Abstract
Dispersal is a fundamental process defining the distribution of organisms and has long been a topic of inquiry in ecology and evolution. Emerging research points to an interdependency of dispersal with a diverse suite of traits in terrestrial organisms, however the extent to which such dispersal syndromes exist in freshwater species remains uncertain. Here, we test whether dispersal in freshwater fishes (1) is a fixed property of species, and (2) correlates with life-history, morphological, ecological and behavioural traits, using a global dataset of dispersal distances collected from the literature encompassing 116 riverine species and 196 locations. Our meta-analysis revealed a high degree of repeatability and heritability in the dispersal estimates and strong associations with traits related to life-history strategies, energy allocation to reproduction, ecological specialization and swimming skills. Together, these results demonstrate that similar to terrestrial organisms, the multi-dimensional nature of dispersal syndromes in freshwater species offer opportunities for the development of a unifying paradigm of movement ecology that transcend taxonomic and biogeographical realms. The high explanatory power of the models also suggests that trait-based and phylogenetic approaches hold considerable promises to inform conservation efforts in a rapidly changing world.
Keywords: dispersal ability, life-history strategies, ecological specialization, co-adaptation, evolutionary trade-offs, repeatability
1. Introduction
Understanding the causes, consequences and mechanisms of dispersal—the movement of organisms between successive habitat patches with potential consequences for gene flow across space—is a central topic in biogeography, ecology and evolution [1,2]. Theoretical and empirical investigations suggest that dispersal is the reflection of syndromes emerging from complex trade-offs and covariation among traits [3–7]. Identifying so-called dispersal syndromes provides critical insight into the evolution of dispersal and holds considerable promise to enhance the biological realism of species forecasts under future environmental change (e.g. [8]).
Evolutionary biologists have long recognized that the evolution of dispersal has multiple causes [1–3,9]. In active dispersers, any trait reducing the costs of movement—including energetic, time, risk and opportunities costs—is expected to select for increased dispersal [6,10]. However, dispersal syndromes may emerge for a variety of other reasons. For instance, escaping conspecific competition is recognized as a major potential benefit of dispersal, leading to patterns of covariation between dispersal and competition-related traits [1,10]. Environmentally driven dispersal syndromes may also result from the joint selection of dispersal and other phenotypic traits in varying environments [3,7]. In addition, movements are associated with multiple ecological functions (e.g. feeding, escape, exploration, migration and reproduction), such that dispersal may emerge as a side product of the evolution of other traits [11]. There is therefore a need to consider the multi-dimensionality of dispersal evolution that includes both phenotypic traits and the environmental factors modulating the strength and directionality of syndromes.
A recent effort demonstrated that species' dispersal ability can be predicted successfully according to a suite of life-history, behavioural, ecological and morphological traits in a wide range of terrestrial and semi-terrestrial organisms [4]. However, this research also points to idiosyncratic relationships among different taxonomic groups, illustrating the complex nature and multiple origins of dispersal syndromes. There is also compelling evidence that dispersal is a context-dependent (e.g. environment) and condition-dependent (e.g. age, sex) trait, exhibiting strong intra-specific variability in many cases [2,12,13]. Thus, it is questionable whether inferences and predictions garnered from the terrestrial realm are informative in freshwater systems, where connectivity and flow directionality represent fundamental constraints to strictly aquatic organisms, and may lead to very different pressures on life-history and ecological traits that mediate dispersal [14–17].
Here, we investigate the existence of dispersal syndromes in freshwater fishes. We first assess the predictability of dispersal by quantifying the importance of intra-specific and inter-specific variability in movement patterns. We then test for systematic covariations between dispersal ability and a suite of morphological, life-history, ecological and behavioural traits. Rather than focusing on a single descriptor of dispersal (e.g. mean or maximum dispersal distances), we assess the consistency of dispersal syndromes in different parts of the dispersal kernel by using empirical and fitted estimates of dispersal distances. Results from our research contribute to a unified perspective on dispersal syndromes that considers whether, and if so to what extent, uncoupled selection may occur for short- (explorative or routine movement) versus long-distance dispersal [1,9,18].
2. Material and methods
(a). Dispersal data collection
We performed a comprehensive literature review to identify studies that quantified dispersal of riverine fishes according to direct (i.e. capture–recapture, radio-tracking) methods. We searched the literature using various combinations of the following keywords in Google Scholar and ISI Web of Knowledge: fish* AND (freshwater OR stream* OR river*), (dispersal OR ‘dispersal distance’ OR dispersion OR swim* OR movement* OR redistribution), (CMR OR MRR OR mark-release OR mark-recapture OR radio-track*). Additional studies were gathered from the reference section of the relevant articles and previous meta-analyses (e.g. [19]).
Our initial literature search identified 448 studies, including peer-reviewed articles and grey literature sources (e.g. reports, graduate school theses). Each study was subsequently screened and selected only if it provided a quantitative estimate of dispersal distance at the population or individual level (figures were extracted using PlotDigitizer). Manipulative experiments (e.g. translocation) and studies performed on hatchery individuals were not considered. We further excluded studies explicitly investigating migration (e.g. distance between wintering and spawning habitat) and those conducted over less than 24 h (e.g. diel movements). To account for differences in the temporal scale at which movement patterns were recorded, all dispersal distances were expressed as dispersal rates in m day−1 according to the individual records when available or the average time between sightings across all recaptured individuals in all other cases (mean time between sightings = 96.34 days, range = 1–21912; see electronic supplementary material, figure S1).
(b). Dispersal metrics
We considered a broad definition of dispersal as any movement of an individual between habitat patches, irrespective of the distance between them or their underlying causes (e.g. feeding, escape, exploration, reproduction [10]). From the studies, we extracted empirical estimates of mean and maximum dispersal distances as they were the most commonly reported statistics. When these metrics were not given in the text and raw data were available (e.g. individual dispersal distance, frequency distribution), we recalculated them when possible. If dispersal distances were reported separately for different subsamples of the population (e.g. small and large body sizes), they were pooled together after accounting for differences in sample sizes (e.g. weighted mean dispersal distance).
