Abstract
Understanding how human activities influence immune response to environmental stressors can support biodiversity conservation across increasingly urbanizing landscapes. We studied a bobcat (Lynx rufus) population in urban southern California that experienced a rapid population decline from 2002–2005 due to notoedric mange. Because anticoagulant rodenticide (AR) exposure was an underlying complication in mange deaths, we aimed to understand sublethal contributions of urbanization and ARs on 65 biochemical markers of immune and organ function. Variance in immunological variables was primarily associated with AR exposure and secondarily with urbanization. Use of urban habitat and AR exposure has pervasive, complex and predictable effects on biochemical markers of immune and organ function in free-ranging bobcats that include impacts on neutrophil, lymphocyte and cytokine populations, total bilirubin and phosphorus. We find evidence of both inflammatory response and immune suppression associated with urban land use and rat poison exposure that could influence susceptibility to opportunistic infections. Consequently, AR exposure may influence mortality and has population-level effects, as previous work in the focal population has revealed substantial mortality caused by mange infection. The secondary effects of anticoagulant exposure may be a worldwide, largely unrecognized problem affecting a variety of vertebrate species in human-dominated environments.
Keywords: anticoagulant rodenticide, inflammation, immune suppression, bobcat, Lynx rufus, urbanization
1. Introduction
Urbanization presents natural populations with evolutionarily novel stressors [1–4]. Although toxicant use is an inevitable consequence of urbanization, their impacts are often cryptic and may be more widespread than we realize. Toxicants directly kill wildlife [5–7] and cause population declines worldwide [8,9]. Yet their sublethal effects, which can include impaired immune competence [10,11] and increased disease susceptibility [3,12], are difficult to study in natural populations and are thus poorly understood [7].
Secondary toxicant exposure, when toxicants are transmitted among species, is common [7]. One example is when exposed rodents transfer anticoagulant rodenticides (ARs) to their predators. Globally, non-target wildlife exhibits widespread secondary exposure to ARs [5,13–16]. In some populations, more than 80% of individuals test positive for ARs [5,13–17], pointing to the potential for ecosystem-wide effects. This secondary exposure can be directly lethal to some mammalian and avian predators [5,13–17], including endangered species [14,16].
As vitamin K antagonists, ARs are intended to disrupt coagulation and cause fatal haemorrhage [16]. The sensitivity of species to the vitamin K antagonistic effects can vary by orders of magnitude [18] and some species may tolerate long-term sublethal exposure. Yet, understanding the potential health effects of long-term sublethal exposure has been difficult, because doing so requires (i) long-term monitoring, (ii) methods to measure exposure, (iii) ability to sample both exposed and unexposed animals while they are alive and measure appropriate health biomarkers, and (iv) information to control for potential confounding variables such as age, sex or sampling conditions.
We assess the sublethal health effects of exposure to ARs in an urban bobcat (Lynx rufus) population that precipitously declined due to mange caused by the mite Notoedres cati (hereafter termed ‘mange’). Although previously reported only in isolated cases in wild felids, mange was the greatest source of mortality for urban bobcats at Santa Monica Mountains National Recreation Area (SMMNRA), a national park in southern California, during 2002–2005 [17]. After mange was detected in 2001, average annual survival plummeted by 49%. In 2003, 51% of radio-collared animals died of mange and transect surveys showed a 90% decline in urban bobcat scats post-epizootic [17]. The epizootic caused a significant genetic bottleneck [4]. Necropsies of mange-infected bobcats showed 98% of individuals exposed to ARs, and to significantly greater amounts than bobcats that did not die with mange [17,19,20]. Severe mange in wildlife and domestic animals is often associated with decreased immune competence [21]. Carnivore exposure to toxicants other than ARs (including heavy metals) has rarely been documented in our study area or more broadly across the State of California (S. P. D. Riley 2017, unpublished data; S. McMillin 2017, unpublished data; R. Poppenga 2017, personal communication). Therefore, we hypothesized that urbanization exposes bobcats to novel immunotoxic effects, mediated principally by ARs that are widely used in nearby urban areas [19].
Here, we test for effects of AR exposure and proximity to urbanization on 65 measures of immune and organ function in a population of wild bobcats that has been the subject of long-term study. We used a battery of health assays to accomplish three objectives: (i) to identify individual markers of potential immune impairment or organ cellular damage that correlate with AR exposure or urban proximity (controlling for potential confounding variables in a linear model framework), (ii) to test whether there is a system-wide predictable relationship between ARs and health parameters such that immune and organ function parameters themselves can be used to indicate AR exposure (using a random forests classifier approach), and (iii) to propose a mechanism by which life at the urban edge could influence mange susceptibility in bobcats. Using this multifaceted approach, we provide new understanding of the cryptic threat toxicants pose at the urban–wildland interface.
2. Material and methods
See electronic supplementary material S1 for detailed methods.
(a). Study area and sample collection
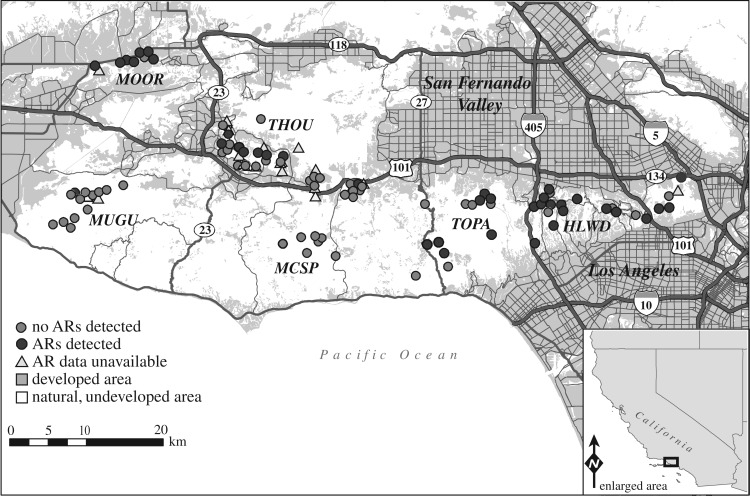
Bobcats (n = 124) were sampled in and around SMMNRA (figure 1) in southern California during 2007–2012 as previously described [4,17,19,20]. Sampling occurred within fragmented natural habitat interspersed within urban areas (THOU, HLWD), fragmented natural habitat within a predominantly agricultural area (MOOR) and unfragmented natural areas (protected state and national park lands; MUGU, MCSP). Bobcats were sexed, measured and classified as juveniles or adults [19]. Blood samples were collected in EDTA, serum separating and sodium citrate tubes via jugular, cephalic or saphenous venipuncture.
