |
| Clindamycin | 52/248 (21%);33 14/32 (44%)27 | ·· |
| Erythromycin | 0/13 (0%; CA);14 2/9 (23%; CA);34 5/17 (29%; HA);14 144/248 (58%);33 21/32 (66%)27 | 29% (12–62) |
| Ciprofloxacin | NA/32 (14%);27 10/31 (32%)32 | ·· |
| Chloramphenicol | 2/13 (15%; CA);14 5/17 (29%; HA);14 6/9 (67%; CA);17 30/32 (94%)32 | 47% (21–81) |
| Meticillin | 58/131 (44%)19 | ·· |
| Meticillin-resistant S aureus | ||
| Oxacillin plus cefoxitin | 9/32 (28%);27 14/95 (15%; CA);19 23/36 (65%; HA)19 | ·· |
Median and IQR could not be calculated if there were fewer than three papers assessing non-susceptibility rates. CA=community-acquired, where specified in the literature (blank=not specified). HA=hospital-acquired, where specified in the literature (blank=not specified). NA=not available.
The studies covered the full paediatric age range of 0–18 years, with a focus on young childhood (0–5 years). Four studies30, 45, 46, 48 exclusively investigated infections in infants within the first 90 days of life.
Ten studies31, 32, 34, 36, 37, 38, 39, 40, 48, 50 specifically examined community-acquired infections only, and three studies14, 19, 26 investigated antibiotic susceptibility patterns that distinguished community-acquired and hospital-acquired infection (and although the incidence of different infectious aetiologies could have been clarified within these studies, only two studies14, 19 analysed the resistance patterns for each subset independently). The remaining five studies27, 28, 29, 33, 35 did not identify whether the infections were community-acquired or nosocomial in nature.
Gram-negative organisms (Escherichia coli, Klebsiella spp) and (less commonly) Streptococcus agalactiae were the predominant causes of early-onset neonatal sepsis in sub-Saharan Africa, which is defined as sepsis occurring at younger than 72 h of age (aside from sepsis due to S agalactiae, which was defined as occurring from 0–6 days).52 S aureus is an important cause of late-onset sepsis (with an ongoing burden caused by E coli, Klebsiella spp, S agalactiae, and other Gram-positive organisms such as Streptococcus pyogenes).52, 53, 54, 55, 56, 57, 58 Early-onset infections are usually vertically transmitted, yet they might also be secondary to nosocomial acquisition (in which case resistance is more likely to be an issue), whereas late-onset infections are due to horizontal infection (either community-acquired or hospital-acquired).44 Although the understanding of susceptibility patterns according to the time of onset of neonatal infection is important, most of the included studies that investigated invasive neonatal infections did not clearly delineate whether these were early-onset or late-onset, and whether the patient population was transferred from the delivery ward or presenting for admission from the community.52, 57
Four studies26, 27, 28, 35 specifically investigated neonatal patient populations born within hospital environments and at home. These studies found a predominance of infections caused by Gram-negative bacteria and in particular Klebsiella spp, which was responsible for approximately half of all bloodstream infections (especially in early-onset illness).27, 28 Other common neonatal pathogens identified included S aureus (range 27–39%),27, 28, 53 E coli (21%),26 and S agalactiae (6·9%26 and 20%53). A high prevalence of MDR organisms was documented in a prospective cross-sectional study27 of 300 neonates in Tanzania, with 40% (36 of 91 isolates) of Gram-negative organisms showing ESBLs, and 30% (nine of 30 isolates) of S aureus classified as meticillin resistant. However, these isolates were not identified as community-acquired or hospital-acquired.27 MDR organisms were associated with increased mortality for both populations (52% vs 25% in ESBL-producing organisms, p=0·0008; and 55% vs 21% mortality in meticillin-resistant S aureus organisms; p=0·0008).
Studies31, 35 that isolated S agalactiae from 57 neonates in Malawi and 37 in Mozambique revealed an approximately equal incidence of early-onset and late-onset disease, with a higher case fatality rate for early-onset disease. All isolates were susceptible to β-lactams. One further study26 based in a rural setting investigated invasive bacterial infections in infants born outside hospital, but did not delineate infections as community-acquired or hospital-acquired. An important finding in this study26 was diminishing in-vitro susceptibility of all isolates to WHO recommended ampicillin and gentamicin over the study period (from 88% susceptibility in 2001 to 66% in 2009; p<0·001).