Dispersal movements were further described by fitting dispersal kernels to the empirical cumulative frequency distributions (i.e. the number of individuals recaptured per distance class when given for at least three distance classes). We used the most common probability density functions (i.e. Cauchy, exponential, Gaussian, logistic, log-normal and Weibull) [20] as well as a Gaussian mixture kernel that has been suggested to more accurately describe heterogeneous freshwater fish movements [19]. We compared model fits using the small sample-size corrected Akaike's information criterion (AICc) and chose the model with the lowest AICc. Owing to the uncertainty surrounding the definition of short- versus long-distance dispersal [21], we then described movement patterns using the 10th, 20th, 30th, 40th, 50th, 60th, 70th, 80th, 90th and 99th percentiles of the fitted probability distributions.
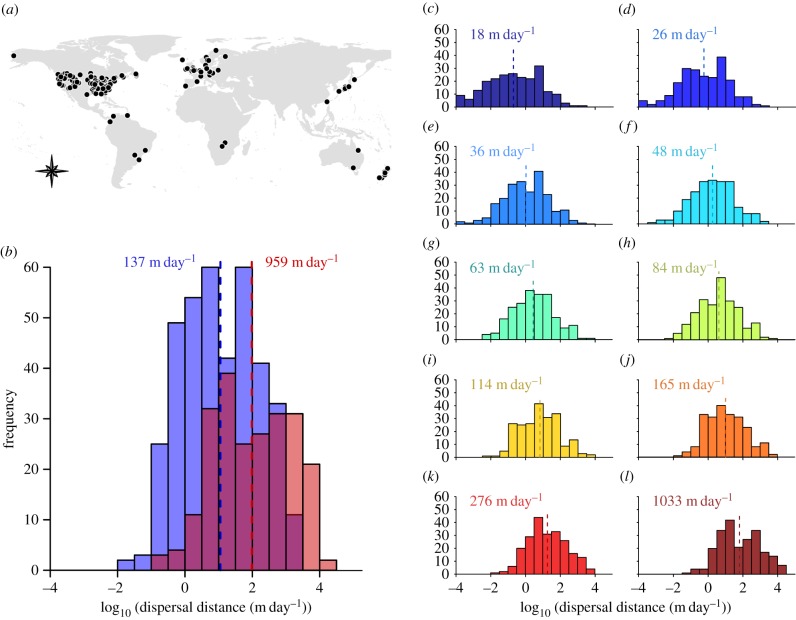
The final dataset comprised 154 studies encompassing 116 fish species (from 14 taxonomic orders and 27 families) and 196 locations (mean number of studies per species = 3.78, range = 1–48), located mostly in the Northern Hemisphere (figure 1a). The empirical estimates were available for 109 species for the mean dispersal distance and 92 species for the maximum dispersal distance (electronic supplementary material, figure S2). The fitted estimates were derived from the dispersal kernels fitted to empirical cumulative frequency distributions for 82 species (electronic supplementary material, figure S3). The Weibull, log-normal and Gaussian mixture dispersal kernels were selected as the best fitting functions for most species and locations (see electronic supplementary material, table S1). Across all studies, empirical dispersal distances showed a mean and maximum of 137 and 959 m day−1 (figure 1b), respectively, whereas the fitted dispersal distances ranged from 18 m day−1 for the 10th percentile to 1033 m day−1 for the 99th percentile (figure 1c–l).
Figure 1.
(a) Locations where movement-based studies included in the final dataset were performed. (b) Distribution of empirical dispersal distances defined according to the mean (blue) and maximum (red) dispersal distances and the fitted dispersal distances defined according to the (c) 10th, (d) 20th, (e) 30th, (f) 40th, (g) 50th, (h) 60th, (i) 70th, (j) 80th, (k) 90th and (l) 99th percentiles of the dispersal kernels. The vertical lines indicate the mean values across all species and locations.
(c). Species’ traits
We considered traits related to five main hypotheses. All correlations among traits belonging to the different hypotheses were <0.55 (see electronic supplementary material, table S2 and figure S4).
(i). Life-history strategies
Syndromes may emerge when the environment jointly influences the expression of dispersal and other phenotypic traits [3]. Accordingly, we expect high dispersal ability to be selected under high habitat instability and be integrated into life-history strategies promoting species resilience, as observed among the basic demographic parameters of survival, fecundity and onset and duration of reproduction [9,22]. In riverine ecosystems, many life-history adaptations are shaped by long-term flow dynamics—the major form of environmental variability encountered in these ecosystems [23,24]. As such, the so-called periodic strategists [25] are predicted to disperse over larger distances as they display both the energy (large body size) and generation time (long-lived and delayed maturity) to escape high duration flow events and the reproductive effort (high fecundity) to increase juvenile survivorship during favourable environmental conditions [26].
Traits considered for this hypothesis were body length (maximum, mm), length at first maturity (female, mm), age at first maturity (female, years), longevity (maximum, years) and fecundity (total number of eggs or offspring per breeding season).
(ii). Energy allocation to reproduction
We expect a negative relationship between dispersal distances and most traits related to the energy/time invested in reproduction due to the allocation trade-off of limiting resources between competing traits [6]. Both spatial and temporal dispersal strategies may also represent alternative strategies allowing species to cope with environmental stochasticity [5]. Accordingly, we expect dispersal through space to constrain the evolution of life-history traits that distribute individuals into separate reproductive events.
Traits considered for this hypothesis were parental care (open substrate spawners, brood hiders, nest guarders; increasing order), spawning frequency (one or more times per season) and egg size (mean diameter of mature (fully yolked) ovarian oocytes, mm).