Figure 1.
Map of sampling areas in Santa Monica Mountains National Recreation Area and the surrounding region. Circles and triangles represent capture locations for individuals for which health parameter data were available.
(b). Immune and organ measures
We measured a total of 71 blood cell properties (table 1). We used whole blood to measure complete blood counts (CBC; n = 118) and serum for serum chemistry (n = 116) as described in [20]. At the UCLA Center for AIDS Research (CFAR) ImmunoBioSpot Core we used serum to measure circulating cytokine levels (n = 93), using the 19 cytokine Feline Cytokine Panel (EMD Millipore, Billerica, MA). We isolated peripheral blood mononuclear cells for immunophenotyping and analysed the samples (n = 64) in the UCLA CFAR Flow Cytometry Core. We measured the absolute number and proportion of total T lymphocytes, helper and cytotoxic T lymphocytes, activated helper and cytotoxic T lymphocytes, natural killer cells, monocytes/macrophages, total B and CD5+ B lymphocytes and CD4 + CD8 + lymphocytes (electronic supplementary material, table S1). We removed health parameters with more than one-third of missing data from the dataset, leaving us with 65 measures of immune or organ function (hereafter ‘health parameters’; electronic supplementary material, tables S2–S5). For reference comparison, we accessed CBC and serum chemistry values for captive bobcats using the International Species Information System (ISIS; 2011, Apple Valley, Minnesota 55124–8151, USA). Reference values for cytokines and immunophenotypes are not available because to our knowledge, we are the first to generate data on bobcat immune parameters.
Table 1.
Sample sizes and information for use of the samples in varied tests.
| data parameter | test (sample type) | n |
|---|---|---|
| anticoagulants | residue analysis for exposure (whole blood and serum) | 99 |
| PIVKA clotting time (plasma) | 50 | |
| prothrombin clotting time (whole blood) | 24 | |
| health parameters | general (whole blood and serum) | 124 |
| immunophenotype (lymphocytes in whole blood profiled by flow cytometry) | 64 | |
| cytokine concentrations (serum) | 93 | |
| complete blood counts (whole blood) | 118 | |
| blood chemistries (serum) | 116 | |
| B cell clonality | immunofixation (serum) | 6 |
| PARR (whole blood) | 7 | |
| pathogen infection or exposure | exposure/infection (whole blood and serum) | 93 |
| land use | percentage urban area (buffer zones) | 118 |
(c). Pathogen surveys, anticoagulant rodenticide screening and land use
The presence of AR residues for warfarin, coumachlor, bromadiolone, brodifacoum, diphacinone, chlorophacinone and difethialone was assessed in whole blood or serum (n = 99) at the Center for Animal Health and Food Safety (CAHFS) as described in [19].
We assessed clotting times using two measures: prothrombin times (PT) [22] and the more sensitive proteins induced in vitamin K absence (PIVKA) [23]. We measured PT (n = 24) using the Coag DX machine according to the manufacturer's instructions (IDEXX, Irvine, CA). PIVKAs were assessed at CAHFS using plasma samples frozen in liquid nitrogen as described in [23].
In addition to testing the effects of AR exposure on our dataset of health measures, we also tested the potential effects of urban land use. We previously documented that testing blood only indicates recent AR exposure events, thus leading to frequent false negatives (approximately 62% of the time; see [19] for more detail) respective to an individual's history of exposure. Urbanization, therefore, is arguably a more sensitive measure of AR exposure than AR levels in the tissues we are able to sample (i.e. peripheral tissues such as blood) [19], but it can also reflect potential exposure to other toxicants from urban environments. To quantify ‘urbanization’ or ‘land use’ for each individual, we used a standardized metric previously described in [19]. We calculated the proportion of natural and developed area within a radius (buffer zone) approximating one home range (females 2.3 km2; males 5.2 km2) around each individual's capture location (n = 118).
We tested individuals (n = 93) for exposure (i.e. seropositivity) to the bacterial pathogen bartonella (Bartonella spp.), the protozoan Toxoplasma gondii, and the viral pathogens feline immunodeficiency virus, puma lentivirus, feline calicivirus and feline herpesvirus. We also tested for infection with bacterial pathogens Mycoplasma haemofelis, M. haemominutum, Bartonella henselae and B. clarridgeaie. All testing was done at the Center for Companion Animals Studies or in the Feline Retrovirus Research Laboratory at Colorado State University as described in [24]. All individuals were inspected for signs of clinical mange as described in [17,20]. Four bobcats were captured with clinical signs of mild-to-severe mange. We excluded these individuals from further analyses because our goal was to isolate the effects of ARs, without introducing the noise from immune response to mange.
(d). Statistical analyses
(i). Identifying individual immune measures associated with AR exposure or land use
Our primary goal was to identify individual immune or organ function parameters associated with AR exposure or land use. However, because demography (age class and sex), season of sample collection and disease exposure or infection status may also have strong effects on immunity, we first used principal components analysis (PCA) to determine which of these parameters explained significant variance in our dataset. To do so, we first imputed missing values in our dataset of 65 health measures (using a k-nearest-neighbours algorithm from the impute package [25]; n = 75 individuals), and used a Spearman's rank correlation to test for a relationship between each potential covariate and the top 20 principal components (PCs). We identified three covariates—age class, M. haemominutum infection and Bartonella sp. exposure—that explained significant variance in the overall dataset (i.e. that correlated with one or more of the top 20 PCs at a 20% false discovery rate (FDR) threshold [26,27]). We controlled for these three parameters in all downstream analyses.
Next, we used linear models to test for a relationship between each of the 65 health parameters and AR exposure or urban land use, controlling for age class, M. haemominutum infection and Bartonella sp. infection. Each variable was normalized via z-score transformation prior to linear modelling using the ‘scale’ function in R. While AR exposure is the primary predictor of interest, we included urban land use in each of our linear models to capture the spectrum of health effects at the urban edge (see electronic supplementary material, S1 results and table S6 for analyses that test for effects of AR exposure and urban land use separately). For each model, we extracted the p-value associated with the AR exposure or urban land use effect and corrected for multiple hypothesis testing using an FDR approach [27] implemented in the R package ‘qvalue’ [26].