Gram-negative bacteria
Salmonella spp are the most frequently isolated Gram-negative pathogens in children older than 1 month in sub-Saharan Africa, with a predominance in the wet season.9, 23, 24, 31, 37 Few studies analysed Salmonella enterica serotype Typhi (S typhi) and non-typhoidal species independently for susceptibility patterns against individual antibiotics. Nine of the included papers14, 19, 29, 31, 32, 37, 38, 39, 40 investigated susceptibility patterns to Salmonella spp, revealing non-susceptibility to penicillin and ampicillin (median 66%, IQR 39–73), gentamicin (28%, 23–32), co-trimoxazole (60%, 48–67), amoxicillin-clavulanate (20%,29 38%,31 and 74%32), and chloramphenicol (27%, 15–54). Only one paper14 delineated community-acquired and hospital-acquired infections, with a slightly higher prevalence of non-susceptibility in hospital-acquired isolates. MDR organisms are of increasing concern, with up to 65% of S typhi and up to 98% of non-typhoidal salmonella isolates showing combined resistance to ampicillin, co-trimoxazole, and chloramphenicol.32, 33, 45
Klebsiella spp cause a substantial amount of morbidity among paediatric patients in sub-Saharan Africa, accounting for almost half of all Gram-negative infections in neonates and a substantial overall burden of hospital-acquired infection.9, 25, 55, 56 Nine studies18, 19, 21, 28, 36, 40, 41, 43, 46 assessed Klebsiella spp susceptibility patterns, of which two delineated hospital-acquired and community-acquired acquisition,18, 19 whereas other research specifically evaluated hospital-acquired strains,41 community-acquired strains,46 or did not clarify the place of acquisition. This research revealed a consistently high prevalence of non-susceptibility to commonly used antimicrobial therapies, including gentamicin (median 49%, IQR 48–58%) and ceftriaxone (range 33–50%36, 40, 47). Non-susceptibility was similar between community-acquired and hospital-acquired strains, and high frequencies of ESBL-producing Klebsiella spp were documented (from 76% for community-acquired isolates to 82% among hospital-acquired isolates30, 36).
E coli causes a substantial burden of disease in sub-Saharan Africa; it is responsible for approximately 11% of all paediatric bloodstream infections19 and is a predominant cause of community-acquired sepsis.24, 42 Eight papers19, 25, 26, 28, 29, 34, 36, 40 assessed non-susceptibility of E coli, documenting non-susceptibility to penicillin and ampicillin of 50–100% (median 93%, IQR 78–96), gentamicin (29%, 20–46), and ceftriaxone (16%, 12–34). One paper14 delineated community-acquired and hospital-acquired infection, revealing a higher frequency of non-susceptibility among hospital-acquired isolates (gentamicin non-susceptibility of 29% among community-acquired isolates compared with 46% among hospital-acquired isolates). ESBL-producing E coli infections were also more common among hospital-acquired isolates (22%19 and 58%36) compared with community-acquired isolates (12%19).
Although Shigella spp are an important cause of community-acquired bacteraemia,37, 45, 59 only one paper37 assessed susceptibility of Shigella spp to commonly available antimicrobials, documenting resistance to co-trimoxazole (87%), ampicillin (56%), and chloramphenicol (52%) alongside high levels of MDR (non-susceptibility to more than two antimicrobials from different classes). However, when analysed together with other Enterobacteriaceae, there was evidence of sensitivity to ciprofloxacin.14
Although the advent of the conjugate vaccine has considerably diminished the burden of Haemophilus influenzae type b,41 its case fatality rate has the potential to remain high because of substantial antimicrobial resistance to first-line therapies. Three papers31, 34, 48 assessed resistance among Haemophilus spp isolates, documenting non-susceptibility to ampicillin and chloramphenicol ranging from 50% to 100%, rendering these antimicrobials largely ineffective in treating H influenzae meningitis.
Although a rare cause of sepsis, Acinetobacter spp are nonetheless clinically significant because of their high mortality when causing bacteraemia (up to 25%), with 78% of hospital-acquired Acinetobacter spp isolates (and 25% of community-acquired isolates) being MDR in a large study19 of paediatric bloodstream infections in South Africa (which included a small cohort of patients [13%] who were HIV-positive, in whom there was no statistically significant difference in the likelihood of bloodstream infections). A large case series26 of 4849 neonates in rural Kenya identified Acinetobacter spp as a cause of 10% of positive blood cultures in infants not born in hospital, with resistance to penicillin and ampicillin (56%, 95% CI 42–70), gentamicin (27%, 14–39), and ceftriaxone (35%, 22–48). A further review14 of 1787 paediatric patients in Tanzania reported increased non-susceptibility to ampicillin (100% for both community-acquired and hospital-acquired isolates), gentamicin (44% for hospital-acquired isolates and 67% for community-acquired isolates), and ceftazidime (22% among hospital-acquired isolates and 33% among community-acquired isolates; susceptibility profiles tested for three community-acquired invasive isolates and nine hospital-acquired isolates).
