Abstract
The timing of sex in facultatively sexual organisms is critical to fitness, due to the differing demographic consequences of sexual versus asexual reproduction. In addition to the costs of sex itself, an association of sex with the production of dormant life stages also influences the optimal use of sex, especially in environments where resting eggs are essential to survive unfavourable conditions. Here we document population dynamics and the occurrence of sexual reproduction in natural populations of Daphnia magna across their growing season. The frequency of sexually reproducing females and males increased with population density and with decreasing asexual clutch sizes. The frequency of sexually reproducing females additionally increased as population growth rates decreased. Consistent with population dynamic models showing that the opportunity cost of sexual reproduction (foregoing contribution to current population growth) diminishes as populations approach carrying capacity, we found that investment in sexual reproduction was highest when asexual population growth was low or negative. Our results support the idea that the timing of sex is linked with periods when the relative cost of sex is reduced due to low potential asexual growth at high population densities. Thus, a combination of ecological and demographic factors affect the optimal timing of sexual reproduction, allowing D. magna to balance the necessity of sex against its costs.
Keywords: timing of sex, Daphnia, population density, cost of sex
1. Introduction
Many treatments of the evolution of sex consider sexual and asexual forms as discrete lineages (reviewed in [1,2]). In nature, however, competition between these types is often more subtle. Organisms that use sex facultatively potentially gain the ‘best of both worlds’, as they avoid paying costs of sexual reproduction much of the time, while maintaining access to the benefits of genetic recombination [3–5]. This flexibility brings about a suite of life-history consequences: the ability to reproduce either sexually or asexually, depending on current conditions, means that the frequency and timing of the sexual life cycle is an evolvable trait [6].
The timing of sex in facultative sexual organisms is critical to fitness for several reasons stemming from the differing demographic consequences of sexual versus asexual reproduction. Asexual reproduction is usually the more efficient strategy in terms of converting resources into offspring, as it avoids the ‘twofold cost’ of male production [7,8]. A genotype's asexual and sexual success are not independent, and strongly traded off against each other, because a female can only do one at a time. Switching from asex to sex too early also entails potentially large opportunity costs: foregoing the opportunity to contribute to asexual generations (i.e. current population growth) can, in an exponentially growing population, drastically reduce the representation of a clonal genotype in the mating pool, and hence in the sexually produced offspring generation. This opportunity cost (birth rate disadvantage) of sexual reproduction may be particularly pronounced when offspring gained through sex are not equivalent to offspring produced asexually, as often observed in nature. For example, in facultative sexual organisms sexual offspring are often dormant or dispersing life stages (e.g. Cladocera: [9]; rotifers [10,11]; aphids [12]) that do not contribute to current local population growth.
In populations that undergo periods of inhospitable conditions, an association between sexual reproduction and dormancy results in potentially complex selection on the timing of sex. Sexually produced, diapausing offspring are vital for the persistence of a lineage across favourable (‘growing’) seasons, and must be produced before the intervening periods when the habitat is unsuitable. In such a system, the measure of fitness that is expected to be maximized is the total count of sexually produced dormant stages at the end of the growing season [13,14]. In contrast, during the growing season, selection in the short term favours asexual reproduction due to its efficiency [15]. If individuals can precisely predict the duration of each favourable period, we expect a simple switch from asexual to sexual reproduction towards the end of the growing season (N Gerber, H Kokko, I Booksmythe 2018, unpublished data) [16,17]. However, in unpredictable environments individuals might hedge their bets with regards to reproductive mode, while in more predictable environments the use of environmental cues could allow this plasticity [18]. Indeed, in various facultatively sexual species, changes in temperature [12], population density or crowding [19,20], food quality [21,22], photoperiod [22] and predation [23] contribute to the induction of sexual reproduction. All of these cues potentially inform females about seasonal changes and/or deteriorating conditions, under which sex may be advantageous [24]. The reproductive response to such cues can be complicated by the need to produce both males and sexually reproductive females so that they coincide at sexual maturity during the period optimal for the production of sexual offspring [25,26].
The cost of sex is not a fixed parameter, but may depend itself on current conditions. Investment in each reproductive mode is expected to be influenced by the relative costs of sexual and asexual reproduction. For example, mate-finding costs may be higher at low densities, when encounter rates between individuals are low [27,28]. However, this cost may be diminished if individuals can flexibly switch to asexuality should a mate not be found. Similarly, the cost of sex may depend on the current scope for asexual population growth [29–31]. Assuming that adults are better able than offspring to survive at high densities, when a population approaches carrying capacity the recruitment rate declines.
A handful of population dynamic models highlight the consequences of these dynamics for the demographic advantage of asexual lineages over sexuals [32–34]. They show that asexuality cannot always realize its demographic advantage: if high densities prevent immediate population growth, the opportunity cost of sex diminishes for populations nearing carrying capacity. Although this is not always sufficient to modify the cost if sex and asex occur simultaneously [8], the prediction for facultative sexuals with dormant sexual eggs is clear: they should switch to sex as resources become limiting and the opportunity cost of sexual reproduction is reduced. This important prediction has, to date, been largely overlooked by empirical studies of the costs and benefits of sex.
In facultative sexual Daphnia (Cladocera: Daphniidae) we expect strong selection on the timing of sex. Daphnia sex appears costly relative to asexual reproduction in the short term due to its demographic effects. The largest asexual clutches recorded for D. magna contain approximately 110 eggs [35], whereas sexual clutches contain at most two eggs. This clutch size difference represents a potentially extreme opportunity cost of reproducing sexually, which necessarily entails foregoing an asexual reproductive bout. Non-equivalence of sexually and asexually produced offspring holds for this system: asexually produced eggs develop immediately in the maternal brood chamber into free-swimming plankton, whereas fertilized sexual eggs must undergo a period of dormancy, encased in a hardy capsule known as an ephippium [9]. Additionally, sexual reproduction requires the (asexual) production of males, which reduces the asexual growth rate of a lineage. However, in terms of resource allocation, the extent to which investment in sex trades off with other life-history traits, including asexual investment, is not clear. While production times for sexual and asexual clutches are equal, the large number of eggs in an asexual clutch could translate into higher resource requirements compared to the two eggs per sexual clutch. Alternatively, melanization of the ephippium and provisioning for dormancy might require additional resources when producing a sexual clutch.