(ii). Testing for systemic, predictable differences between AR-exposed and unexposed individuals
We observed strong associations between AR exposure and many individual health parameters. We next wanted to test if these associations were system-wide and predictable, such that the health parameters themselves could be used to indicate AR exposure. To test this possibility, we used a random forests classifier (see [28–30] for information on random forests and their application to ecological datasets). This algorithm proceeds by randomly sampling a training set from the original data and a test set comprised the remaining individuals. The training set is used to grow a series of decision trees that accurately classify individuals with respect to the variable of interest, and the test set is used to test the predictive accuracy of the model. Further, this framework can be used to estimate the importance of individual variables by quantifying the mean decrease in accuracy for a random forests classifier that does versus does not include the focal variable. Importantly, for our purposes, a random forests approach allowed us to evaluate the importance of all 65 health parameters in one analysis simultaneously, using a method that is well-suited to handle a range of statistical relationships among predictor variables (e.g. correlation, interaction or context dependency [28–30]). Thus, while linear models allowed us to test for an association between each immune measure and AR exposure independently, random forests provide a complementary perspective by accounting for the complex relationships among the health parameters themselves.
To implement random forests, we first regressed out the effects of major covariates—age class, M. haemominutum infection and Bartonella sp. infection—from the filtered, imputed dataset of 65 health measures (n = 75 individuals; note that we again z-score normalized each measure prior to linear modelling). We used these residuals, combined initially with information on land use, to predict AR exposure, using the R package ‘randomForest’ [31] and default parameter settings; note that we also used the R package ‘ROCR’ to evaluate model sensitivity/specificity as quantified by area under the curve (AUC) [32]. To identify important variables, we used the package ‘rfPermute’ to quantify the mean decrease in accuracy associated with the removal of each predictor variable [33]. We considered variables to be significant if they passed a 10% FDR threshold. We also repeated these analyses without land use information in the model, and report those results in electronic supplementary material, table S7 and figure S1.
Testing blood for AR residues leads to 62% false negatives because blood measures only recent exposure [19]. We therefore hypothesized that (i) some individuals with no detectable levels of ARs in blood would be classified by the random forest as AR-exposed, and (ii) these individuals represent a set of truly AR-exposed individuals for whom the blood tests produced a false negative. If true, we would expect individuals living in more urbanized areas (where AR exposure is widespread) to fall into the misclassified group (i.e. to have immune profiles that are similar to known AR-exposed individuals, even though ARs were not detected in blood). To test this possibility, we used a Wilcoxon-signed rank test to ask whether individuals categorized as AR-exposed by random forests (but unexposed based on blood tests) had higher proportions of urbanization in their buffer zones than individuals classified as unexposed by both blood tests and random forests.
(iii). Hypergeometric assessment to assess overlap with model-organism studies
Finally, we used a hypergeometric test to ask whether our findings were in accordance with those from human or model-organism laboratory studies [34–46]. Specifically, we asked whether the set of immune measures we identified as (i) associated with AR exposure in our linear model analyses or (ii) important for predicting AR exposure in our random forests were significantly enriched for immune measures previously associated with therapeutic doses of anticoagulants. The list of immune measures previously reported to be associated with the anticoagulants that we examined in this study is provided in electronic supplementary material, table S8. All statistical analyses described here were performed in R v. 3.2.3 [47].
3. Results
(a). Sampling and measures of immune and organ function
Between 2007 and 2012, we collected samples from 124 apparently healthy bobcats (table 1) across a 1264 km2 study area of natural, urban and agricultural land (figure 1). We report descriptive values for the 65 health parameters, sample sizes and comparative reference ISIS values in electronic supplementary material, tables S2–S5.
(b). AR exposure, land use and pathogens
Blood samples from 98 bobcats were available for anticoagulant residue testing of which 38 were positive in blood, indicating recent exposure [19]. Bobcats were exposed to 1–5 compounds (electronic supplementary material, table S9), and for 32 individuals, we detected only diphacinone. The proportion of urban land use (residential, agricultural or developed open space areas) near each individual's habitat ranged between 0% and 92% (mean = 28.13%, s.d. = 25.04%, median = 23.96%; n = 118). AR exposure detected in blood was significantly positively associated with urban proximity (β = 1.57, p < 0.001), but not individual age or sex.
We tested 93 individuals for exposure to or infection with nine common feline pathogens. Prevalences were generally low (less than 25%; electronic supplementary material, table S10), with the exceptions of M. haemominutum (55%) and Bartonella henselae (65%). No pathogen was associated with urban proximity.
(c). AR exposure rarely affects clotting factors
Neither PT (t = −0.51; p = 0.307) nor PIVKA clotting times (t = −0.76; p = 0.227) differed significantly between AR-exposed or unexposed bobcats (electronic supplementary material, table S11), suggesting that bobcats are tolerant to vitamin K antagonistic effects of ARs at levels to which they were exposed.
(d). Analyses of individual variables reveal immune perturbations associated with urbanization and ARs
Linear models indicated associations between both AR exposure and urban proximity and multiple parameters reflective of immune and organ function (table 2; electronic supplementary material, table S6 and figure S1). Cell signalling proteins keratinocyte-derived chemokines (KC; β = −1.1; p = 0.029) were negatively associated with urbanization suggesting that as urban land use increases, there is a decrease in KC cytokines that mobilize granulocytic white blood cells (e.g. neutrophils) key to nonspecific immune responses. Although the association with AR exposure and KC was not significant, KC expression was 50% lower in AR-exposed bobcats (electronic supplementary material, table S5). By contrast, stem-cell factor (SCF; β = 1.2; p = 0.019) and interleukin (IL)-12p40 (β = 1.1; p = 0.029) were elevated in bobcats captured closer to the urban edge. Positive relationships with SCF and IL-12 indicate potential heightened activity of cytokines linked with the earliest stages of blood cell formation and development and differentiation of T lymphocytes in urban areas [48].
Table 2.
Linear regression results. We present the β- and p-values for tests that were significant for one or both predictor variable, and the mean and standard errors (s.e.) for the parameters shown. NR indicates values not reported.