Gram-positive bacteria
S pneumoniae is the most common Gram-positive organism isolated in positive blood cultures in children in sub-Saharan Africa9, 24, 53 and is responsible for up to 35% of clinical episodes of sepsis, with a predominance in the dry season.9 Although the burden of disease caused by this pathogen is declining as the pneumococcal conjugate vaccine is introduced, it nevertheless continues to cause substantial morbidity and mortality.60, 61 Three papers32, 34, 38 analysed susceptibility patterns of S pneumoniae, documenting non-susceptibility (which was not classified into intermediate-level vs high-level resistance) to penicillin and ampicillin (range 6–24%) and chloramphenicol (11–25%); however, two studies showed full susceptibility to ceftriaxone.32, 38 Although no longer part of WHO treatment guidelines, co-trimoxazole and macrolide antibiotics are still often prescribed in low-income and middle-income countries to treat pneumonia (and as prophylaxis for children with HIV). A median prevalence of 100% (IQR 56–100) non-susceptibility to co-trimoxazole was documented,31, 32, 37, 38, 50 although susceptibility to erythromycin remains adequate.34, 49
S aureus causes a substantial burden of bloodstream infections in paediatric patients in sub-Saharan Africa.19, 23, 31, 32, 37, 40, 47 WHO's recommendation is for first-line treatment with cloxacillin, for which median non-susceptibility was 20% (IQR 10–55%), with similar susceptibility patterns between community-acquired and hospital-acquired isolates.14, 25, 28 Chloramphenicol and flucloxacillin are the treatment of choice for osteomyelitis, with median reported non-susceptibility of 47% (21–81) for chloramphenicol and 17% for flucloxacillin (based on a sample of 32 positive blood cultures in children aged <5 years in rural Ghana).14, 32, 34
Alongside its effect within the community, S aureus is the most common hospital-acquired infection,14 and there is an increased propensity for these strains to be multiresistant (defined as having both oxacillin and cefoxitin resistance; identified among 20 [15%] of 131 community-acquired isolates and 85 [65%] of 131 hospital-acquired isolates from a study19 of invasive infection in children in South Africa; however, this research did not identify if previous antibiotic exposure confounded these blood culture results). A laboratory review33 of 248 meticillin-resistant isolates (not differentiated by community-acquired vs hospital-acquired) collected throughout South Africa revealed high frequencies of non-susceptibility to gentamicin (85%), erythromycin (58%), nitrofurantoin (38%), and clindamycin (21%); however, isolates were fully sensitive to vancomycin.
Analysis of data from a Tanzanian cohort study of 1828 bloodstream infections showed that enterococci were responsible for 15% of culture-confirmed causes of bacteraemia and resulted in case fatality rates of 29% for Enterococcus faecalis and 7% for Enterococcus faecium. A small number of invasive isolates (21 for E faecium and 15 for E faecalis) suggested more frequent non-susceptibility in hospital-acquired infection to ampicillin (89% hospital-acquired, 75% community-acquired) and gentamicin (67% hospital-acquired, 33% community-acquired) for E faecium, although E faecalis showed ampicillin susceptibility.14
Discussion
Our results highlight a dramatic lack of data on antimicrobial non-susceptibility patterns in the general paediatric population of sub-Saharan Africa, particularly for community-acquired infection. Based on the estimated prevalence of non-susceptibility among positive cultures, empirical treatment guidelines—which rely heavily on commonly available antibiotics such as penicillin and gentamicin—need review (Table 1, Table 2). Considering that about 429 million children live in sub-Saharan Africa,62 the 67 451 cultures tested in the studies identified in this Review (of which approximately 8% were culture-positive) reveal the paucity of investigations (particularly for community-acquired infections) for such a large population at risk. Furthermore, a large proportion of research does not clearly delineate the denominator of the study population, making attribution of the prevalence of non-susceptible pathogens difficult. Although our Review focused on a generalised paediatric population, estimates of non-susceptibility are likely to be higher in specific populations at risk (such as children with HIV or tuberculosis) and warrant further reviews. Children with immunocompromising conditions are a unique population in their acquisition of antimicrobial-resistant infections because of their exposure to empirical antimicrobials, frequent encounters with health-care settings, and overall immune dysfunction.63, 64, 65, 66
There is increasing evidence of antimicrobial resistance to drugs recommended in WHO antibiotic guidelines,23, 25 and together with the data presented here (Table 1, Table 2), a review of recommended empirical therapies is warranted. In the 2013 WHO guideline revisions, recommendations for some organisms were changed on the basis of susceptibility (eg, from chloramphenicol to ciprofloxacin23 to treat Shigella spp and Salmonella spp infections); however, many common organisms continue to be treated with regimens with reportedly high frequencies of in-vitro non-susceptibility because of insufficient evidence (or local data) to support further changes. Such an evidence base needs to comprise antimicrobial susceptibility patterns (identified from standardised reporting of defined populations) and the results of clinical trials that include safety data and patient outcomes.