Ultimately, only sexual, dormant eggs are able to withstand harsh conditions, including freezing and desiccation, so sexual reproduction is vital for the long-term persistence of a lineage over inhospitable periods. At the start of each growing season, when environmental conditions become suitable, dormant eggs hatch into females that found the planktonic population anew. Male production and the female switch to sexual reproduction may occur in response to different cues, or with different sensitivity to the same cues: in D. magna, production of males and of sexual clutches responded differently to manipulations of photoperiod [36], and male production has been observed to occur more stochastically throughout the growing season compared to ephippia production [25]. The relative roles of environment and genotype in determining the likelihood of male and ephippia production also vary: for example, in Daphnia pulex inhabiting temporary ponds over a short growing season, substantial male production occurred very early while population densities were still low, and was also not linked to other environmental factors such as pond temperature [37].
Most studies on the timing of sex and male production in Daphnia have focused on cues that allow dormant eggs to be produced before environmental deterioration or the end of the season, and have been conducted under laboratory conditions (e.g. [22,26,36,38,39], but see [37]). We aim to add the costs and consequences of sex itself to this picture, and focus on population density as a variable connecting the ecological and demographic influences on the timing of sex. We highlight the hypothesis of demographically varying costs of sex [32–34] as an important alternative to the prevailing emphasis on sex as a response to deteriorating conditions. This prevailing view sees sex either providing a direct escape route (e.g. dormancy [26,36]) or generating diversified offspring through recombination, to explain why particularly stressful conditions induce sex [40].
Of these three options, we focus on the first two (the demographic cost hypothesis, and the habitat deterioration hypothesis). The third hypothesis appears unlikely to explain the precise scheduling of sex in the current context. While high density (and its correlates, e.g. increased resource limitation or disease risk) may constitute a stressful environment, it is difficult to envisage a benefit of producing diverse offspring genotypes in response to this transient stress. Offspring hatch in subsequent seasons under benign density conditions; the range of densities a lineage may later encounter is independent of the density when the lineage-founding ephippia were produced.
Returning to the two focal hypotheses, previous work has shown that crowding promotes sex induction and reduces asexual fecundity in laboratory populations of Daphnia [41]. Observing these patterns in natural populations would support the habitat deterioration hypothesis, with support strengthening if populations do not persist after reaching high density. The demographic cost hypothesis, in contrast, predicts that density directly modifies the relative costs of sex and asexual reproduction through its relationship with the population's capacity for growth [32–34]. In this case, we would expect sex induction to be related to population growth rates in addition to density.
We used an intensive longitudinal sampling regime to document population dynamics and the occurrence of sexual reproduction over the main part of the growing season in natural populations of cyclically parthenogenetic Daphnia magna. We investigated the interacting effects of population density, asexual reproductive investment and growth rates on the frequency of sexually reproducing individuals. Additionally, in the laboratory we estimated resource allocation trade-offs between the production of ephippia and asexual fecundity over the lifespan of individual females, to clarify whether investment in sex imposes costs beyond its immediate demographic disadvantage.
2. Methods
(a). Population sampling
We sampled 11 natural D. magna populations every 3–4 days for 60 days (May 30–July 28, 2015). Populations inhabited separate rock pools distributed over six islands (FU1, HA, K, LON, N and SMF) in the Finnish archipelago near Tvärminne Zoological Station (59.8420° N, 23.2018° E). We recorded density and demographic structure (‘stage-structure’) of the populations at each sampling point. To assess population density, 350 ml water samples were collected at 15 haphazardly chosen locations spanning the pool area and depth. These were combined in a bucket and stirred to distribute individuals evenly, and a 350-ml subsample was taken as the final density sample. The remaining animals were returned to the rock pool. After collecting the density sample a small hand net was swept through the pond to take a representative population sample.
Live samples were brought back to the laboratory and analysed the same day. All D. magna individuals in the 350-ml density sample were counted under a dissecting microscope and converted to an estimate of individuals per litre. The stage-structure samples were variable in size; to make larger samples manageable (less than 1000 individuals) they were split using a Folsom plankton sample divider. The sample was then sieved through a 0.6 mm nylon mesh to separate the smallest individuals. Individuals that remained in the sieve were counted and classified into the following categories under a dissecting microscope: females with asexual eggs or embryos in the brood pouch, females with an empty brood pouch but filled ovaries, females with ephippia, adult females without eggs, embryos or filled ovaries, juvenile females (indicated by short first abdominal process [9]); adult males (prolonged first antenna, copulatory hook on the first thoracic leg [9,42]) and juvenile males. After assessing stage-structure, up to 10 females (where possible; median = 10, mean ± s.e. = 8.72 ± 0.17) with asexual eggs in the brood pouch were isolated from the sample and maintained in individual 35 ml jars until they released their clutch. The number and sex of offspring was determined under a dissecting microscope. This paper's focus is the timing of investment in sexual reproduction, and does not present the data on offspring sex allocation, which is addressed in a second study using the population density and stage-structure data collected here (I Booksmythe, N Gerber, D Ebert, H Kokko 2018, unpublished data).