| parameter | function | urban proximity |
AR exposure |
mean value (s.e.) |
||||
|---|---|---|---|---|---|---|---|---|
| β | pa | β | pa | unexposed | AR-exposed | captiveb | ||
| keratinocyte-derived chemokine | involved in chemotaxis and activation of neutrophils and monocytes/macrophages | −1.1 | 0.029* | −0.2 | 0.551 | 2058.2 (399.9) | 979.6 (288.1) | NR |
| stem-cell factor | promotes cell survival, proliferation, and functional activation of cells during haematopoiesis | 1.2 | 0.019* | −0.1 | 0.827 | 578.04 (28.3) | 642.1 (28.9) | NR |
| interleukin-12p40 | stimulates growth and functional differentiation of T lymphocytes | 1.1 | 0.029* | 0.0 | 0.285 | 807.2 (70.4) | 885.5 (83.3) | NR |
| lymphocytes (k cells µl−1) | major cellular component of adaptive cell-mediated and humoral immunity | −0.6 | 0.194 | 0.7 | 0.008** | 1.6 (0.1) | 2.3 (0.3) | 2.1 (0.2) |
| neutrophils (%) | first cells to respond to inflammation | 0.4 | 0.366 | −0.7 | 0.003** | 79.7 (1.7) | 73.4 (2.5) | 62.3c |
| basophils (k cells µl−1) | responsible for inflammatory reactions; key role in acute and chronic alleric diseases | −1.3 | 0.007** | 0.7 | 0.004** | 0.0 (0.0) | 0.01 (0.0) | 0.3 (0.1) |
| basophils (%) | −1.2 | 0.009** | 0.6 | 0.012* | 0.02 (0.01) | 0.1 (0.04) | 4.3c | |
| eosinophils (k cells µl−1) | target multicellular parasites; associated with allergic responses | −0.8 | 0.118 | 0.6 | 0.016* | 0.5 (0.1) | 1.0 (0.3) | 0.5 (0.04) |
| eosinophils (%) | −0.6 | 0.207 | 0.6 | 0.024* | 3.9 (0.6) | 6.9 (1.6) | 7.2c | |
| B lymphocytes (cells µl−1) | humoral immune component of adaptive immune system; secrete antibodies and cytokines | 0.7 | 0.039* | 0.6 | 0.267 | 331.8 (39.2) | 686.6 (122.2) | NR |
| CD4 + CD8 + T (cells µl−1) | T lymphocyte that targets viral-infected or damaged cells | −1.6 | 0.019* | 0.7 | 0.057 | 25.1 (4.8) | 30.5 (7.1) | NR |
| CD4 + CD8 + T lym. % | −1.6 | 0.021* | 0.4 | 0.310 | 1.6 (0.2) | 1.4 (0.2) | NR | |
| haemoglobin | indicators of iron sufficiency, oxygen-carrying capacity of red blood cells | 1.1 | 0.027* | −0.2 | 0.422 | 12.7 (0.2) | 12.9 (0.2) | 12.0 (0.2) |
| MCHC | 1.3 | 0.011* | −0.5 | 0.043* | 32.2 (0.4) | 31.6 (0.4) | 32.7 (0.2) | |
| alkaline phosphatase | enzyme indicative of liver function | 0.9 | 0.015* | −0.1 | 0.556 | 27.3 (3.8) | 31.5 (8.3) | 19.0 (1.9) |
| blood urea nitrogen | marker of kidney function | −1.0 | 0.046* | 0.1 | 0.713 | 36.1 (1.6) | 34.6 (1.5) | 32.0 (0.9) |
| calcium | integral to bone, muscle, nerve function | 0.8 | 0.028* | 0.1 | 0.527 | 9.4 (0.1) | 9.5 (0.1) | 9.8 (0.1) |
| phosphorus | integral to bone, muscle, nerve, a kidney function; tissue repair and maintenance | 0.9 | 0.007** | 0.3 | 0.110 | 5.8 (0.2) | 6.48 (0.2) | 5.4 (0.1) |
| creatinine | protein indicator of kidney function | 0.2 | 0.613 | −0.5 | 0.049* | 1.7 (0.1) | 1.5 (0.1) | 2.0 (0.1) |
aFalse discovery rate-corrected p-values; *p ≤ 0.05, **p ≤ 0.01.
b2011, International Species Information System (ISIS), Apple Valley, Minnesota 55124–8151, USA.
cValues not available in ISIS records and so approximated using available data calculated with reported mean absolute cellular counts.
On a cellular level (table 2; electronic supplementary material , tables S2–S6 and figure S2), AR exposure and urban land use were similarly linked with both immune stimulatory and suppressive effects. Lymphocyte (β = 0.7; p = 0.008), basophil (β = 0.7; p = 0.004) and eosinophil (β = 0.6; p = 0.016) counts were positively associated with AR exposure. In particular, lymphocyte counts were on average 44% greater in AR-exposed bobcats, suggesting a link between AR exposure and generalized inflammation [48]. The fraction of neutrophils, relative to the distribution of other white blood cells, was suppressed by 10% in AR-exposed bobcats (p = 0.004). While mild suppression of neutrophils may not directly impede an ability to mount immune responses, the suppression may signal that larger autoimmune or cytokine-mediated inflammatory processes of concern are present [49].
We used immunophenotyping to identify and quantify specific lymphocyte populations central to the adaptive immune response (electronic supplementary material, tables S1, S4 and S6). CD4 + CD8 + T lymphocytes associated with cell-mediated immunity to tumour and viral-infected cells [48] decreased with urban land use (β = −1.6; p = 0.019). Urbanization was also associated with greater B lymphocyte activity (β = 0.7; p = 0.039), and was associated with AR exposure in joint AR and urban models, albeit not significantly (table 2); when we tested for association with AR exposure alone, B cells were significantly associated with AR exposure (electronic supplementary material, tables S4 and S6). Specifically, B cell counts were 48% higher in AR-exposed bobcats (β = 0.9; p = 0.005) and B cell fractions were 36% higher (β = 0.7; p = 0.025). B lymphocytes are the humoral adaptive immune component that targets invading pathogens [48]. We verified that B cells were polyclonal to rule out lymphocytic leukaemia (see electronic supplementary material, S1 methods).
AR exposure and urban proximity were also associated with anomalies in biochemical markers associated with organ function (table 2; electronic supplementary material, table S6 and figure S2). Creatinine, a protein indicator of kidney function, was negatively associated with AR exposure (β = −0.5; p = 0.049). The serum concentrations of the minerals calcium (β = 0.8; p = 0.028) and phosphorus (β = 0.9; p = 0.007) were positively associated with urbanization. Blood urea nitrogen (β = −1.0; p = 0.046), a marker of kidney function, was negatively associated with urban land use, whereas alkaline phosphatase, a marker of liver function, was positively associated with urban land use (β = 0.9; p = 0.015). The clinical implications of these associations are unknown, but highlight that ARs and urbanization may have system-wide physiological consequences for species that persist at the urban–wildland edge.