(iii). Specialization
We expect a joint evolution of dispersal and specialization, so that dispersal is counter-selected for specialist species due to the higher costs of movement across unsuitable habitat patches, or conversely poor dispersal ability favours the evolution of specialization to local conditions [27–30]. We also expect high temporal variability in environmental conditions to prevent specialization and select for increased dispersal to allow organisms to track their optimal habitat through space and time [31]. Hence, we predict a negative correlation between spatial/temporal niche specialization and dispersal distances [32].
Traits considered for this hypothesis were temperature seasonality (coefficient of variation of monthly mean temperature across species range), precipitation seasonality (as a proxy of flow variation; coefficient of variation of monthly cumulated precipitation across species range), climatic niche breadth based on the index of tolerance of an outlying mean index analysis [33] that measures the amplitude in the distribution of species according to four climatic variables (mean temperature of warmest and coldest quarters,°C; cumulated precipitation of wettest and driest quarters, mm) and range size (as a proxy of habitat niche breadth; km2).
(iv). Swimming skill
We expect the costs of dispersal to decrease for species displaying better locomotion and orientation skills [34]. We thus predict a positive relationship between dispersal distances and swimming ability. Traits considered for this hypothesis were aspect ratio of the caudal fin (A = h2/s, h = height of the caudal fin; s = surface area of fin) and body shape (deep/short, fusiform/normal, elongated; increasing order).
(v). Routine (feeding) behaviour
We expect dispersal to result from a by-product of selection favouring larger-scale exploration and resource exploitation [11]. We thus predict greater dispersal distances for species at higher trophic position [35,36]. The trait considered for this hypothesis was trophic position (continuous).
(d). Statistical analyses
Covariations between empirical or fitted dispersal estimates and species traits, which putatively form a syndrome, were estimated using multivariate generalized linear mixed models accounting for both phylogenetic relatedness and repeated measurements within species. This method provides the advantage of controlling for phylogenetic dependency in the dataset (which is highly valuable in the context of complex trait syndromes), while also effectively partitioning other sources of variation such as environmental effects and measurement errors [37,38]. More specifically, we fitted multivariate Gaussian models including the empirical or fitted dispersal estimates (mean and maximum or percentiles of dispersal distance) as response variables and fitted unstructured phylogenetic, species identity and residual covariance matrices as random effects. The phylogenetic covariance matrix was estimated using the time-calibrated phylogeny of ray-finned fishes from Rabosky et al. [39] including all the ancestral nodes. Whereas the phylogenetic effect accounts for shared ancestry between species, species identity accounts for any specific effect that would be independent from the phylogenetic relationships (e.g. permanent environmental effect). In this case, the residual variance corresponds to the sum of measurement error and intra-specific variance in response to local conditions, and thus encompasses both the within- and between-population variation. We did not include location as a random effect due to the limited number of studies performed on multiple species at a given location.
We first fitted models including no fixed effects and estimated the extent to which dispersal estimates were consistent between species by calculating the agreement repeatability (i.e. without accounting for fixed effects) as Rsp = (phylogenetic + species identity variances)/(phylogenetic + species identity + residual variances) [40]. We also estimated the amount of variation in the dispersal estimates explained by shared ancestry between species (i.e. phylogenetic signal) by calculating the phylogenetic heritability as h2 = phylogenetic variance/(phylogenetic + species identity + residual variances) [38]. The contribution of within-species variability was estimated through the residual variance as Rwithin = (residual variance)/(phylogenetic + species identity + residual variances).
We then included the individual traits as fixed effects after removing the global intercept and estimated the strength of the relationships with the dispersal estimates using the standardized β-coefficients. To assess whether the effects differ for short- versus long-distance dispersal, we compared models with separate or common regression slopes for the different dispersal estimates using the deviance information criterion (DIC). We considered differences in DIC between the models with separate and common slope (DICseparate − DICcommon) to indicate the level of support for the model with separate slopes as follows: ΔDIC ≤ 4, substantial support; 4 < ΔDIC ≤ 7, considerably less support; ΔDIC > 10, essentially no support. We also calculated the marginal (i.e. the variance explained by the fixed effects) and conditional (i.e. the variance explained by both fixed and random effects) R2 for each model [41].
The models were implemented in a Bayesian framework using Markov chain Monte Carlo (MCMC) sampling in the package MCMCglmm [42] in R version 3.2.0 [43]. We used inverse-Wishart distributed priors for the residuals and random effects with V = 2 and ν = 1.002 for the empirical estimates and V = 10 and ν = 9.002 for the fitted estimates, and independent normal priors with a mean of zero and large variance (1010) for the fixed effects. Each model was run three times for 3 000 000 iterations, with a burn-in period of 150 000 and a sampling interval of 1000. The convergence of the models was confirmed by examining the effective sample size (>1000) and autocorrelation between samples (less than 0.10) for each chain, as well as the Gelman–Rubin statistics (less than 1.1) among chains [44]. Dispersal estimates and species' traits were log10-transformed to satisfy assumptions of normality and linearity when required. To facilitate model convergence, all predictor and response variables were converted to z-scores and outliers (less than 3 s.d. from the mean) were removed prior to analyses. Parameter estimates from models are reported as the posterior modes with 95% lower and upper credible intervals (CIs).
3. Results
We found high levels of repeatability (approx. 65–80%) across species for both the empirical and fitted dispersal distances (table 1). The results also indicated that a large portion of the variance in the empirical estimates was explained by the shared ancestry between species (approx. 60–70%), whereas fitted estimates showed comparatively lower heritability values (approx. 30–40%). In both cases, intra-specific variability accounted for approximately 20–35% of the total variance in the dispersal estimates.
Table 1.