Our Review has several limitations, including heterogeneity among the included studies and a possible sampling bias, with most studies arising from tertiary centres in urban settings, and underestimating the substantial burden of community-acquired infections. This sampling bias could overestimate the burden of morbidity caused by Gram-negative bacteria, which have a higher propensity to result in hospital presentation because of more severe clinical features and failure of oral therapy in the community, which introduces the possibility of non-representative population selection, as increased population density might be independently associated with antimicrobial resistance.67 Most research did not identify whether isolates were secondary to community-acquired or hospital-acquired infections, an issue previously highlighted in analysis of resistance patterns in paediatric patients in Africa,54, 56, 68 and although previous exposure to antimicrobials was rarely documented, it is uncertain how pretreatment (a common practice before tertiary presentation in sub-Saharan Africa) affects the validity of the findings of these studies.
Publication bias is also likely to be an issue, and although our search generated a large number of results, papers about individual pathogens might have not been captured by our search terms—for example, while susceptibility for Shigella spp to ciprofloxacin was revealed, the possibility of increasing non-susceptibility should be considered in light of the increasing burden of the S typhi MDR haplotype H58, which is widely evident throughout Asia and is reported to be present in parts of sub-Saharan Africa.69 An element of geographical publication bias is also likely since a third of countries were in southern Africa and, despite their large populations, central and west African nations were underrepresented, an issue previously noted by other reviews of antimicrobial data in Africa.9, 25 Finally, non-susceptibility estimates were calculated from a small number of isolates, which are representative of the proportions documented through the cascade of hospital-based admissions—that is, of the large number of hospital presentations, a very small proportion will have positive blood cultures, of which an even smaller proportion will be positive for a particular pathogen for which non-susceptibility to antimicrobials can be tested. This cascade might result in imprecise results, and has been documented previously.70 The tension between high prevalence of non-susceptibility in a few isolates and a low overall incidence among all seriously ill children poses a further challenge for interpretation.
Nevertheless, the available data are conclusive that antimicrobial resistance is an increasing and real threat among children admitted to hospital in sub-Saharan Africa, and prevalent MDR organisms are likely to become progressively pathogenic because of their swift spread within both the community and in hospital.19, 25, 30, 32, 36, 38, 39, 40, 51 Community carriage of ESBLs is common (up to 45%), and nosocomial acquisition occurs at a rate of 20% for every 48 h spent in hospital.30 In light of the increasing prevalence of MDR organisms in hospitals, simple improvements in local hospital-based infection control measures are important.14, 51 Our findings support a systematic review and meta-analysis71 that assessed the most effective strategies for implementing antimicrobial stewardship policies in local settings, which identified strategies that could be extrapolated to low-income and middle-income countries to tackle antimicrobial resistance. These strategies include more rigorous use of empirical therapy that follows appropriately formulated local antimicrobial guidelines, consistently taking blood cultures (where possible) before the start of antimicrobial therapy (to allow earlier cessation of antibiotics if negative), and de-escalation of therapy (from intravenous to oral) as soon as clinical improvement occurs.71
There are few antimicrobial resistance awareness programmes in sub-Saharan Africa, with low frequencies of national and regional coordination.7 These considerations should be incorporated into revisions of international treatment guidelines and monitoring of antimicrobial use. At the community level, infection control requires addressing of more pervasive and challenging issues that are inextricably linked with underdevelopment, such as poor sanitation and hygiene, overcrowding, and strategies aimed at limiting the availability of freely available over-the-counter antibiotics. Several effective surveillance systems have successfully been instituted for high-profile diseases (such as malaria, HIV, and MDR tuberculosis), providing evidence that a paediatric-focused antimicrobial resistance surveillance programme could be achieved with adequate commitment.71
It is difficult to draw firm conclusions about how increasing antimicrobial resistance contributes to neonatal and child mortality in light of the challenges of attributing mortality to antimicrobial resistance versus the underlying condition (which might be nosocomial in nature or a more severe illness), or due to insufficient access to appropriate antibiotics. Additionally, antimicrobial resistance has been increasing over the past two decades while child mortality has fallen greatly in low-income and middle-income countries. Furthermore, in-vitro non-susceptibility does not necessarily correlate with a lack of clinical therapeutic effect. Nevertheless, excessive mortality rates attributable to antimicrobial resistance have been reported,72, 73 highlighting the importance of enhanced research in this area.