(e). Urbanization and ARs have systemic, predictable effects on health parameters
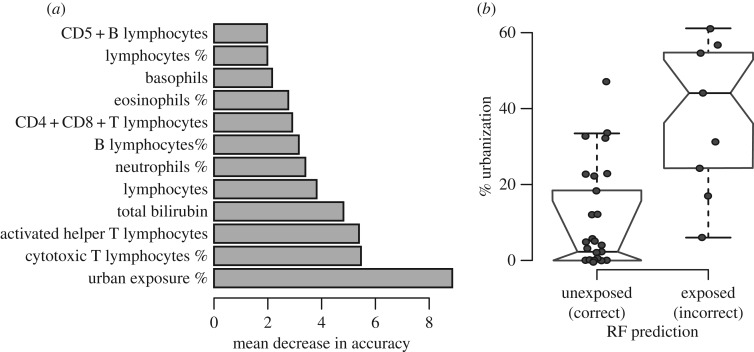
We used random forests to explore the cumulative, system-wide relationship between AR exposure and health effects. Random forests revealed that the differences between AR-exposed and unexposed individuals were systemic and predictable such that the parameters themselves can be used to predict an individual's exposure status (predictive accuracy = 67.3%, error rate = 32.7% and AUC = 0.68, electronic supplementary material, figure S2a–b; proportion of individuals correctly classified as exposed and unexposed = 18/29 and 31/46). The parameters important for predicting whether an animal was exposed to ARs were linked with both immune and organ function (figure 2a; electronic supplementary material, tables S7 and S11). For example, the number of activated helper T lymphocytes, the number and fraction of cytotoxic T cells and the fraction of B cells significantly contributed to model accuracy (figure 2a; electronic supplementary material, tables S7 and S11). These findings were similar across models that did not incorporate urban land use (electronic supplementary material, tables S7 and S11).
Figure 2.
(a) Results of random forest analyses ranking variable importance scores for parameters key to predictive accuracy. All parameters are absolute counts unless indicated as a percentage. (b) Boxplot comparing land use info for individuals correctly classified versus misclassified individuals.
We predicted that systemic health parameter changes may be a better indicator of AR exposure than blood residue tests. Our random forests predicted that 11 bobcats testing negative for ARs in blood were actually AR-exposed; in support of the idea that these individuals represent false negatives for AR blood tests (and truly were exposed), these animals had greater urban proximity compared with individuals classified as unexposed to ARs by both random forest and blood tests (Wilcoxon rank sum test, p = 0.001; figure 2b).
(f). Bobcat response to ARs mirrors model-organism response to therapeutic anticoagulants
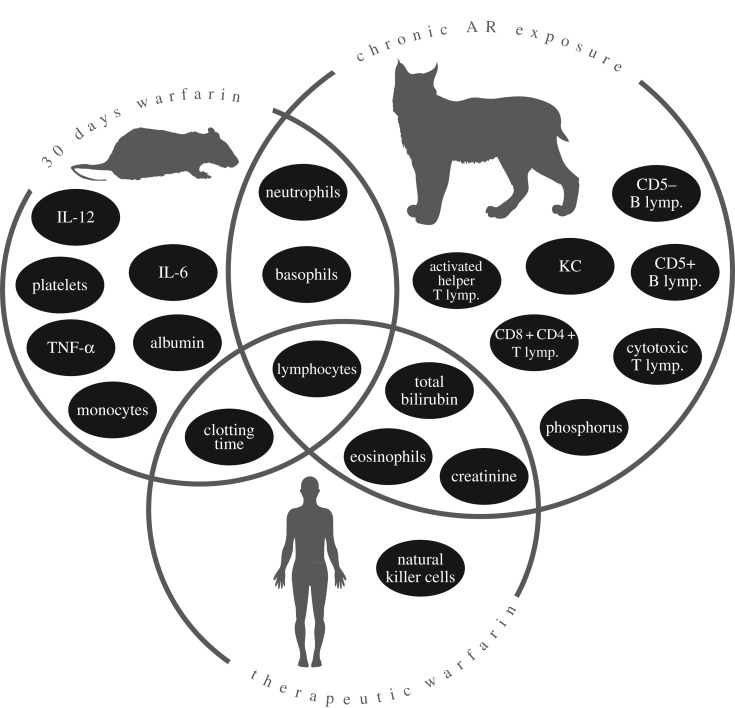
We tested whether our findings were in accordance with those from human and model-organism laboratory studies [34–39] (electronic supplementary material, table S8). For example, concordant with published studies, we found increased lymphocyte activity and a decrease in neutrophil fractions associated with anticoagulants (figure 3). The overlap we observe is more so than expected by chance (p = 0.005, hypergeometric test).
Figure 3.
A Venn diagram illustration of significant health parameters that overlap with published rodent and human laboratory studies, as well as the novel parameters discovered in the bobcat population. See electronic supplementary material, table S8 for citations.
4. Discussion
Carnivores in SMMNRA are widely and chronically exposed to ARs [5,17,19,50], but the sublethal consequences of this exposure were unknown. Here, we focused our analyses directly on the effects of ARs, while also incorporating the urban environmental conditions. We found that exposure to urbanization and ARs is associated with health parameter anomalies that range from subtle responses (i.e. 10% decrease in neutrophil fractions) to more pronounced associations with potential clinical implications (i.e. 50% elevated KC and lymphocytes). We posit that, cumulatively, these health parameter changes may increase bobcat vulnerability to environmental stressors such as opportunistic notoedric mange. Our findings reveal a complex response to sublethal toxicosis, and underscore the harmful toxic effects associated with urbanization for natural populations at the urban–wildland interface.
We focused on a free-ranging, non-model organism to evaluate the immunotoxic effects of urbanization and ARs. While the immunotoxic effects of ARs were our primary interest, we also more broadly characterized the immunotoxic effects of urban proximity for three reasons: (i) it is impossible when studying free-ranging animals to control for every toxic component of the urban edge; (ii) analyses that rely on AR exposure in live-captured animals using blood vastly underestimate the true pervasiveness of AR exposure [19]; and (iii) we previously found that exposure to different individual AR compounds, the concentrations of compounds detected and exposure to multiple compounds (an indicator of repeat exposure events and thus likely chronic exposure) were strongly correlated with the percentage developed area in buffer zones [19]. Clotting times, the most frequent method of AR detection in controlled veterinary settings (e.g. [22]), were not prolonged in AR-exposed bobcats, confirming the difficulty of assessing AR exposure using conventional methods. We therefore conclude that the use of both blood residues and percentage urbanization more robustly evaluates the effects of AR exposure and including of chronic exposure, the latter being immeasurable without frequent sampling of the same individuals. Further, our random forest analyses suggest that the strength and characteristics of the physiological response itself may be more informative for identifying individuals who are truly AR-exposed in cases where blood tests fail to detect ARs.