Proportion of variance explained by consistent between-species differences (repeatability, Rsp), shared ancestry between species (heritability, h2) and within-species differences (residual variance, Rwithin). Estimates are the modes of the posterior distributions with 95% CIs.
| Rsp | h2 | Rwithin | |
|---|---|---|---|
| empirical | |||
| mean | 0.83 [0.70–0.90] | 0.69 [0.38–0.85] | 0.17 [0.10–0.30] |
| maximum | 0.74 [0.58–0.85] | 0.56 [0.26–0.80] | 0.26 [0.15–0.42] |
| fitted | |||
| p10th | 0.64 [0.55–0.76] | 0.33 [0.22–0.52] | 0.36 [0.24–0.45] |
| p20th | 0.65 [0.55–0.76] | 0.31 [0.19–0.49] | 0.35 [0.24–0.45] |
| p30th | 0.70 [0.58–0.79] | 0.32 [0.19–0.49] | 0.30 [0.21–0.42] |
| p40th | 0.71 [0.59–0.79] | 0.29 [0.19–0.50] | 0.29 [0.21–0.41] |
| p50th | 0.71 [0.60–0.80] | 0.31 [0.18–0.50] | 0.29 [0.20–0.40] |
| p60th | 0.72 [0.62–0.81] | 0.31 [0.19–0.51] | 0.28 [0.19–0.38] |
| p70th | 0.73 [0.62–0.82] | 0.31 [0.19–0.52] | 0.27 [0.18–0.38] |
| p80th | 0.75 [0.63–0.83] | 0.32 [0.19–0.52] | 0.25 [0.17–0.37] |
| p90th | 0.73 [0.64–0.83] | 0.34 [0.20–0.54] | 0.27 [0.17–0.36] |
| p99th | 0.72 [0.62–0.82] | 0.36 [0.22–0.57] | 0.28 [0.18–0.38] |
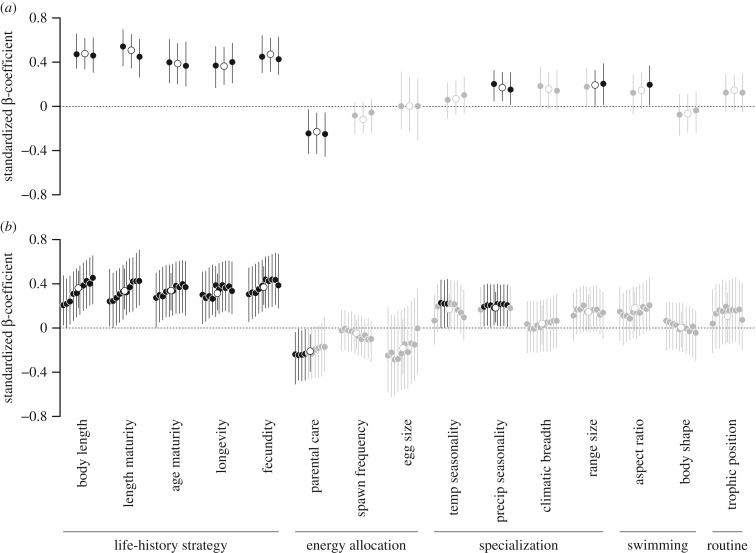
Strong inter-dependencies were also revealed between dispersal distances and species’ traits (figure 2). In particular, we found strong associations between empirical or fitted dispersal distances and life-history strategy traits, with the strength of the associations increasing from short- to long-distance movements, particularly for body length and length at maturity. This indicated that highly fecund and large-bodied species displaying longer generation time tend to disperse over larger distances. The degree of parental care was also negatively associated with the dispersal distances, except for the longer distance movements for which the 95% CIs overlapped zero. This provided support for the energy allocation hypothesis where species investing more time into egg or juvenile protection show lower dispersal ability. We also found evidence for an association with the degree of specialization, with generalist species displaying the longest movements. In particular, we found positive relationships with the temporal variations in temperature and precipitation experienced by species across their ranges as well as with their range size. Nonetheless, the strength of these associations varied according to the dispersal estimates (empirical or fitted) and the structure of the model (including or not separate slopes) considered, reflecting to some extent the higher uncertainty associated with the most complex models. Finally, although the results did not provide strong support for the swimming skill hypothesis, the aspect ratio appeared to be increasingly important to explain long distance movements, notably the maximum dispersal distance for which 95% CI did not overlap zero.
Figure 2.
Standardized β-coefficient between (a) empirical and (b) fitted dispersal estimates and individual species' traits for multivariate models including (solid dots) or not (open dots) a separate slope for the different dispersal estimates. Results are sorted from left to right according to the mean and maximum empirical dispersal distances and increasing percentiles (10th, 20th, 30th, 40th, 50th, 60th, 70th, 80th, 90th, 99th), respectively. Results for the single slope estimate are arbitrarily represented as the mid-interval values. Dots and associated bars are the posterior modes and 95% CIs. Grey shades indicate coefficients whose CIs overlap zero.
Differences in DIC values for the empirical estimates indicated substantial, albeit ambiguous, support for models with separate slopes, especially regarding the aspect ratio (electronic supplementary material, table S3). Model comparison for the fitted estimates revealed that models including separate slopes had relatively similar (ΔDIC ≤ 4) levels of support to the models including a common slope for body size and egg size, and considerably less support (4 < ΔDIC ≤ 7) for the other traits. Lastly, we found that the full models explained approximately 65–80% of the variance (conditional R2), with the fixed effects alone accounting for up to 15% of the total variance (marginal R2) (electronic supplementary material, figures S5–S6). The conditional R2 increased for long- versus short-distances movements for all models including or not a separate slope, although the CIs showed a high degree of overlap. Using a higher threshold to exclude case studies based on the recorded time between sightings (i.e. 7 or 30 days) did not modify our conclusions (results not shown).