(b). Reproductive life-history trade-offs
We collected large population samples from five additional rock pool populations and isolated 60 females (F0) carrying asexual clutches. Females were kept individually in 50 ml Falcon tubes filled with artificial Daphnia medium (ADaM [43]) and fed daily with Scenedesmus algae (approximately 5 million cells per individual per day) until they released their first clutch. We isolated four F1 daughters per F0 female and housed them in pairs in 50 ml falcon tubes until they produced their first clutch. Twenty-two of these groups of four sister F1 females (henceforth ‘clones’) synchronously produced enough daughters that we could isolate 10 F2 females per clone, half of which were assigned to a long day length treatment (18:6 hours light:dark) and the other half to a short day length treatment (6:18 hours light:dark) on the day of their release from the maternal brood pouch. We used extreme day lengths (naturally occurring at midsummer and midwinter at the study site) to induce propensities for sex that were as different as possible between treatment groups. Over the 35-day experimental period, females experienced these photoperiod treatments under otherwise standardized conditions in climate chambers (20°C, with Daphnia placed approximately 20 cm below the fluorescent light source). Individual F2 females were fed and checked daily for the release of asexual clutches or sexually produced ephippia. When an asexual clutch was released, the date, number and sex of offspring were recorded, the offspring removed and the water changed. When an ephippium was produced, the date was recorded, the ephippium removed and the water changed. We recorded the date of any deaths. Females that did not reproduce were excluded from the analysis. We also excluded seven females that produced an ephippium in the very first clutch, which needed twice the time to produce their first clutch compared to other females, indicating very unusual behaviour.
(c). Statistical analysis
We were interested in how the frequency of sex relates to population density, population growth and asexual reproductive effort. We ran separate models to predict the frequencies of sexual females and males, as they could respond differently to these predictors. Models for the frequency of sexual females in a sample used population density from the previous sampling point (‘lag density’) as a predictor, because these prior conditions (3–4 days before) coincide with the point at which female reproductive mode would have been determined [9]. However, models for the frequency of adult males used current density, as conditions at the previous sampling point do not coincide with the production of these males. The appropriate lag period (the amount of time males need to mature) is at least 10 days/three sampling points, and a predictor variable using this lag would have unacceptably reduced our sample size. Using current density in the analysis instead allows us to examine whether males are produced so as to coincide with periods of high density in adulthood. As density varied by orders of magnitude across populations, and within populations over time, we used log-transformed density in all analyses. We calculated the intrinsic rate of per capita population growth per time step as 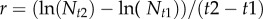 [44].
[44].
Asexual reproductive effort was estimated from the clutch size of approximately 10 females, carrying asexual eggs, per population sample. The clutch size among sampled females overestimates the mean population asexual reproductive effort, as a small but variable proportion of mature females in each population sample showed no current reproductive investment (with neither eggs in the brood pouch nor filled ovaries). We weighted the mean clutch size of sampled females by the proportion of currently reproductive females among all mature, non-sexually reproducing females to estimate the mean asexual reproductive effort in the population. We used log-transformed clutch size and reproductive effort in our analyses to normalize their distribution. Because asexual reproductive effort and growth are related (collinearity) we included them separately in models with density as the only other predictor. To determine the threshold asexual reproductive effort at which populations switch to sexual reproduction, we created a binary dependent variable for whether a population sample contained females investing in sexual reproduction or not. We fitted a logistic regression of this variable over asexual reproductive effort and determined its inflection point.
Statistical analyses were performed in R (v. 3.2.2) [45]. We used linear mixed-effects models in the package lme4 [46] for analyses of density, clutch size, and growth rates in the natural populations, and of asexual clutch size and mean interval between clutches in the laboratory experiment. For analyses of proportions of males and sexual females we used generalized linear mixed-effects models (GLMMs) with binomial error and logit link in lme4. To account for repeated measurements the population ID (natural populations) or family ID (laboratory experiment) was included as random factor. If binomial models were overdispersed an observation-level random factor was included [47]. Predictor variables in binomial GLMMs were standardized to aid in interpretation of parameter estimates, reported on the log odds scale; as an indication of effect sizes, we also present the odds ratio for each parameter, and marginal and conditional R2 [48] for each GLMM. Summary statistics are presented as mean ± 1 standard error (s.e.), unless otherwise specified.
3. Results
(a). Sex is associated with high density and low asexual reproductive effort
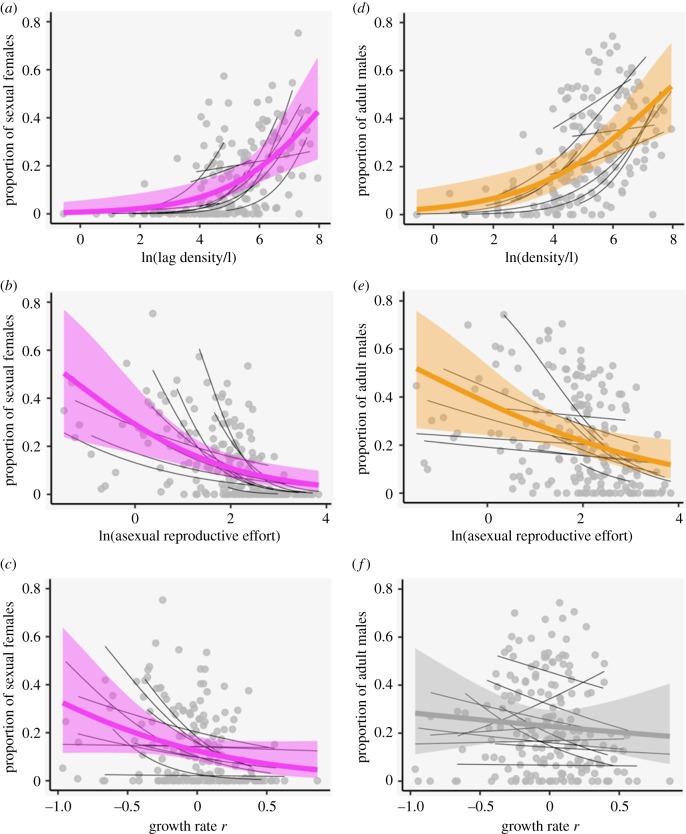
The frequency of sexual females (adult females carrying sexual eggs) was larger following high population densities, and when mean reproductive effort among asexual females was low (figure 1, table 1). The value for asexual reproductive effort at which the majority of populations contained females investing in sexual eggs was 11.47 offspring (electronic supplementary material, figure S1). The frequency of sexual females also increased with decreasing growth rate r (figure 1, table 1). The frequency of males in the adult population was larger when density was high, and when asexual effort was low, but was not significantly related to population growth rate (figure 1, table 1). Odds ratios in table 1 show the predicted change in odds with each standard deviation increase in the predictor, for a constant (mean) value of the covariate. For example, the odds of a female carrying an ephippium were 0.035 at the intercept (i.e. for mean values of density and asexual effort). For each standard deviation increase in density, keeping asexual effort constant, these odds increased by a factor of 4.75.