Bobcats, like domestic cats [18], seem tolerant to the vitamin K antagonistic effects of ARs, as indicated by the normal clotting times in AR-exposed bobcats. However, this tolerance may lead to chronic sublethal exposure that influences other physiological parameters. Our findings related to AR impacts in free-ranging bobcats are remarkably consistent with other published studies (figure 3). Humans and laboratory rodents show simultaneous immunostimulatory and immunosuppressive effects of anticoagulants, such as increased lymphocyte, IL-12 and IL-6 activity, and decreased TNF-α and neutrophils. We document similar immune responses, but also novel systemic effects of AR exposure. These AR-induced changes were in a wide-range of immune parameters associated with responses to allergens (eosinophils and basophils), tumours and viral infections (CD4 + CD8 + T lymphocytes), and novel pathogens (helper T lymphocytes). Because the immune response is energetically costly, and non-target immune activation may impair organismal function, fitness may be reduced by regular immune stimulation [51–53]. Thus, understanding the role of ARs in regulating immunity is essential to the conservation of wild populations.
Bobcats living on the urban edge where they are chronically exposed to ARs may also experience persistent immune and organ perturbations. Chronic activation of immune function can lead to immune exhaustion, characterized by the dysfunction and depletion of immune cells [54,55]. This kind of activation increases susceptibility to opportunistic infections, accelerates disease progression, and even causes disease itself [54–56]. In a hallmark example, the mechanism by which human immunodeficiency virus infection causes acquired immunodeficiency is chronic immune activation that depletes helper T lymphocytes with time, and thus causes an erosion of immune function [57]. Within our study system, the association between AR exposure and immune discord may explain the mechanism linking chronic AR exposure to susceptibility to notoedric mange, which has precipitated regional declines in bobcat populations and decimated our focal population [17,20]. Similar cascading effects could occur in other species where ARs cause sublethal effects.
Given the observed immune system effects, the dearth of evidence of clinical disease related to any pathogens other than mange is surprising. However, when challenged with pathogens, the early activation of specific immune cells and proteins determines the rate of disease progression and outcome [58,59]. Although little is known about immune pathways associated with notoedric mange, the secretion of specific cytokines during early-stage infection is pivotal to disease progression in humans infected with the closely related mite, Sarcoptes scabiei. If helper T lymphocytes secrete IFN-γ and IL-2 in response to infection, the infection is overcome. However, if those same helper T lymphocytes secrete other cytokines IL-4, IL-5 and IL-13 [58], an uncontrolled response occurs. The broad-spectrum immune activation that we observe in AR-exposed bobcats may compromise the ability to mount specific immune responses to certain pathogens such as N. cati.
Bobcats may also encounter this mite more regularly than other pathogens. In general, we have found low levels of exposure (low seropositivity) to other common feline pathogens [24]. However, N. cati is present on other species in the ecosystem including rabbits and ground squirrels [60], and rabbits are the predominant prey item of bobcats in the study area [61]. In our study area overall, bobcats may simply be at risk of infection from N. cati more than from other pathogens, and if ARs increase their vulnerability to the pathogen, urban bobcats would be more likely to succumb to infection.
Exposure of non-target wildlife to ARs is increasingly recognized as a global conservation issue [16]. Within the bobcat population, we have also shown that AR exposure can begin during prenatal development, and persist for the duration of an individual's life [19]. While we have rarely documented direct AR toxicity mortality in bobcats (n = 2 over a 20-year period), AR toxicity was the second leading cause of death in coyotes over a 9-year study in SMMNRA [5]. These contrasting mortality findings show that toxicants produce myriad effects that differentially influence species within ecosystems. Toxicant exposure can indirectly have fatal impacts, even if it is not a source of direct mortality. Our analyses suggest that sublethal toxicant exposure may indirectly cause mortality by severely weakening the immune system in a free-ranging carnivore, and thus mitigation of the effects of mange will require reduction of ARs at the urban–wildlands interface. While evaluating the sublethal effects of toxicants on the health of free-ranging animals that may experience a lifetime of poisoning is difficult and complex, our findings of measurable and consequential sublethal effect of ARs in a wild population imply that environmental agencies must re-evaluate the harm these toxicants pose to wild species and the ecosystems they inhabit.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Special thanks to the many NPS biologists and interns that assisted with fieldwork. We thank S. Weldy, K. Krause, D. Tom, Fund for Animals, B. Gordon, M. A. Hausner, I. Schmid, J. Dow, M. Tompkins, L. Hutlin, P. Hutlin, W. Sprague, A. Aviary, S. Schlotmann, M. Lappin, CSU Center for Companion Animal Studies, and for their contribution to optimizing immunological assays. Thanks to J. Tung and M. S. Rogan for reviewing previous versions of this manuscript.
Ethics
Animal capture, handling and sample collection protocols were approved by the Office of Animal Research Oversight (UCLA: ARC#2007-167-12) and California Department of Fish and Wildlife (SC-9791).
Data accessibility
All data are available on Dryad: http://dx.doi.org/10.5061/dryad.36rf5 [62].
Authors' contributions
L.E.K.S. and A.J.L. wrote the manuscript and analysed data. M.E. and C.H.U. aided with the development, use and interpretation of immune assays. L.E.K.S., T.C.A., J.M., S.V. and S.C. generated data. J.F., R.K.W. and S.P.D.R. assisted L.E.K.S. with sampling design and manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
The research was supported by the NSF GRF, NSF EID 0723676 and 1413925, Summerlee Foundation, Christine Stevens Wildlife Award, Panthera, Santa Monica Bay Audubon Society, G2 Gallery, the NPS, SAMO Fund, Poison Free Malibu, HESKA Corporation. The UCLA Flow Cytometry and Immuno/BioSpot Cores are supported by NIH (AI-28697 and CA-16042) grants.