Until new antimicrobial strategies are discovered and tested, the focus must remain on adherence to tailored local guidelines, education of physicians on prescribing practices, improvement of laboratory infrastructure, and promotion of collaboration between regional sites. Future research should focus on identification of appropriate local empirical therapies with improved susceptibility profiles, provision of clear clinical indications for timely second-line therapy when empirical therapy fails, establishment of guidelines for the de-escalation and cessation of antibiotic therapy, and regular surveillance of antimicrobial use within integrated, coordinated, international surveillance programmes. Standardised research methods adhering to WHO's Global Antimicrobial Resistance Surveillance System74 should be used, clearly delineating resistance patterns for community-acquired versus hospital-acquired infections, while assessing for possible biases, such as prior antibiotic exposure, and ensuring systematic selection of patients for inclusion, with clearly identified population denominators. This strategy will allow non-susceptibility patterns and antimicrobial use to be monitored on a continental scale, and will ensure this issue of utmost public health concern is effectively addressed.
Acknowledgments
Acknowledgments
Funding was provided from Nuffield Department of Medicine (The University of Oxford), General Sir John Monash Foundation, The Medical Research Council/Department for International Development/Wellcome Trust Joint Global Health Trials Scheme (MR/M007367/1), and the Bill & Melinda Gates Foundation (OPP1131320).
Contributors
PCMW did the literature search, designed figure 1, and wrote the first draft of the paper. PCMW and JAB carried out data analysis and interpretation. DI and JAB reviewed and helped revise the report. JAB conceptualised the paper, the study, and figure 2.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Laxminarayan R, Matsoso P, Pant S. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 2.Laxminarayan R, Duse A, Wattal C. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2017;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Antimicrobial resistance. Global report on surveillance. World Health Organization; Geneva: 2014. [Google Scholar]
- 4.The World Bank Databank. World development indicators. 2015. http://databank.worldbank.org/data/reports.aspx?source=2&country=SSF (accessed Feb 13, 2017).
- 5.Ashley E, Lubell Y, White N, Turner P. Antimicrobial susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asian low and middle income countries. Trop Med Int Health. 2011;16:1167–1179. doi: 10.1111/j.1365-3156.2011.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leopold S, ven Leth F, Terekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother. 2014;69:2337–2353. doi: 10.1093/jac/dku176. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Worldwide country situation analysis: response to antimicrobial resistance. World Health Organization; Geneva: 2015. [Google Scholar]
- 8.Bahwere P, Levy J, Hennart P. Community-acquired bacteraemia among hospitalized children in rural central Africa. Int J Infect Dis. 2001;5:180–188. doi: 10.1016/s1201-9712(01)90067-0. [DOI] [PubMed] [Google Scholar]
- 9.Reddy E, Shaw A, Crump J. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale A, Davies M, Anampiu K. Invasive group A streptococcus infection among children, rural Kenya. Emerg Infect Dis. 2016;22:224–233. doi: 10.3201/eid2202.151358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissoon N, Uyeki T. Sepsis and the global burden of disease in children. JAMA Pediatr. 2016;170:107–108. doi: 10.1001/jamapediatrics.2015.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omulo S, Thumbi SM, Njenga MK, Call DR. A review of 40 years of enteric antimicrobial resistance research in Eastern Africa: what can be done better? Antimicrob Resist Infect Control. 2015;4:1. doi: 10.1186/s13756-014-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eager H, Swan G, van Vuuren M. A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. J S Afr Vet Assoc. 2012;83:16. doi: 10.4102/jsava.v83i1.16. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg B, Manji K, Urassam W. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis. 2007;22:43. doi: 10.1186/1471-2334-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storberg V. ESBL-producing Enterobacteriaceae in Africa—a non-systematic literature review of research published 2008–2012. Infect Ecol Epidemiol. 2014;4:20342. doi: 10.3402/iee.v4.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 17.Murunga EM, Reriani M, Otieno CF, Wanyoike NM. Comparison of antibiotic use between an ‘open’ and a ‘closed’ intensive care unit. East Afr Med J. 2005;82:414–417. doi: 10.4314/eamj.v82i8.9326. [DOI] [PubMed] [Google Scholar]