Figure 1.
The relationship of the proportion of sexual females (a–c) and males (d–f) with lag (a) or current (d) population density (Daphnia/l), asexual reproductive effort (b,e), and growth rate (c,f). Bold lines show the global logistic regression with 95% confidence intervals, with significant relationships in colour and non-significant in grey. Thin black lines show regressions for each population, and light grey points show raw data. (Online version in colour.)
Table 1.
Effects of population density, mean asexual reproductive effort (RE), and growth rate on the proportions of sexual females and adult males in a population; estimated by binomial GLMM with logit link. Parameter estimates are presented as the log odds ratio (β) and its standard error (s.e.); we additionally present the odds ratio (OR) as a measure of effect size. 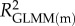 , marginal R2 (variance explained by fixed effects);
, marginal R2 (variance explained by fixed effects); 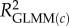 , conditional R2 (variance explained by fixed + random effects) [48].
, conditional R2 (variance explained by fixed + random effects) [48].
| model: density and asexual reproductive effort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| proportion of sexual females (n = 174) |
proportion of adult males (n = 185) |
||||||||||
| fixed effects: | β | s.e. | z | p | OR | fixed effects: | β | s.e. | z | p | OR |
| (intercept) | −3.344 | 0.333 | −10.04 | <0.001 | 0.035 | (intercept) | −1.878 | 0.172 | −10.91 | <0.001 | 0.153 |
| ln(lag density) | 1.558 | 0.221 | 7.04 | <0.001 | 4.751 | ln(density) | 0.976 | 0.129 | 7.56 | <0.001 | 2.654 |
| ln(mean asexual RE) | −0.914 | 0.155 | −5.91 | <0.001 | 0.401 | ln(mean asexual RE) | −0.470 | 0.108 | −4.36 | <0.001 | 0.625 |
| random effects: | s.d. | random effects: | s.d. | ||||||||
| population ID | 0.983 | population ID | 0.466 | ||||||||
| observation ID | 1.412 | observation ID | 1.206 | ||||||||
| R2: | 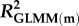 |
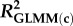 |
R2: | 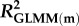 |
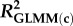 |
||||||
| 0.426 | 0.514 | 0.224 | 0.258 | ||||||||
|
model: density and growth rate | |||||||||||
| proportion of sexual females (n = 166) |
proportion of adult males (n = 177) |
||||||||||
| fixed effects: | β | s.e. | z | p | OR | fixed effects: | β | s.e. | z | p | OR |
| (intercept) | −3.299 | 0.274 | −12.05 | <0.001 | 0.037 | (intercept) | −1.910 | 0.171 | −11.15 | <0.001 | 0.148 |
| ln(lag density) | 1.879 | 0.240 | 7.83 | <0.001 | 6.544 | ln(density) | 1.177 | 0.144 | 8.19 | <0.001 | 3.243 |
| growth rate r | −0.389 | 0.155 | −2.52 | 0.012 | 0.678 | growth rate r | 0.161 | 0.114 | 1.41 | 0.16 | 1.175 |
| random effects: | s.d. | random effects: | s.d. | ||||||||
| population ID | 0.721 | population ID | 0.443 | ||||||||
| observation ID | 1.586 | observation ID | 1.277 | ||||||||
| R2: | 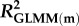 |
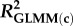 |
R2: | 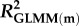 |
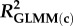 |
||||||
| 0.394 | 0.444 | 0.191 | 0.222 | ||||||||
The association of higher frequencies of sexual females with high population density and low growth rates can also be seen when looking at patterns across the growing season (electronic supplementary material, figure S2). Across all populations, there were two main peaks in density during our sampling period. These peaks are followed by periods of reduced growth rate (electronic supplementary material, figure S2) reflecting the negative relationship between population growth and population density (LMM: slope = −0.053 ± 0.014, χ2 = 13.83, p < 0.001). Lag population density was also negatively related to asexual clutch size (LMM: slope = −0.26 ± 0.047, χ2 = 31.02, p < 0.001) (electronic supplementary material, figure S2).