References
- 1.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/34073) [DOI] [PubMed] [Google Scholar]
- 2.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006. From patterns to emerging processes in mechanistic urban ecology. TREE 21, 186–191. ( 10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 3.Bradley CA, Altizer S. 2007. Urbanization and the ecology of wildlife diseases. TREE 22, 95–102. ( 10.1016/j.tree.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serieys L, Lea A, Pollinger JP, Riley SP. D, Wayne RK. 2015. Disease and freeways drive genetic change in urban bobcat populations. Evol. App. 8, 75–92. ( 10.1111/eva.12226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley SPD, Sauvajot RM, Fuller TK, York EC, Kamradt DA, Bromley C. 2003. Effects of urbanization and habitat fragmentation on bobcats and coyotes in southern California. Conserv. Biol. 17, 566–576. ( 10.1046/j.1523-1739.2003.01458.x) [DOI] [Google Scholar]
- 6.Mendelssohn H, Paz U. 1977. Mass mortality of birds of prey caused by Azodrin, an organophosphorus insecticide. Biol. Conserv. 11, 163–170. ( 10.1016/0006-3207(77)90001-5) [DOI] [Google Scholar]
- 7.Berny P. 2007. Pesticides and the intoxication of wild animals. J. Vet. Pharmacol. Ther. 30, 93–100. ( 10.1111/j.1365-2885.2007.00836.x) [DOI] [PubMed] [Google Scholar]
- 8.Davidson C. 2004. Declining downwind: amphibian population declines in California and historical pesticide use. Ecol. Appl. 14, 1892–1902. ( 10.1890/03-5224) [DOI] [Google Scholar]
- 9.Brittain CA, Vighi M, Bommarco R, Settele J, Potts SG. 2010. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 11, 106–115. ( 10.1016/j.baae.2009.11.007) [DOI] [Google Scholar]
- 10.Ross P. 2002. The role of immunotoxic environmental contaminants in facilitating the emergence of infectious diseases in marine mammals. Hum. Ecol. Risk Assess. 2, 272–292. ( 10.1080/20028091056917) [DOI] [Google Scholar]
- 11.Galloway T, Handy R. 2003. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12, 345–363. ( 10.1023/A:1022579416322) [DOI] [PubMed] [Google Scholar]
- 12.Presley SM, Austin GP, Dabbert CB. 2010. Influence of pesticides and environmental contaminants on emerging diseases of wildlife. In Wildlife toxicology: emerging contaminant and biodiversity issues (eds Kendall RJ, Lacher TE, Cobb GP, Cox SB), pp. 74–109. Boca Raton, FL: CRC Press. [Google Scholar]
- 13.Berny PJ, Buronfosse T, Buronfosse F, Lamarque F, Lorgue G. 1997. Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 35, 1817–1829. ( 10.1016/S0045-6535(97)00242-7) [DOI] [PubMed] [Google Scholar]
- 14.McMillin SC, Hosea RC, Finlayson BF. 2008. Anticoagulant rodenticide exposure in an urban population of San Joaquin kit fox In Proc. 23rd V.P.C., San Diego, CA, 17–20 March, pp. 163–165. Davis, CA: University of California, Davis. [Google Scholar]
- 15.Albert CA, Wilson LK, Mineau P, Trudeau S, Elliott JE. 2009. Anticoagulant rodenticides in three owl species from Western Canada, 1988–2003. Arch. Environ. Contam. Toxicol. 58, 451–459. ( 10.1007/s00244-009-9402-z) [DOI] [PubMed] [Google Scholar]
- 16.Erickson W, Urban D. 2004. Potential risks of nine rodenticides to birds and nontarget mammals: a comparative approach. Washington, DC: United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substance. [Google Scholar]
- 17.Riley SPD, Bromley C, Poppenga R, Uzal FA, Whited L, Sauvajot RM. 2007. Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban southern California. J. Wildl. Manag. 71, 1874–1884. ( 10.2193/2005-615) [DOI] [Google Scholar]
- 18.Petterino C, Paolo B. 2001. Toxicology of various anticoagulant rodenticides in animals. Vet. Hum. Toxicol. 43, 353–360. [PubMed] [Google Scholar]
- 19.Serieys LEK, Armenta T, Moriarty JG, Boydston EE, Lyren LM, Poppenga R, Crooks KR, Wayne RK, Riley S. 2015. Anticoagulant rodenticides in urban bobcats: exposure, risk factors and potential effects based on a 16-year study. Ecotoxicology 24, 844–862. ( 10.1007/s10646-015-1429-5) [DOI] [PubMed] [Google Scholar]
- 20.Serieys LEK, et al. 2013. Serum chemistry, hematologic and post-mortem findings in bobcats (Lynx rufus) with notoedric mange. J. Parasitol. 99, 989–996. ( 10.1645/12-175.1) [DOI] [PubMed] [Google Scholar]
- 21.Pence D, Ueckermann E. 2002. Sarcoptic mange in wildlife. Rev. Sci. Tech. 21, 385–398. ( 10.20506/rst.21.2.1335) [DOI] [PubMed] [Google Scholar]
- 22.Sheafor SE, Couto CG. 1999. Anticoagulant rodenticide toxicity in 21 dogs. J. Am. Anim. Hosp. Assoc. 35, 38–46. ( 10.5326/15473317-35-1-38) [DOI] [PubMed] [Google Scholar]
- 23.Whisson DA, Salmon TP. 2002. Effect of diphacinone on blood coagulation in Spermophilus beecheyi as a basis for determining optimal timing of field bait applications. Pest Manag. Sci. 58, 736–738. ( 10.1002/ps.516) [DOI] [PubMed] [Google Scholar]
- 24.Carver S, et al. 2015. Pathogen exposure varies widely among sympatric populations of wild and domestic felids across the United States. Ecol. Appl. 26, 367–381. ( 10.1890/15-0445) [DOI] [PubMed] [Google Scholar]
- 25.Hastie T, Tibshirani R, Narasimhan B, Chu G. 2015. Impute: imputation for microarray data. R package version 1.42.0. [DOI] [PMC free article] [PubMed]
- 26.Dabney A, Storey JD. 2015. qvalue: Q-value estimation for false discovery rate control. R Package version 1.43.0.
- 27.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA 100, 9440–9445. ( 10.1073/pnas.1530509100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutler DR, Edwards TC Jr, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. 2007. Random forests for classification in ecology. Ecology 88, 2783–2792. ( 10.1016/S0034-4257(00)00145-0) [DOI] [PubMed] [Google Scholar]
- 29.Olden JD, Lawler JJ, Poff NL. 2008. Machine learning methods without tears: a primer for ecologists. Q. Rev. Biol. 83, 171–193. ( 10.1086/587826) [DOI] [PubMed] [Google Scholar]
- 30.Breiman L. 2001. Random forests. Mach. Learn. 45, 5–32. ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 31.Liaw A, Wiener M. 2002. Classification and regression by random forest. R. News 2, 18–22. [Google Scholar]
- 32.Sing T, Sander O, Beerenwinkel N, Lengauer T. 2005. ROCR: visualizing classifier performance in R. Bioinformatics 21, 3940–3941. ( 10.1093/bioinformatics/bti623) [DOI] [PubMed] [Google Scholar]
- 33.Archer E. 2015. rfPermute: estimate Permutation p-Values for Random Forest Importance Metrics. R package version 1.42.0.