- 18.Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H. The worldwide antibiotic resistance and prescribing in European children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71:1106–1117. doi: 10.1093/jac/dkv418. [DOI] [PubMed] [Google Scholar]
- 19.Dramowski A, Cotton M, Rabie H, Whitelaw A. Trends in paediatric bloodstream infections at a South African referral hospital. BMC Pediatr. 2015;15:33. doi: 10.1186/s12887-015-0354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493. doi: 10.1136/bmj.f1493. [DOI] [PubMed] [Google Scholar]
- 21.WHO . Pocket book of hospital care for children. Guidelines for the management of common illnesses with limited resources. World Health Organization; Geneva: 2005. [PubMed] [Google Scholar]
- 22.WHO . Guideline. Managing possible serious bacterial infection in young infants when referral is not feasible. World Health Organization; Geneva: 2015. [PubMed] [Google Scholar]
- 23.Downie L, Armiento R, Subhi R, Kelly J, Clifford V, Duke T. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics—systematic review and meta-analysis. Arch Dis Childhood. 2013;98:146–154. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 24.Alcoba G, Kerac M, Breysse S. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PLoS One. 2013;8:e53184. doi: 10.1371/journal.pone.0053184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Doare J, Bielicki J, Heath PT, Sharland M. Systematic review of antibiotic resistance rates among Gram-negative bacteria in children with sepsis in resource-limited countries. J Paediatric Infect Dis Soc. 2014;4:11–20. doi: 10.1093/jpids/piu014. [DOI] [PubMed] [Google Scholar]
- 26.Talbert AW, Mwaniki M, Mwarumba S, Newton CR, Berkley JA. Invasive bacterial infections in neonates and young infants born outside hospital admitted to a rural hospital in Kenya. Pediatr Infect Dis J. 2010;29:945–950. doi: 10.1097/INF.0b013e3181dfca8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayange N, Kamugisha E, Mwizamholya D, Jeremiah S, Mshana S. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010;10:39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mhada TV, Fredrick F, Matee MI, Massawe A. Neonatal sepsis at Muhimbili National Hospital, Dar es Salaam, Tanzania: aetiology, antimicrobial sensitivity pattern and clinical outcome. BMC Public Health. 2012;12:904. doi: 10.1186/1471-2458-12-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwadioha I, Nwokedi E, Kashibu E, Odimayo MS, Okwori EE. A review of bacterial isolates in blood cultures of children with suspected septicaemia in a Nigerian tertiary hospital. African J Microbiol Res. 2010;4:222–225. [Google Scholar]
- 30.Schaumberg F, Alabi A, Kokou C. High burden of extended-spectrum β-lactamase-producing Enterobacteriaceae in Gabon. J Antimicrob Chemother. 2013;68:2140–2143. doi: 10.1093/jac/dkt164. [DOI] [PubMed] [Google Scholar]
- 31.Sigaúque B, Roca A, Mandomando I. Community-acquired bacteraemia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen M, Sarpong N, Krumkamp R. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One. 2012;7:e44063. doi: 10.1371/journal.pone.0044063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marais E, Aithma N, Perovic O, Oosthuysen W, Musenge E, Dusé AG. Antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus isolates from South Africa. S Afr Med J. 2009;99:170–173. [PubMed] [Google Scholar]
- 34.Nantanda R, Hildenwall H, Peterson S, Kaddu-Mulindwa D, Kalyesubula I, Tumwine JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Ann Trop Paediatr. 2008;28:253–260. doi: 10.1179/146532808X375404. [DOI] [PubMed] [Google Scholar]
- 35.Gray MJ, Bennett SL, French N, Phiri AJ, Graham SM. Invasive group B streptococcal infection in infants, Malawi. Emerg Infect Dis. 2007;13:223–230. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndir A, Diop A, Faye PM, Cissé MF, Ndoye B, Astagneau P. Epidemiology and burden of bloodstream infections caused by extended-spectrum β-lactamase producing Enterobacteriaceae in a pediatric hospital in Senegal. PLoS One. 2016;11:e0143729. doi: 10.1371/journal.pone.0143729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enwere G, Biney E, Cheung Y. Epidemiologic and clinical characterstics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J. 2006;25:700–705. doi: 10.1097/01.inf.0000226839.30925.a5. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz N, Sarpong N, Hünger F. Systemic bacteraemia in children presenting with clinical pneumonia and the impact of non-typhoid salmonella (NTS) BMC Infect Dis. 2010;10:319. doi: 10.1186/1471-2334-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandomando I, Jaintilal D, Pons MJ. Antimicrobial susceptibility and mechanisms of resistance in Shigella and Salmonella isolates from children under five years of age with diarrhoea in rural Mozambique. Antimicrob Agents Chemother. 2009;53:2450–2454. doi: 10.1128/AAC.01282-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phoba MF, De Boeck H, Ifeka BB. Epidemic increase in Salmonella bloodstream infection in children, Bwamanda, the Democratic Republic of Congo. Eur J Clin Microbiol Infect Dis. 2014;33:79–87. doi: 10.1007/s10096-013-1931-8. [DOI] [PubMed] [Google Scholar]