(b). Ephippia production trades off with asexual clutch size
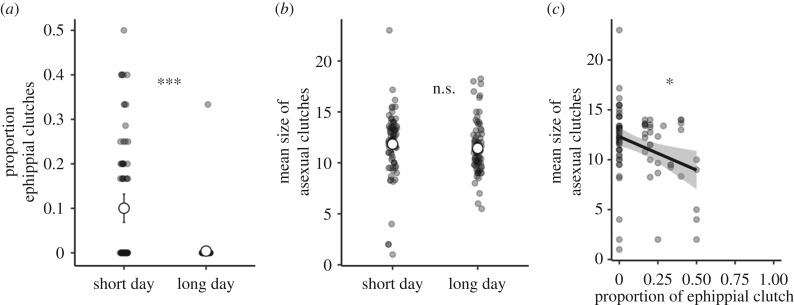
The number of females that reproduced in the long-day and short-day experimental treatments did not differ (83 of 110 and 88 of 110, respectively; z = 0.81, p = 0.42). The short-day treatment successfully induced ephippia production, with 41 of 88 females producing at least one ephippium, compared to one of 83 females in the long-day treatment (GLMM: slope = −4.700 ± 1.199, z = −3.92, p < 0.001; figure 2). Total asexual reproductive success (number of offspring) was higher in the long-day treatment (LMM: slope = 7.507 ± 2.475, χ2 = 9.20, p = 0.002; figure 2). Day length did not affect the total number of reproductive bouts, the latency to first reproduction, or the mean interval between clutches (all p > 0.05). Nor did day length affect the mean asexual clutch size when calculated across all of a female's asexual clutches (LMM: slope = 0.178 ± 0.420, χ2 = 0.18, p = 0.67). Within the short-day treatment, we could compare individuals that reproduced only asexually with those that produced at least one sexual clutch. The latter had fewer total asexual offspring, losing on average nine offspring compared to their clone mates that reproduced only asexually (asexual, 54.1 ± 1.9, sexual, 45.4 ± 3.2; LMM: slope = −9.167 ± 3.856, χ2 = 5.65, p = 0.02), but whether or not females reproduced sexually did not affect their mean asexual clutch size (−0.974 ± 0.801, χ2 = 1.48, p = 0.22). The absolute number of sexual clutches (0, 1 or 2) was not related to mean asexual clutch size (−0.523 ± 0.588, χ2 = 0.79, p = 0.37). However, the proportion of a female's reproductive events that were sexual was negatively related to her mean asexual clutch size (LMM: slope = −5.958 ± 2.535, χ2 = 5.52, p = 0.019); if half of a female's clutches were sexual, the mean size of her asexual clutches was reduced by around three eggs compared to females producing only asexual clutches (figure 2c). Females that produced relatively many ephippia had smaller clutches when they reproduced asexually.
Figure 2.
The effect of day length treatment (short-day, 6:18 hours light:dark; long-day, 18:6 hours light:dark) on (a) the mean proportion of a female's clutches that were sexual and (b) the mean size of asexual clutches; and (c) the relationship between the proportion of a female's clutches that were sexual and her mean asexual clutch size, for the short-day treatment only. Asterisks indicate significance (n.s., p > 0.05; *p < 0.05; ***p < 0.001).
4. Discussion
Daphnia magna in the rock pool habitat studied here experience a short growing season followed by completely inhospitable conditions in which only dormant, sexually produced eggs persist. Besides winter freezing, pools often experience summer droughts [49]. The resulting strong selection for the production of dormant eggs is therefore expected to shape the timing of sexual and asexual reproduction during the growing season. While approaching inhospitable conditions do influence investment in sexual reproduction [36], here we argue that in line with theoretical predictions, the reduced cost of sexual relative to asexual reproduction at high densities may plausibly favour density as a cue for the switch to sexual reproduction in D. magna. Our results suggest that an understanding of how population dynamics affect the costs of sex can inform predictions of when sex should occur, and highlight the importance of considering variation in the costs of sex when seeking explanations for the maintenance of sexual reproduction.
Investment in sexual reproduction in our sampled populations was strongly predicted by population density: when density was high at the previous sampling point, a greater proportion of females in the current sample carried sexual eggs. The frequency of males in the adult population also increased with density. These relationships were consistent over the sampled time period, where we observed two density peaks (electronic supplementary material, figure S2). However, neither of these peaks in density and sexual reproduction was followed by extinction of the planktonic population, indicating that sex did not immediately precede inhospitable periods where the dormant stage would be vital. This conflicts with the habitat deterioration hypothesis, suggesting that the approach of inhospitable conditions alone cannot explain the marked increase in investment in sex at these points.
We suggest, in accordance with the demographic cost hypothesis, that the association of sexual reproduction and population density results from declining efficiency of asexual reproduction as populations approach carrying capacity—newborn offspring may then have difficulty recruiting into the current population, which reduces the returns on asexual offspring. Consequently, the relative profitability of sexual reproduction (which does not contribute to current population growth) increases with population density. Furthermore, sexually produced offspring are not affected by current conditions (e.g. competition), as they do not hatch until subsequent growing seasons. We could not measure juvenile survival, which is predicted to decrease with population density under this scenario. However, the frequency of sexual females increased with decreasing (or negative) population growth, and decreasing asexual reproductive effort. Thus, for a given population density, females increased their investment in sexual reproduction when potential growth was low or populations were declining. This supports the demographic cost hypothesis that sex in ephemeral D. magna populations is timed to coincide with periods when the opportunity cost of sexual reproduction is reduced [32–34] (in addition to the likelihood of sex increasing with ecological cues, such as changes in day length, ensuring that it occurs before the season end [36]). Our data on the clutches of females taken from natural populations suggest that this cost is balanced when the mean asexual reproductive effort in a population is around 11.5 eggs. Below this value, sexually reproducing females could be found in the majority of populations (electronic supplementary material, figure S1).
Interestingly, in other facultative sexual systems showing an association between population density and sex induction (e.g. rotifers [14,20,50]), density-dependent induction of sex has been shown to contribute to regulating population density in a controlled laboratory setting [51]. Because of the production of males and dormant stages, which require resource investment and do not contribute to current population growth, increasing rates of sex can feed back negatively on growth rates [16,17]. In rotifers the density threshold for sex induction is low, and sex is directly related to population density, rather than indirectly through resource depletion [20], suggesting that the induction of sex influences the growth potential of the population [50]. This effect on population growth has also been demonstrated in laboratory Daphnia populations [52]. Such negative feedback is also possible in our dataset, but is much more difficult to detect in natural populations due to the many other uncontrolled variables (e.g. pool volume, algal productivity) likely to affect rates of sex, density and their relationship.
