Abstract
For sexually reproducing species, functionally competent sperm are critical to reproduction. While high atmospheric temperatures are known to influence the timing of breeding, incubation and reproductive success in birds, the effect of temperature on sperm quality remains largely unexplored. Here, we experimentally investigated the impact of ecologically relevant extreme temperatures on cloacal temperature and sperm morphology and motility in zebra finches Taeniopygia guttata. We periodically sampled males exposed to 30°C or 40°C temperatures daily for 14 consecutive days. Following a 12-day (23°C) recovery period, birds were again exposed to heat, but under the alternate treatment (e.g. birds initially exposed to 40°C were exposed to 30°C). Elevated temperatures led to an increase in cloacal temperature and a reduction in the proportion of sperm with normal morphology; these effects were most notable under 40°C conditions, and were influenced by the duration of heat exposure and prior exposure to high temperature. Our findings highlight the potential role of temperature in determining male fertility in birds, and perhaps also in constraining the timing of avian breeding. Given the increased frequency of heatwaves in a warming world, our results suggest the need for further work on climatic influences on sperm quality and male fertility.
Keywords: avian reproduction, climate change, heatwave, male fertility, sperm swimming speed, sperm morphology
1. Introduction
Normal sperm function is critical to reproductive success as only functionally competent sperm are capable of fertilizing eggs. Over the past few decades, understanding how post-copulatory sexual selection (i.e. sperm competition and cryptic female choice) shapes sperm morphology and performance has been a key focus of studies in a wide range of taxa. It is now widely accepted that sperm competition can drive evolutionary changes in sperm size, morphology, swimming speed, metabolic performance, viability and longevity [1,2]. By contrast, the impact of external environmental effects on sperm function has received relatively little attention in the evolutionary and ecological literature. This is surprising given the considerable body of evidence suggesting that a range of environmental factors (e.g. temperature, diet) may impact sperm quality in both externally and internally fertilizing species [3].
Given warming global temperatures and an increasing frequency of heatwaves [4], it is particularly important to understand the consequences of temperature variation for sperm function and quality. Environmental temperature variation affects organisms across all life stages, influencing physiology, behaviour and global distribution. In birds, ambient temperature influences breeding phenology [5], incubation behaviour [6] and reproductive success [7]. In some taxa, temperature impacts sperm function and fertilizing ability [3]. For example, mammals exposed to high environmental temperatures exhibit reductions in sperm motile performance and an increase in sperm morphological defects [8,9]. However, studies examining the impact of temperature on sperm quality primarily assess the effects of temperature variation under in vitro sperm incubation conditions and mostly address seasonal temperature variation effects or effects due to freeze/thaw conditions associated with cryopreservation methods [3]. Heat stress and high environmental temperatures (approx. 32–35°C) have also been linked to male infertility and reduced sperm quality in domestic poultry [10–12]. The impact of extreme temperatures (i.e. those experienced during heatwaves) on avian sperm quality, however, has not been previously considered.
The zebra finch Taeniopygia guttata is a model system for avian sperm biology and sperm competition [13]. In the wild, they are opportunistic breeders, capable of breeding year round given suitable environmental conditions [14]. Breeding activity occurs under average ambient temperatures ranging from 2.2°C [14] to approximately 36°C, but maximum temperatures regularly exceed 40°C during breeding periods [15]. Despite being adapted to the hot, arid interior of Australia, atmospheric temperatures of over 40°C are likely to be physiologically stressful for zebra finches, and while mild hyperthermia is tolerated, body temperature of 46°C is lethal [16]. To contextualize the ecological relevance of extreme temperatures, we considered temperature records for Fowlers Gap, New South Wales (data for 2004–2017, Australian Bureau of Meteorology), the site of the longest running study of wild breeding zebra finches [17]. At Fowlers Gap, temperatures reached or exceeded 40°C, on an average of 18.31 ± 8.32 (s.d.) days per year, with a maximum recorded atmospheric temperature of 46.8°C.
While heatwaves have been defined in many ways [18], we consider a heatwave to be any period when atmospheric temperature exceeds 40°C on two or more consecutive days. Heatwaves occur regularly in the Australian arid zone. For example, at Fowlers Gap, between one and seven heatwaves occur each year between November and March (with the highest frequency of heatwaves in January). Such heatwaves can last for 2–8 days, when temperatures can exceed 40°C for up to 10 h per day with cooler conditions at night (see electronic supplementary material, S1). Thus, Australian birds are regularly exposed to extreme ambient temperatures [4,19] during late Austral spring through summer. For numerous bird species in the Australian arid zone (including the zebra finch), breeding activity appears to be suppressed during the hottest summer months [20]. One of the potential costs of breeding during periods of extreme heat may be the negative effect of this heat on sperm quality, similar to the effects observed in mammals [8,9].