4. Discussion
Our study reveals the existence of dispersal syndromes in freshwater fishes, through strong associations between dispersal distances and a suite of morphological, life-history and ecological traits. The relatively high level of repeatability and heritability observed across fish species also suggest considerable promises to incorporate a more realistic portrayal of dispersal in the modelling of freshwater biodiversity [45].
Our findings show strong interdependency of dispersal with multiple life-history traits. As predicted, we found that periodic strategists associated with high environmental seasonality and characterized by large body size, long generation time and high fecundity display higher dispersal ability. In fish, dispersal may thus have been selected by the same environmental pressures that shaped life-history strategies, namely long-term flow dynamics. However, we also found evidence, albeit tenuous, that morphological-related attributes were more tightly associated with long-distance dispersal, whereas the strength of the associations with the other life-history traits was less likely to differ for short- versus long-distance dispersal. This indicates that the same syndrome may emerge from distinct mechanisms operating at different spatial scales. For example, the scale at which competition and reproduction affects metapopulation dynamics is generally assumed to be more localized than those of habitat perturbation. It follows that the benefits of temporally escaping from periods of adverse conditions may be responsible for the dispersal-fecundity syndrome observed for long-distance movements, whereas the selection for increased dispersal under high kin competition may explain the association with shorter distance movements [9].
Despite highlighting the potentially complex origins of dispersal syndromes, our results expand previous terrestrial evidence for the existence of a universal, positive association between dispersiveness, fecundity and longevity [4]. Whether such covariation holds in marine environments, however, where passive dispersal is easier and specific dispersal adaptations are rare remains an open question. Indeed, some aspects of dispersal-associated movement for marine species may be more likely to evolve as an incidental outcome in response to selective forces related to other functions [32]. Nonetheless, Bradbury et al. [46] found that isolation by distance parameters were consistently correlated with many life-history traits in marine fishes, including body length, fecundity and age at maturity, suggesting a reduced role of pelagic larval stage to effective dispersal. This indicates that the distinct perspectives on dispersal among realms may be reconciled and provides new exciting research avenues for the development of a unifying paradigm of movement ecology that may transcend taxonomic and system boundaries [47].
The negative associations between dispersal distances and parental care are also in accordance with the traditional time/energy allocation hypothesis, predicting that investing energy into reproduction is done at the expense of less energy being available for other functions [6]. By contrast, we found no support for a trade-off between traits favouring dispersal in space and time, although the lack of pattern may also reflect the simplicity of the scoring system adopted to describe temporal aspects of reproduction. Moreover, allocation trade-offs can induce dispersal syndromes at the individual or population level and between the different phases of the dispersal process, although potentially for different reasons, thus obscuring the patterns of covariation at higher levels of organization [5,48].
Beyond life-history strategies, we show that dispersal might be tightly associated with the degree of ecological specialization. Species characterized by broader climatic and habitat niche breadth were found to display higher dispersal ability, confirming what is often a priori assumed but seldom evaluated [49]: the fact that dispersal is an important process moderating species distributions. Whitmee & Orme [50] similarly found a strong correlation between maximum dispersal distance and range size in mammals, suggesting that the degree of generalism favours long distance dispersal through access to distant habitat [32], and subsequently leads to range expansion. Nonetheless, it is important to note that in natural populations, both phenotype and dispersal evolve together and that correlative studies such as ours do not enable an investigation of the causes or consequences of the identified covariations. Beyond biogeographical considerations, these results have also important conservation implications. They indicate that under global environmental change, geographically restricted species are exposed to the double jeopardy of having a narrow range of tolerance and low dispersal ability.
Among the traits associated with swimming skills only aspect ratio was found to be correlated with the maximum dispersal distance. In fish, the aspect ratio of the caudal fin closely correlates with the level of activity [51] and swimming efficiency [52], with higher aspects ratios facilitating both sustained swimming and rapid accelerations. An indirect relationship might have arisen through allometric relationships with body size, but is consistent with the hypothesis that better swimming skill may translate to less energy expenditure per unit of time that may facilitate long-distance movements [34]. However, the existence of correlations linking both life-history and morphological traits does not allow to discriminate between the two non-mutually exclusive hypotheses that dispersal evolved as part of the life-history strategies in response to habitat instability or that dispersal is facilitated for species with attributes that decrease the costs of movements. Lastly, and contrary to what we expected, trophic position demonstrated no significant relationship with dispersal. The hypothesis that dispersal may arise from a by-product of feeding routine movements thus did not find support in freshwater fish.
We found rather weak evidence that short- versus long-distance dispersal events rely on distinct mechanisms. Although this might indicate that these movements are accomplished by different types of individuals [2], other potential explanations include (1) our ability to separate genuine dispersal from exploratory movements, (2) the use of fixed dispersal kernels, and (3) the presence of taxonomic and spatial biases. Critics have suggested that direct field-based studies underestimate infrequent long-distance dispersal because of temporally/spatially restricted sampling [53]. In turn, the similarity of the coefficients estimated for the different distance classes, and especially between the empirical estimates, may reflect these biases towards routine movements when pooling the short- and long-distance components of these movement patterns [54]. Also, by constraining the dispersal-distance distributions to a fixed unique function for most species, we assume that the evolution of the different parts of the dispersal kernels are linked [1].
Dispersal is a complex process involving three interrelated phases (i.e. departure, transience and settlement) that are affected by multiple factors acting at different spatial and temporal scales [55]. However, the intrinsic complexity of dispersal dynamics may be lost upon oversimplified kernels [20], which may explain to some extent the comparatively lower level of repeatability and heritability observed in the fitted estimates. In this respect, the use of more flexible dispersal kernels (see [20] for a review) and indirect genetic-based methods (e.g. [46]) may hold considerable promise to improve our ability to quantify spatial and temporal patterns in dispersal. Alternatively, we cannot rule out the possibility that our results may have been affected by including non-random species pools in the analysis. Although known to be detrimental, taxonomic and spatial biases are pervasive in the scientific literature. As such, the strong spatial skew towards the Nearctic realm may have overemphasized taxa whose evolution have strongly been imprinted by repeated glaciation history [56].