- 41.Ginsburg AS, Tinkham L, Riley K, Kay NA, Klugman KP, Gill CJ. Antibiotic non-susceptibility among Streptococcus pneumoniae and Haemophilus influenzae isolates identified in African cohorts: a meta-analysis of three decades of published studies. Int J Antimicrob Agents. 2013;42:482–491. doi: 10.1016/j.ijantimicag.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Aamodt H, Mohn SC, Maselle S. Genetic relatedness and risk factor analysis of ampicillin-resistant and high-level gentamicin-resistant enterococci causing bloodstream infections in Tanzanian children. BMC Infect Dis. 2015;15:107. doi: 10.1186/s12879-015-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usha G, Chunderika M, Prashini M, Willem S, Yusuf E. Characterisation of extended-spectrum beta-lactamases in Salmonella spp at a tertiary hospital in Durban, South Africa. Diagn Microbiol Infect Dis. 2008;62:86–91. doi: 10.1016/j.diagmicrobio.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 45.Lubell Y, Turner P, Ashley E, White N. Susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asia to macrolide antibiotics. Trop Med Int Health. 2011;16:1192–1205. doi: 10.1111/j.1365-3156.2011.02837.x. [DOI] [PubMed] [Google Scholar]
- 46.Balshem H, Helfand M, Schünemann HJ. GRADE guidelines: 3. rating the quality of the evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Vlieghe E, Phoba M, Tamfun J, Jacobs J. Antibiotic resistance among bacterial pathogens in Central Africa: a review of the published literature between 1955 and 2008. Int J Antimicrob Agents. 2009;34:295–303. doi: 10.1016/j.ijantimicag.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Roca A, Bassat Q, Morais L. Surveillance of acute bacterial meningitis among children admitted to a district hospital in rural Mozambique. Clin Infect Dis. 2009;48(suppl 2):S172–S181. doi: 10.1086/596497. [DOI] [PubMed] [Google Scholar]
- 49.Falade AG, Lagunju IA, Bakare RA, Odekanmi AA, Adegbola RA. Invasive pneumococcal disease in children aged <5 years admitted to 3 urban hospitals in Ibadan, Nigeria. Clin Infect Dis. 2009;48(suppl 2):S190–S196. doi: 10.1086/596500. [DOI] [PubMed] [Google Scholar]
- 50.Falade AG, Ayede AI. Epidemiology, aetiology and management of childhood acute community-acquired pneumonia in developing countries—a review. Afr J Med Med Sci. 2011;40:293–308. [PubMed] [Google Scholar]
- 51.Woerther PL, Angebault C, Jacquier H. Massive increase, spread and exchange of extended spectrum β-lactamase-encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin Infect Dis. 2011;53:677–685. doi: 10.1093/cid/cir522. [DOI] [PubMed] [Google Scholar]
- 52.Huynh BT, Padget M, Garin B. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Infect Dis. 2015;15:127. doi: 10.1186/s12879-015-0843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha L, Russell LB, Tomcysk S. Disease burden of group B Streptococcus among infants in sub-Saharan Africa: a systematic review and meta-analysis. Pediatr Infect Dis J. 2016;35:933–942. doi: 10.1097/INF.0000000000001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamer G, Darmstadt GL, Carlin JB. Etiology of bacteraemia in young infants in six countries. Pediatr Infect Dis J. 2015;34:e1–e8. doi: 10.1097/INF.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaidi AK, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J. 2009;28(suppl 1):S10–S18. doi: 10.1097/INF.0b013e3181958769. [DOI] [PubMed] [Google Scholar]
- 56.Waters D, Jawad I, Ahmad A. Aetiology of community-acquired neonatal sepsis in low and middle-income countries. J Glob Health. 2011;1:154–170. [PMC free article] [PubMed] [Google Scholar]
- 57.Kabwe M, Tembo J, Chilukutu L. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral centre in Zambia. Pediatr Infect Dis J. 2016;35:e191–e198. doi: 10.1097/INF.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 58.The World Health Organization Causes of Child Mortality. 2015. http://www.who.int/gho/child_health/mortality/causes/en (accessed Jan 10, 2016).