- 34.Belij S, Miljkovic D, Popov A, Subota V, Timotijevic G, Slavic M, Mirkov I, Kataranovski D, Kataranovski M. 2012. Effects of subacute oral warfarin administration on peripheral blood granulocytes in rats. Food Chem. Toxicol. 50, 1499–1507. ( 10.1016/j.fct.2012.01.049) [DOI] [PubMed] [Google Scholar]
- 35.Berkarda B, Bouffard-Eyüboğlu H, Derman U. 1983. The effect of coumarin derivatives on the immunological system of man. Agents Actions 13, 50–52. ( 10.1007/BF01994281) [DOI] [PubMed] [Google Scholar]
- 36.Berkarda B, Marrack P, Kappler JW, Bakemeier RF. 1978. Effects of warfarin administration on the immune response of mice. Arzneimittelforschung 28, 1407–1410. [PubMed] [Google Scholar]
- 37.Popov A, Belij S, Subota V, Zolotarevski L, Mirkov I, Kataranovski D, Kataranovski M. 2013. Oral warfarin affects peripheral blood leukocyte IL-6 and TNFα production in rats. J. Immunotoxicol. 10, 17–24. ( 10.3109/1547691X.2012.684159) [DOI] [PubMed] [Google Scholar]
- 38.Kurohara M, et al. 2008. Low-dose warfarin functions as an immunomodulator to prevent cyclophosphamide-induced NOD diabetes. Kobe J. Med. Sci. 54, E1–13. [PubMed] [Google Scholar]
- 39.Takahashi O. 1991. Some properties of rat platelet aggregation and effects of butylated hydroxytoluene, warfarin and aspirin. Food Chem. Toxicol. 29, 173–183. ( 10.1016/0278-6915(91)90035-6) [DOI] [PubMed] [Google Scholar]
- 40.Goudarzipour K, Ghazizadeh F, Tavassol HH, Behnam B. 2015. Warfarin-induced eosinophilia in a child with urkitt Lymphoma: a case report. Iran. J. Pharm. Res. 14, 887. [PMC free article] [PubMed] [Google Scholar]
- 41.Bobek V, Boubelik M, Fišerová A, L'uptovcová M, Vannucci L, Kacprzak G, Kolodzej J, Majewski AM, Hoffman RM. 2005. Anticoagulant drugs increase natural killer cell activity in lung cancer. Lung Cancer 47, 215–223. ( 10.1016/j.lungcan.2004.06.012) [DOI] [PubMed] [Google Scholar]
- 42.Teragaki M, Kawano H, Makino R, Inoue K, Sai Y, Hosono M, Suehiro S, Okamura M. 2012. A case of Warfarin-induced eosinophilia. Int. Med. 51, 1627–1629. ( 10.2169/internalmedicine.51.7138) [DOI] [PubMed] [Google Scholar]
- 43.Mikhail MW, Abdel-Hamid YM. 2007. Effect of warfarin anticoagulant rodenticide on the blood cell counts of Rattus norvegicus and Rattus rattus. J. Egypt. Soc. Parasitol. 37, 853–861.18383788 [Google Scholar]
- 44.Doyle JJ, Koren G, Cheng MY, Blanchette VS. 1988. Anticoagulant with sodium warfarin in children: effect of a loading regimen. J. Pediatr. 113, 1095–1097. ( 10.1016/S0022-3476(88)80589-4) [DOI] [PubMed] [Google Scholar]
- 45.Kuwahara T, Hamada M, Inoue Y, Aono S, Hiwada K. 1995. Warfarin-induced eosinophilic pleurisy. Int. Med. 34, 794–796. ( 10.2169/internalmedicine.34.794) [DOI] [PubMed] [Google Scholar]
- 46.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. 2011. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 80, 181–189. ( 10.1038/ki.2011.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R Core Team. 2016. R: A language and environment for statistical computing.
- 48.Murphy D. 2012. Janeway's immunobiology, 8th edn New York, NY: Garland Science. [Google Scholar]
- 49.Newburger PE, Dale DC. 2013. Evaluation and management of patients with isolated neutropenia. Semin. Hematol. 50, 198–206. ( 10.1053/j.seminhematol.2013.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beier P, Riley S, Sauvajot R. 2010. Mountain Lions (Puma concolor). In Urban carnviores (eds Gehrt S, Riley S, Cypher BL), pp. 141–155. Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 51.Sadd BM, Schmid-Hempel P. 2008. Principles of ecological immunology. Evol. Appl. 2, 113–121. ( 10.1111/j.1752-4571.2008.00057.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derting TL, Compton S. 2003. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiol. Biochem. Zool. 76, 744–752. ( 10.1086/375662) [DOI] [PubMed] [Google Scholar]
- 53.Hanssen SA, Hasselquist D, Folstad I, Erikstad KE. 2004. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. Lond. B 271, 925–930. ( 10.1098/rspb.2004.2678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leligdowicz A, Feldmann J, Jaye A, Cotten M, Dong T, McMichael A, Whittle H, Rowland Jones S. 2010. Direct relationship between virus load and systemic immune activation in HIV-2 infection. J. Infect. Dis. 201, 114–122. ( 10.1086/648733) [DOI] [PubMed] [Google Scholar]
- 55.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247. ( 10.1038/ni.1703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauce D, et al. 2011. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood 117, 5142–5151. ( 10.1182/blood-2011-01-331306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Appay V, Sauce D. 2008. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214, 231–241. ( 10.1002/path.2276) [DOI] [PubMed] [Google Scholar]
- 58.Walton SF. 2010. The immunology of susceptibility and resistance to scabies. Parasite Immunol. 32, 532–540. ( 10.1111/j.1365-3024.2010.01218.x) [DOI] [PubMed] [Google Scholar]
- 59.Darwich LD, et al. 2008. The magnitude of interferon-gamma responses to human cytomegalovirus is predictive for HIV-1 disease progression. JAIDS 49, 501–512. ( 10.1097/QAI.0b013e318189a7af) [DOI] [PubMed] [Google Scholar]
- 60.Foley J, et al. 2016. A synthetic review of notoedres species mites and mange. Parasitol. 143, 1847–1861. ( 10.1017/S0031182016001505) [DOI] [PubMed] [Google Scholar]
- 61.Riley S, Boydston EE, Crooks KR, Lyren LM. 2010. Bobcats (Lynx rufus). In Urban carnivores (eds Gehrt SD, Riley S, Cypher BL), pp. 121–138. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 62.Serieys LEK, et al. 2018. Data from: Urbanization and anticoagulant poisons promote immune dysfunction in bobcats Dryad Digital Repository. ( 10.5061/dryad.36rf5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Serieys LEK, et al. 2018. Data from: Urbanization and anticoagulant poisons promote immune dysfunction in bobcats Dryad Digital Repository. ( 10.5061/dryad.36rf5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available on Dryad: http://dx.doi.org/10.5061/dryad.36rf5 [62].