Despite these considerations, we found a strong species-specific and phylogenetic signal in the dispersal estimates with the full models explaining up to approximately 80% of the total variance. By contrast, a similar analysis on European butterflies reveals at least as much variation within than between species and a low degree of phylogenetic signal [12]. These differences could reflect different selective pressures and phylogenetic pathways among different clades. In fish, life-history traits such as body length, length at first maturity and fecundity show a relatively low level of intra-specific variability (approximately 25% [57]) and a strong degree of phylogenetic signal [58], whereas for butterfly species, traits associated with dispersal may be comparatively more labile (e.g. [59]).
Irrespective of the underlying mechanisms, our results point out the great potential for phylogenetic and trait-based approaches to inform conservation efforts by predicting dispersal for species for which data are lacking. Radinger & Wolter [19] previously demonstrated that morphological traits offer a powerful way to infer movement patterns in riverine fishes. Here, we show that other life-history and ecological traits may offer complementary, if not more promising, opportunities to improve our understanding of the determinants of dispersal. Nonetheless, it is noteworthy that short-distance dispersal was less predictable than long-distance dispersal. This confirmed that individuals that engage in routine, explorative movements are more likely to respond to extrinsic stimuli such as feeding resources, predation risk and habitat boundaries than those involved in directed, longer distance movements [18].
More generally, the non-negligible proportion of variance explained by intra-specific factors advocates for more mechanistic approaches to model dispersal that considers how the environment or the biotic context affect the redistribution of animals [60–62]. Context-dependent dispersal syndromes are likely to arise at the different phases of the dispersal process in response to external factors such as predation risk, conspecific density, climatic conditions or habitat structure [63]. The sign of covariations of the dispersal syndromes may even be reversed due to habitat matching [64] or when compared both within and among species [2]. Unfortunately, we were not able to gather information about the local environmental conditions or intra-specific trait variation from the case studies extracted from the literature, which precluded the incorporation of these potentially important factors in our analyses. Assessing the consistency of dispersal syndromes at the intra-specific level by considering both the multi-dimensional nature and context dependency of traits covariation thus represents an important area for future research [65].
Determining the underlying determinants of dispersal is a fundamental but challenging question in evolutionary ecology. Our study highlights the pervasiveness but multi-dimensional nature of dispersal syndromes and calls for the development of more integrated framework to better predict the evolution of dispersal and the consequences of changing environments across a wide range of taxa and ecosystems.
Supplementary Material
Acknowledgements
We thank Rebekah Stiling and Joelle Blais to help with data collection as well as Juan Manuel Morales and three anonymous reviewers for their insightful comments that greatly improved the manuscript.
Data accessibility
The datasets supporting this article are available at https://figshare.com/s/f639441c6e16c39c50a3.
Authors' contributions
L.C. and J.D.O. designed the study. L.C. collected the data, led the analyses and wrote the manuscript with substantial help from J.D.O.
Competing interests
We declare we have no competing interests.
Funding
Financial support was provided by a H. Mason Keeler Endowed Professorship (School of Aquatic and Fishery Sciences, University of Washington) to J.D.O. (also supporting L.C.).
References
- 1.Ronce O. 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 38, 231–253. ( 10.1146/annurev.ecolsys.38.091206.095611) [DOI] [Google Scholar]
- 2.Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209. ( 10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 3.Ronce O, Clobert J. 2013. Dispersal syndromes. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 119–138. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Stevens VM, et al. 2014. A comparative analysis of dispersal syndromes in terrestrial and semi-terrestrial animals. Ecol. Lett. 17, 1039–1052. ( 10.1111/ele.12303) [DOI] [PubMed] [Google Scholar]
- 5.Buoro M, Carlson SM. 2014. Life-history syndromes: integrating dispersal through space and time. Ecol. Lett. 17, 756–767. ( 10.1111/ele.12275) [DOI] [PubMed] [Google Scholar]
- 6.Bonte D, et al. 2012. Costs of dispersal. Biol. Rev. 87, 290–312. ( 10.1111/j.1469-185X.2011.00201.x) [DOI] [PubMed] [Google Scholar]
- 7.Stevens VM, Trochet A, Van Dyck H, Clobert J, Baguette M. 2011. How is dispersal integrated in life histories: a quantitative analysis using butterflies. Ecol. Lett. 15, 74–86. ( 10.1111/j.1461-0248.2011.01709.x) [DOI] [PubMed] [Google Scholar]
- 8.Santini L, Cornulier T, Bullock JM, Palmer SC. F., White SM, Hodgson JA, Bocedi G, Travis JMJ. 2016. A trait-based approach for predicting species responses to environmental change from sparse data: how well might terrestrial mammals track climate change? Glob. Change Biol. 22, 2415–2424. ( 10.1111/gcb.13271) [DOI] [PubMed] [Google Scholar]
- 9.Duputié A, Massol F. 2013. An empiricist's guide to theoretical predictions on the evolution of dispersal. Interface Focus 3, 20130028 ( 10.1098/rsfs.2013.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowler DE, Benton TG. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225. ( 10.1017/s1464793104006645) [DOI] [PubMed] [Google Scholar]
- 11.Burgess SC, Baskett ML, Grosberg RK, Morgan SG, Strathmann RR. 2016. When is dispersal for dispersal? Unifying marine and terrestrial perspectives. Biol. Rev. 91, 867–882. ( 10.1111/brv.12198) [DOI] [PubMed] [Google Scholar]