In contrast to the frequency of sexual females, the frequency of adult males was not related to population growth. High male frequencies coincided with periods of high density and low asexual reproductive effort, but generally male occurrence appears to be timed less precisely than ephippia production. This is perhaps not surprising when considering that males require time (approx. 10 days at 20°C) to mature, which reduces the likelihood that cues available when males are produced will reliably predict population dynamics at their maturity. Furthermore, as male lifespan is substantially longer than the moult cycle over which a female bears an ephippium, male frequency increases cumulatively over time while ephippia frequency reflects much more closely the current conditions. Previous studies of Daphnia species in temporary habitats have found similar patterns of male appearance in a population preceding the first production of ephippia [25,37].
High population density leads to increased investment in both males and ephippia in laboratory populations of Daphnia [19,53–55] and to smaller asexual clutch sizes [41]. A negative relationship between density and asexual reproduction was also apparent in our dataset. This could reflect increased competition at high densities, resulting in reduced reproductive condition that restricts female fecundity. However, we observed increased sexual reproduction at high densities, when resources are limited. This finding is difficult to reconcile with the suggestion that the sexual ephippia have a high resource cost [56]. If resources limit the production of large asexual clutches (as shown in many experiments, e.g. [41,57,58]), these conditions should also constrain production of costly ephippia. Our laboratory results on reproductive trade-offs suggest that producing a sexual clutch is costly: individual females producing a greater proportion of sexual clutches over their lifespan produced, on average, smaller asexual clutches. The cost imposed on asexual reproductive potential by a sexual event is thus greater than the loss of one asexual clutch. However, quantifying the absolute cost of producing a sexual clutch requires experiments manipulating asexual clutch sizes by altering resource availability.
The major cost of sex in our experiment appeared to be the immediate trade-off arising from the inability to produce a sexual and asexual clutch simultaneously: females that produced more ephippia had a lower total number of asexual offspring. If a female producing a sexual clutch has fewer opportunities and/or resources left available for asexual reproduction, there are clear consequences for the competitiveness of clonal lineages with different propensities for sexual reproduction in terms of their numerical representation in the population. D. magna clones vary in their propensity to produce males and, independently, ephippia in response to environmental cues [26,36]. Sexually produced, dormant offspring are the measure of long-term fitness in Daphnia and many facultative sexual organisms, but total sexual output depends both on sexual and asexual fecundity. The timing of sexual reproduction is thus expected to optimize investment in the two reproductive modes.
5. Conclusion
In wild populations of facultative sexual D. magna, females invest in sexual reproduction following high population densities and when the population growth rate and asexual reproductive effort are low, conditions that reduce the relative cost of sexual reproduction. We provide empirical support for the idea that a facultative sexual population will show increased rates of sex as it approaches carrying capacity and the cost of sex declines. Combining our new finding with previous results we suggest that three underlying rules determine the induction of sexual reproduction in D. magna on a large biogeographic scale: first, ephemeral, seasonal populations that frequently experience inhospitable periods should generally invest more in sexual reproduction compared to populations in permanent, less seasonal habitats [32]. Second, we have found that within a season, sex induction co-occurs with conditions that are theoretically predicted to reduce its costs relative to asexual reproduction [32–34]. This is the case at high population densities when asexual clutch size is small and the cost of foregoing asexual reproduction is low. Third, previous studies have shown that this pattern can be modified by the timing and predictability of onset of inhospitable conditions, such that investment in sexual reproduction increases towards the anticipated end of the growing season [36]. We conclude that timing of sex in cyclical parthenogens is not only shaped by the approach of inhospitable conditions, but appears to respond to effects of density and population growth on the relative costs of sexual and asexual reproduction.
Supplementary Material
Acknowledgements
We thank Charlotte Narr, Jürgen Hottinger, and the staff at Tvärminne Zoological Station for help in the field and with equipment. David Innes, Maurine Neiman, and an anonymous reviewer provided helpful comments that improved the manuscript.
Data accessibility
All data are archived in the Dryad Digital Repository (doi:10.5061/dryad.1cg39) [59].
Authors' contributions
N.G., I.B., D.E. and H.K. conceived the study; N.G., I.B. and D.E. designed the study; N.G. and I.B. carried out data collection, analysed the data and drafted the manuscript; all authors revised the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Academy of Finland (Finnish Centre of Excellence in Biological Interactions Research) project no. SA-252411 (H.K.).