- 59.Davies NE, Karstaedt AS. Shigella bacteraemia over a decade in Soweto, South Africa. Trans R Soc Trop Med Hyg. 2008;102:1269–1273. doi: 10.1016/j.trstmh.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 60.Usuf E, Bottomley C, Adegbola RA, Hall A. Pneumococcal carriage in sub-Saharan Africa—a systematic review. PLoS One. 2014;9:e85001. doi: 10.1371/journal.pone.0085001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Gottberg A, de Gouveia L, Tempia S. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 62.The World Bank World development indicators: population dynamics. http://data.worldbank.org/indicator/SP.POP.0014.TO.ZS?locations=ZG (accessed Dec 10, 2016).
- 63.McNeil JC. Staphylococcus aureus—antimicrobial resistance and the immunocompromised child. Infect Drug Resist. 2014;7:117–127. doi: 10.2147/IDR.S39639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cotton MF, Wasserman E, Smit J, Whitelaw A, Zar HJ. High incidence of antimicrobial resistant organisms including extended spectrum beta-lactamase producing Enterobacteriaceae and methicillin-resistant Staphylococcus aureus in nasopharyngeal and blood isolates of HIV-infected children from Cape Town, South Africa. BMC Infect Dis. 2008;8:40. doi: 10.1186/1471-2334-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Groome M, Albrich W, Wadula J, Khoosal M, Madhi SA. Community-onset Staphylococcus aureus bacteraemia in hospitalized African children: high incidence in HIV-infected children and high prevalence of multidrug resistance. Paediatr Int Child Health. 2012;32:140–146. doi: 10.1179/1465328111Y.0000000044. [DOI] [PubMed] [Google Scholar]
- 66.Tan CK, Lai CC, Liao CH, Lin SH, Huang YT, Hsueh PR. Increased rifampicin resistance in blood isolates of meticillin-resistant Staphylococcus aureus (MRSA) amongst patients exposed to rifampicin-containing antituberculosis treatment. Int J Antimicrob Agents. 2011;37:550–553. doi: 10.1016/j.ijantimicag.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Bruinsma N, Hutchinson JM, van den Bogaard AE, Giamarellou H, Degener J, Stobberingh EE. Influence of population density on antibiotic resistance. J Antimicrob Chemother. 2003;51:385–390. doi: 10.1093/jac/dkg072. [DOI] [PubMed] [Google Scholar]
- 68.Huynh BT, Padget M, Garin B, Delarocque-Astagneau E, Guillemot D. Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet. 2016;387:533–534. doi: 10.1016/S0140-6736(16)00220-8. [DOI] [PubMed] [Google Scholar]
- 69.Kariuki S, Revathi G, Kiiru J. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in southeast Asia. J Clin Microbiol. 2010;48:2171–2176. doi: 10.1128/JCM.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sangare SA, Rondinaud E, Maataoui N. Very high prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in bacteriemic patients hospitalized in teaching hospitals in Bamako, Mali. PLoS One. 2017;12:e0172652. doi: 10.1371/journal.pone.0172652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuts EC, Hulscher ME, Mouton JW. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 72.Laxminarayan R, Bhutta ZA. Antimicrobial resistance—a threat to neonate survival. Lancet Glob Health. 2017;4:e676–e677. doi: 10.1016/S2214-109X(16)30221-2. [DOI] [PubMed] [Google Scholar]
- 73.Thaver D, Ali SA, Zaidi AK. Antimicrobial resistance among neonatal pathogens in developing countries. Pediatr Infect Dis J. 2009;28(suppl 1):S19–S21. doi: 10.1097/INF.0b013e3181958780. [DOI] [PubMed] [Google Scholar]
- 74.WHO . Global antimicrobial resistance surveillance system. Manual for early implementation. World Health Organization; Geneva: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.