- 12.Stevens VM, Pavoine S, Baguette M. 2010. Variation within and between closely related species uncovers high intra-specific variability in dispersal. PLoS ONE 5, e11123 ( 10.1371/journal.pone.0011123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitume E. V, Bonte D, Ronce O, Bach F, Flaven E, Olivieri I, Nieberding CM. 2013. Density and genetic relatedness increase dispersal distance in a subsocial organism. Ecol. Lett. 16, 430–437. ( 10.1111/ele.12057) [DOI] [PubMed] [Google Scholar]
- 14.Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C. 2013. Individual dispersal, landscape connectivity and ecological networks. Biol. Rev. 88, 310–326. ( 10.1111/brv.12000) [DOI] [PubMed] [Google Scholar]
- 15.Wiens JA. 2002. Riverine landscapes: taking landscape ecology into the water. Freshw. Biol. 47, 501–515. ( 10.1046/j.1365-2427.2002.00887.x) [DOI] [Google Scholar]
- 16.Fullerton AH, Burnett KM, Steel EA, Flitcroft RL, Pess GR, Feist BE, Torgensen CE, Miller DJ, Sanderson BL. 2010. Hydrological connectivity for riverine fish: measurement challenges and research opportunities. Freshw. Biol. 55, 2215–2237. ( 10.1111/j.1365-2427.2010.02448.x) [DOI] [Google Scholar]
- 17.Campbell GEH, Lowe WH, Fagan WF. 2007. Living in the branches: population dynamics and ecological processes in dendritic networks. Ecol. Lett. 10, 165–175. ( 10.1111/j.1461-0248.2006.01007.x) [DOI] [PubMed] [Google Scholar]
- 18.Van Dyck H, Baguette M. 2005. Dispersal behaviour in fragmented landscapes: routine or special movements? Basic Appl. Ecol. 6, 535–545. ( 10.1016/j.baae.2005.03.005) [DOI] [Google Scholar]
- 19.Radinger J, Wolter C. 2014. Patterns and predictors of fish dispersal in rivers. Fish Fish. 15, 456–473. ( 10.1111/faf.12028) [DOI] [Google Scholar]
- 20.Nathan R, Klein EJ, Robledo-Arnuncio JJ, Revilla E. 2013. Dispersal kernels: review. In Dispersal ecology and evolution (eds Clobert J, Baguette M, Benton TG, Bullock JM), pp. 187–210. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Nathan R, Perry G, Cronin JT, Strand AE, Cain ML. 2003. Methods for estimating long-distance dispersal. Oikos 103, 261–273. ( 10.1034/j.1600-0706.2003.12146.x) [DOI] [Google Scholar]
- 22.Southwood TR. E. 1977. Habitat, the templet for ecological strategies? J. Anim. Ecol. 46, 337–365. ( 10.2307/3817) [DOI] [Google Scholar]
- 23.Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC. 1997. The natural flow regime. Bioscience 47, 769–784. ( 10.2307/1313099) [DOI] [Google Scholar]
- 24.Lytle D, Poff N. 2004. Adaptation to natural flow regimes. Trends Ecol. Evol. 19, 94–100. ( 10.1016/j.tree.2003.10.002) [DOI] [PubMed] [Google Scholar]
- 25.Winemiller KO, Rose KA. 1992. Patterns of life-history diversification in North American fishes: implications for population regulation. Can. J. Fish. Aquat. Sci. 49, 2196–2218. ( 10.1139/f92-242) [DOI] [Google Scholar]
- 26.Mims MC, Olden JD, Shattuck ZR, Poff NL. 2010. Life history trait diversity of native freshwater fishes in North America. Ecol. Freshw. Fish 19, 390–400. ( 10.1111/j.1600-0633.2010.00422.x) [DOI] [Google Scholar]
- 27.Kisdi E. 2002. Dispersal: risk spreading versus local adaptation. Am. Nat. 159, 579–596. ( 10.1086/339989) [DOI] [PubMed] [Google Scholar]
- 28.Nurmi T, Parvinen K. 2011. Joint evolution of specialization and dispersal in structured metapopulations. J. Theor. Biol. 275, 78–92. ( 10.1016/j.jtbi.2011.01.023) [DOI] [PubMed] [Google Scholar]
- 29.Poisot T, Bever JD, Nemri A, Thrall PH, Hochberg ME. 2011. A conceptual framework for the evolution of ecological specialisation. Ecol. Lett. 14, 841–851. ( 10.1111/j.1461-0248.2011.01645.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jocque M, Field R, Brendonck L, De Meester L. 2010. Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Glob. Ecol. Biogeogr. 19, 244–252. ( 10.1111/j.1466-8238.2009.00510.x) [DOI] [Google Scholar]
- 31.Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120. ( 10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahirel M, Olivier E, Guiller A, Martin M-C, Madec L, Ansart A. 2015. Movement propensity and ability correlate with ecological specialization in European land snails: comparative analysis of a dispersal syndrome. J. Anim. Ecol. 84, 228–238. ( 10.1111/1365-2656.12276) [DOI] [PubMed] [Google Scholar]
- 33.Dolédec S, Chessel D, Gimaret-Carpentier C. 2000. Niche separation in community analysis: a new method. Ecology 81, 2914–2927. ( 10.1890/0012-9658(2000)081%5B2914:NSICAA%5D2.0.CO;2) [DOI] [Google Scholar]
- 34.Hein AM, Hou C, Gillooly JF. 2012. Energetic and biomechanical constraints on animal migration distance. Ecol. Lett. 15, 104–110. ( 10.1111/j.1461-0248.2011.01714.x) [DOI] [PubMed] [Google Scholar]
- 35.Sutherland GD, Harestad AS, Price K, Lertzman KP. 2000. Scaling of natal dispersal distances in terrestrial birds and mammals. Ecol. Soc. 4, 16. [Google Scholar]
- 36.Berg MP, Kiers ET, Driessen G, van der Heijden M, Kooi BW, Kuenen F, Liefting M, Verhoef HA, Ellers J. 2010. Adapt or disperse: understanding species persistence in a changing world. Glob. Change Biol. 16, 587–598. ( 10.1111/j.1365-2486.2009.02014.x) [DOI] [Google Scholar]
- 37.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 38.de Villemereuil P, Nakagawa S. 2014. General quantitative genetic methods for comparative biology. In Modern phylogenetic comparative methods and their application in evolutionary biology (ed. Garamszegi LZ.), pp. 287–304. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 39.Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, Alfaro ME. 2013. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Commun. 4, 1958 ( 10.1038/ncomms2958) [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa S, Schielzeth H. 2010. Repeatability for gaussian and non-gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 42.Hadfield JD. 2010. MCMC Methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 43.R Development Core Team. 2014. R: a language and environment for statistical computing, v.3.1.2 Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 44.Hadfield J. 2016. MCMCglmm course notes See https://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf.