References
- 1.Lively CM. 2010. A review of Red Queen models for the persistence of obligate sexual reproduction. J. Hered. 101, S13–S20. ( 10.1093/jhered/esq010) [DOI] [PubMed] [Google Scholar]
- 2.Hartfield M, Keightley PD. 2012. Current hypotheses for the evolution of sex and recombination. Int. Zool. 7, 192–209. ( 10.1111/j.1749-4877.2012.00284.x) [DOI] [PubMed] [Google Scholar]
- 3.Green RF, Noakes DLG. 1995. Is a little bit of sex as good as a lot? J. Theor. Biol. 174, 87–96. ( 10.1006/jtbi.1995.0081) [DOI] [Google Scholar]
- 4.Hurst LD, Peck JR. 1996. Recent advances in understanding of the evolution and maintenance of sex. Trends Ecol. Evol. 11, 46–52. ( 10.1016/0169-5347(96)81041-X) [DOI] [PubMed] [Google Scholar]
- 5.Dacks J, Roger AJ. 1999. The first sexual lineage and the relevance of facultative sex. J. Mol. Evol. 48, 779–783. ( 10.1007/PL00013156) [DOI] [PubMed] [Google Scholar]
- 6.Stelzer C-P. 2016. Extremely short diapause in rotifers and its fitness consequences. Hydrobiologia 796, 255–264. ( 10.1007/s10750-016-2937-x) [DOI] [Google Scholar]
- 7.Maynard Smith J. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Lehtonen J, Jennions MD, Kokko H. 2012. The many costs of sex. Trends Ecol. Evol. 27, 172–178. ( 10.1016/j.tree.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 9.Ebert D. 2005. Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda, MA: National Library of Medicine (US), National Center for Biotechnology Information. [Google Scholar]
- 10.Schröder T. 2005. Diapause in monogonont rotifers. In Rotifera X: Rotifer Research: Trends, New Tools and Recent Advances, Proceedings of the Xth International Rotifer Symposium (eds Herzig A, Gulati RD, Jersabek CD, May L), pp. 291–306. Dordrecht, NL: Springer. [Google Scholar]
- 11.Stelzer C-P, Lehtonen J. 2016. Diapause and maintenance of facultative sexual reproductive strategies. Phil. Trans. R. Soc. B 371, 20150536 ( 10.1098/rstb.2015.0536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon J-C, Rispe C, Sunnucks P. 2002. Ecology and evolution of sex in aphids. Trends Ecol. Evol. 17, 34–39. ( 10.1016/S0169-5347(01)02331-X) [DOI] [Google Scholar]
- 13.Taylor BE, Gabriel W. 1993. Optimal adult growth of Daphnia in a seasonal environment. Funct. Ecol. 7, 513–521. ( 10.2307/2390126) [DOI] [Google Scholar]
- 14.Serra M, Aparici E, Carmona M. 2008. When to be sexual: sex allocation theory and population density-dependent growth. J. Evol. Biol. 12, 263–271. ( 10.1093/plankt/fbn077) [DOI] [Google Scholar]
- 15.Carmona MJ, Dimas-Flores N, Garcia-Roger EM, Serra M. 2009. Selection of low investment in sex in a cyclically parthenogenetic rotifer. J. Evol. Biol. 22, 1975–1983. ( 10.1111/j.1420-9101.2009.01811.x) [DOI] [PubMed] [Google Scholar]
- 16.Aparici E, Carmona MJ, Serra M. 1996. Polymorphism in bisexual reproductive patterns of cyclical parthenogens: a simulation approach using a rotifer growth model. Ecol. Model. 88, 133–142. ( 10.1016/0304-3800(95)00076-3) [DOI] [Google Scholar]
- 17.Serra M, King CE. 1999. Optimal rates of bisexual reproduction in cyclical parthenogens with density-dependent growth. J. Evol. Biol. 12, 263–271. ( 10.1046/j.1420-9101.1999.00026.x) [DOI] [Google Scholar]
- 18.Halkett F, Harrington R, Hullé M, Kindlmann P, Menu F, Rispe C, Plantegenest M. 2004. Dynamics of production of sexual forms in aphids: theoretical and experimental evidence for adaptive ‘coin-flipping’ plasticity. Am. Nat. 163, E112–E125. ( 10.1086/383618) [DOI] [PubMed] [Google Scholar]
- 19.Larsson P. 1991. Intraspecific variability in response to stimuli for male and ephippia formation in Daphnia pulex. Hydrobiologia 225, 281–290. ( 10.1007/BF00028406) [DOI] [Google Scholar]
- 20.Stelzer CP, Snell TW. 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnol. Oceanogr. 48, 939–943. ( 10.4319/lo.2003.48.2.0939) [DOI] [Google Scholar]
- 21.Koch U, von Elert E, Straile D. 2009. Food quality triggers the reproductive mode in the cyclical parthenogen Daphnia (Cladocera). Oecologia 159, 317–324. ( 10.1007/s00442-008-1216-6) [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Baer KN. 2000. The influence of feeding, photoperiod and selected solvents on the reproductive strategies of the water flea, Daphnia magna. Environ. Pollut. 110, 425–430. ( 10.1016/S0269-7491(99)00324-3) [DOI] [PubMed] [Google Scholar]
- 23.Hairston NG Jr, Olds EJ. 1984. Population differences in the timing of diapause: adaptation in a spatially heterogeneous environment. Oecologia 61, 42–48. ( 10.1007/BF00378705) [DOI] [PubMed] [Google Scholar]
- 24.Walsh MR. 2013. The link between environmental variation and evolutionary shifts in dormancy in zooplankton. Integr. Comp. Biol. 53, 713–722. ( 10.1093/icb/ict035) [DOI] [PubMed] [Google Scholar]
- 25.Galimov Y, Walser B, Haag CR. 2011. Frequency and inheritance of non-male producing clones in Daphnia magna: evolution towards sex specialization in a cyclical parthenogen? J. Evol. Biol. 24, 1572–1583. ( 10.1111/j.1420-9101.2011.02288.x) [DOI] [PubMed] [Google Scholar]
- 26.Roulin AC, Mariadassou M, Hall MD, Walser J-C, Haag CR, Ebert D. 2015. High genetic variation in resting-stage production in a metapopulation: is there evidence for local adaptation? Evolution 69, 2747–2756. ( 10.1111/evo.12770) [DOI] [PubMed] [Google Scholar]
- 27.Snell TW, Garman BL. 1986. Encounter probabilities between male and female rotifers. J. Exp. Mar. Biol. Ecol. 97, 221–230. ( 10.1016/0022-0981(86)90243-1) [DOI] [Google Scholar]
- 28.Sprenger D, Lange R, Anthes N. 2011. Population density and group size effects on reproductive behaviour in a simultaneous hermaphrodite. BMC Evol. Biol. 11, 107 ( 10.1186/1471-2148-11-107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams GC. 1975. Sex and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 30.Bell G. 1982. The masterpiece of nature. Berkeley, CA: University of California Press. [Google Scholar]
- 31.Burt A. 2000. Perspective: sex, recombination and the efficacy of selection—was Weismann right? Evolution 54, 337–351. ( 10.1111/j.0014-3820.2000.tb00038.x) [DOI] [PubMed] [Google Scholar]
- 32.Doncaster CP, Pound GE, Cox SJ. 2000. The ecological cost of sex. Nature 404, 281–285. ( 10.1038/35005078) [DOI] [PubMed] [Google Scholar]
- 33.Lively CM. 2010. Parasite virulence, host life history, and the costs and benefits of sex. Ecology 91, 3–6. ( 10.1890/09-1158.1) [DOI] [PubMed] [Google Scholar]
- 34.Lively CM. 2011. The cost of males in non-equilibrium populations. Evol. Ecol. Res. 13, 105–111. [Google Scholar]
- 35.Hebert PDN. 1978. The population biology of Daphnia (Crustacea, Daphnidae). Biol. Rev. 53, 387–426. ( 10.1111/j.1469-185X.1978.tb00860.x) [DOI] [Google Scholar]
- 36.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag CR, Ebert D. 2013. Local adaptation of sex induction in a facultative sexual crustacean: insights from QTL mapping and natural populations of Daphnia magna . Mol. Ecol. 22, 3567–3579. ( 10.1111/mec.12308) [DOI] [PubMed] [Google Scholar]
- 37.Innes DJ. 1997. Sexual reproduction of Daphnia pulex in a temporary habitat. Oecologia 111, 53–60. ( 10.1007/s004420050207) [DOI] [PubMed] [Google Scholar]
- 38.Spaak P, Boersma M. 2001. The influence of fish kairomones on the induction and vertical distribution of sexual individuals of the Daphnia galeata species complex. Hydrobiologia 442, 185–193. ( 10.1023/A:1017578221814) [DOI] [Google Scholar]
- 39.Gyllström M, Hanson L-A. 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic–pelagic coupling. Aquat. Sci. 66, 274–295. ( 10.1007/s00027-004-0712-y) [DOI] [Google Scholar]
- 40.Hadany L, Otto SP. 2007. The evolution of condition-dependent sex in the face of high costs. Genetics 176, 1713–1727. ( 10.1534/genetics.107.074203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzsimmons JM, Innes DJ. 2006. Inter-genotype variation in reproductive response to crowding among Daphnia pulex. Hydrobiologia 568, 187–205. ( 10.1007/s10750-006-0104-5) [DOI] [Google Scholar]
- 42.Dodson SI, Frey DG. 2001. Cladocera and other Branchiopoda. In Ecology and classification of North American freshwater invertebrates (eds Thorp A, Covich AP), pp. 849–913, 2nd edn San Diego, CA: Academic Press. [Google Scholar]
- 43.Klüttgen B, Dülmer U, Engels M, Ratte HT. 1994. ADaM, an artificial freshwater for the culture of zooplankton. Wat. Res. 28, 743–746. ( 10.1016/0043-1354(94)90157-0) [DOI] [Google Scholar]
- 44.Begon M, Harper JL, Townsend CR. 1990. Ecology, pp. 150–152, 2nd edn Cambridge MA: Blackwell Scientific. [Google Scholar]
- 45.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 46.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 47.Harrison XA. 2014. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2, e616 ( 10.7717/peerj.616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 49.Altermatt F, Ebert D. 2008. The influence of pool volume and summer desiccation on the production of the resting and dispersal stage in a Daphnia metapopulation. Oecologia 157, 441–452. ( 10.1007/s00442-008-1080-4) [DOI] [PubMed] [Google Scholar]
- 50.Stelzer C-P. 2012. Population regulation in sexual and asexual rotifers: an eco-evolutionary feedback to population size? Funct. Ecol. 26, 180–188. ( 10.1111/j.1365-2435.2011.01918.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schröder T, Gilbert JJ. 2004. Transgenerational plasticity for sexual reproduction and diapause in the life cycle of monogonont rotifers: intraclonal, intraspecific and interspecific variation in the response to crowding. Funct. Ecol. 18, 458–466. ( 10.1111/j.0269-8463.2004.00854.x) [DOI] [Google Scholar]
- 52.McCauley E, Nisbet RM, Murdoch WW, de Roos AM, Gurney WSC. 1999. Large-amplitude cycles of Daphnia and its algal prey in enriched environments. Nature 402, 653–656. ( 10.1038/45223) [DOI] [Google Scholar]
- 53.Kleiven OT, Larsson P, Hobaek A. 1992. Sexual reproduction in Daphnia magna requires three stimuli. Oikos 65, 197–206. ( 10.2307/3545010) [DOI] [Google Scholar]
- 54.Berg LM, Palsson S, Lascoux M. 2001. Fitness and sexual response to population density in Daphnia pulex. Freshwater Biol. 46, 667–677. ( 10.1046/j.1365-2427.2001.00704.x) [DOI] [Google Scholar]
- 55.Olmstead AW, LeBlanc GA. 2001. Temporal and quantitative cycling of the cladoceran Daphnia magna by a juvenile hormone analog. J. Exp. Zool. 290, 148–155. ( 10.1002/jez.1044) [DOI] [PubMed] [Google Scholar]
- 56.Lynch M. 1983. Ecological genetics of Daphnia pulex . Evolution 37, 358–374. ( 10.1111/j.1558-5646.1983.tb05545.x) [DOI] [PubMed] [Google Scholar]
- 57.Lynch M. 1989. The life history consequences of resource depression in Daphnia pulex. Ecology 70, 246–256. ( 10.2307/1938430) [DOI] [Google Scholar]
- 58.Guisande C, Gliwicz ZM. 1992. Egg size and clutch size in two Daphnia species grown at different food levels. J. Plankton Res. 14, 997–1007. ( 10.1093/plankt/14.7.997) [DOI] [Google Scholar]
- 59.Gerber N, Kokko H, Ebert D, Booksmythe I. 2018. Data from: Daphnia invest in sexual reproduction when its relative costs are reduced Dryad Digital Repository. ( 10.5061/dryad.1cg39) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gerber N, Kokko H, Ebert D, Booksmythe I. 2018. Data from: Daphnia invest in sexual reproduction when its relative costs are reduced Dryad Digital Repository. ( 10.5061/dryad.1cg39) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are archived in the Dryad Digital Repository (doi:10.5061/dryad.1cg39) [59].