- 45.Bush A, Hoskins AJ. 2017. Does dispersal capacity matter for freshwater biodiversity under climate change? Freshw. Biol. 62, 382–396. ( 10.1111/fwb.12874) [DOI] [Google Scholar]
- 46.Bradbury IR, Laurel B, Snelgrove PVR, Bentzen P, Campana SE. 2008. Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc. R. Soc. B. 275, 1803–1809. ( 10.1098/rspb.2008.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathan R, et al. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra PA. 2011. Evaluating the life-history trade-off between dispersal capability and reproduction in wing dimorphic insects: a meta-analysis. Biol. Rev. 86, 813–835. ( 10.1111/j.1469-185X.2010.00172.x) [DOI] [PubMed] [Google Scholar]
- 49.Lester SE, Ruttenberg BI, Gaines SD, Kinlan BP. 2007. The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758. ( 10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]
- 50.Whitmee S, Orme CDL. 2013. Predicting dispersal distance in mammals: a trait-based approach. J. Anim. Ecol. 82, 211–221. ( 10.1111/j.1365-2656.2012.02030.x) [DOI] [PubMed] [Google Scholar]
- 51.Pauly D. 1989. A simple index of metabolic level in fishes. Fishbyte 7, 22. [Google Scholar]
- 52.Sambilay V., Jr 1990. Interrelationships between swimming speed, caudal fin aspect ratio and body length of fishes. Fishbyte. 8, 16–20. [Google Scholar]
- 53.Koenig WD, Van Vuren D, Hooge PN. 1996. Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol. Evol. 11, 514–517. ( 10.1016/S0169-5347(96)20074-6) [DOI] [PubMed] [Google Scholar]
- 54.Hovestadt T, Binzenhöfer B, Nowicki P, Settele J. 2011. Do all inter-patch movements represent dispersal? A mixed kernel study of butterfly mobility in fragmented landscapes. J. Anim. Ecol. 80, 1070–1077. ( 10.1111/j.1365-2656.2011.01848.x) [DOI] [PubMed] [Google Scholar]
- 55.Delgado M, Penteriani V, Revilla E, Nams VO. 2010. The effect of phenotypic traits and external cues on natal dispersal movements. J. Anim. Ecol. 79, 620–632. ( 10.1111/j.1365-2656.2009.01655.x) [DOI] [PubMed] [Google Scholar]
- 56.Griffiths D. 2015. Connectivity and vagility determine spatial richness gradients and diversification of freshwater fish in North America and Europe. Biol. J. Linn. Soc. 116, 773–786. ( 10.1111/bij.12638) [DOI] [Google Scholar]
- 57.Blanck A, Lamouroux N. 2006. Large-scale intraspecific variation in life-history traits of European freshwater fish. J. Biogeogr. 34, 862–875. ( 10.1111/j.1365-2699.2006.01654.x) [DOI] [Google Scholar]
- 58.Sternberg D, Kennard MJ. 2014. Phylogenetic effects on functional traits and life history strategies of Australian freshwater fish. Ecography 37, 54–64. ( 10.1111/j.1600-0587.2013.00362.x) [DOI] [Google Scholar]
- 59.Pavoine S, Baguette M, Stevens VM, Leibold MA, Turlure C, Bonsall MB. 2014. Life history traits, but not phylogeny, drive compositional patterns in a butterfly metacommunity. Ecology 95, 3304–3313. ( 10.1890/13-2036.1.sm) [DOI] [Google Scholar]
- 60.Urban MC, Zarnetske PL, Skelly DK. 2013. Moving forward: dispersal and species interactions determine biotic responses to climate change. Ann. N. Y. Acad. Sci. 1297, 44–60. ( 10.1111/nyas.12184) [DOI] [PubMed] [Google Scholar]
- 61.Tesson SV, Edelaar P. 2013. Dispersal in a changing world: opportunities, insights and challenges. Mov. Ecol. 1, 10 ( 10.1186/2051-3933-1-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travis JMJ, et al. 2013. Dispersal and species’ responses to climate change. Oikos 122, 1532–1540. ( 10.1111/j.1600-0706.2013.00399.x) [DOI] [Google Scholar]
- 63.Cote J, Bestion E, Jacob S, Travis J, Legrand D, Baguette M. 2017. Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography 40, 56–73. ( 10.1111/ecog.02538) [DOI] [Google Scholar]
- 64.Edelaar P, Siepielski AM, Clobert J. 2008. Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. ( 10.1111/j.1558-5646.2008.00459.x) [DOI] [PubMed] [Google Scholar]
- 65.Bonte D, Dahirel M. 2017. Dispersal: a central and independent trait in life history. Oikos 126, 472–479. ( 10.1111/oik.03801) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available at https://figshare.com/s/f639441c6e16c39c50a3.