Using domesticated zebra finches, we investigated the impact of extreme environmental temperatures on avian sperm function. The specific nature of the potential damage to sperm is difficult to predict because avian studies considering extreme temperature conditions (i.e. 40°C or more) are lacking and because many studies use a composite measure of sperm quality (i.e. SQI or sperm quality index, e.g. [12]). However, based on findings in other taxa (e.g. mammals) [8,9], we predicted that extreme temperatures would negatively impact sperm motile performance and reduce the number of morphologically normal sperm in samples. We also examined how the duration of heat exposure and prior exposure to high (but not extreme) temperatures might affect potential temperature related changes in sperm function. In mammals, sperm quality is not immediately affected by testicular heat treatment because damaged sperm do not enter the ejaculates for some time after heat stress [8]. In the current study, however, predictions concerning the impact of heat treatment duration based on mammalian taxa are inappropriate, because of the major differences in reproductive anatomy and physiology between mammals and birds; passerine birds store sperm prior to ejaculation in the distal region of the ductus deferens, a site some distance from the testes [21], and spermatogenesis is much faster in birds than it is in mammals [22]. Finally, we examined whether sperm function was restored when birds were returned to milder ambient temperature conditions.
2. Methods
(a). Experimental design
In 2016, 20 male zebra finches from a captive population at Macquarie University (Sydney, Australia) were housed indoors in single-sex cages (dimensions 0.7 × 0.5 × 1.3 m, 5 males per cage) under standardized, baseline climate-controlled conditions (23°C, approximately 50% humidity, 12 L : 12 D cycle) with ad libitum food and water. All birds were sexually mature (15–18 months of age), hatched under the same conditions, and were previously maintained under identical housing conditions in outdoor aviaries. Birds were randomly selected from a single similar-aged cohort, leading to the inclusion of some siblings (3 sets of 2 siblings, 1 set of 3 siblings, and 11 individuals from unique families; controlled for in analysis). Throughout the experiment, males were kept in visual and vocal contact with females; five female zebra finches were housed in cages positioned immediately adjacent to the male cages, such that two cages of five males were each separated by a cage of females at all times.
Birds were held for a three-week acclimation period at baseline conditions. Following this period, males were randomly allocated to one of two heat treatment groups: (i) 30°C and (ii) 40°C (approx. 50% humidity for both treatments). We choose these temperatures as they are representative of (i) relatively normal maximum daily temperatures experienced during active breeding periods (30°C) and (ii) extreme heat conditions experienced intermittently during active breeding periods (40°C). We choose not to exceed 40°C temperature treatments in order to minimize the likelihood of birds dying, as death has been shown to occur if sustained body temperatures reach 45–46°C [14]. Immediately prior to the treatment (07.00–08.00 on day 1), we collected sperm (see below), measured tarsus length and body mass and measured cloacal temperature by gently inserting an internal probe thermometer (QM1601, Digitech, TechBrands, Australia) into the cloacal opening. We chose this approach as it is relatively non-invasive and because it is likely to be representative of the core and testis temperature of males; in other species, cloacal temperature is correlated with core body temperature [23], which in turn is correlated with testis temperature [24]. Birds were then exposed to the heat treatment (30 or 40°C) for an 8 h period (08.00–16.00) each day for 14 consecutive days; we chose a 14-day treatment period as some studies suggest changing climatic conditions may lead to longer lasting heatwaves, as well as more intense and frequent heatwaves (e.g. [25]). Outside of these periods, birds were maintained under baseline conditions (23°C). While, the experimental conditions did not perfectly mimic more variable wild conditions, they accurately reflect the general pattern of heat exposure during natural heatwaves (i.e. individuals are exposed to conditions exceeding 40°C for multiple hours but get some respite at night). More specifically, in an exemplar heatwave in the wild the average daily minimum temperature was 22.6°C, while average maximum was 42.8°C, and the 24 h average temperature was 33.6°C (electronic supplementary material, S1). In our experiment the daily minimum was 23°C, the maximum 40°C and the 24 h average was 28.7°C ± 8.2 (s.d.). Thus, our experimental conditions were ecologically relevant.
Exposure to the different conditions was achieved by moving cages between separate environmental chambers set at 23°C, 30°C and 40°C, and thus exposure to elevated temperatures was immediate. However, to avoid temperature shock, birds in the 40°C treatment were acclimatized for 30 min at 30°C before being placed in 40°C conditions. We collected sperm and measured cloacal temperature and body mass at regular intervals throughout the experiment (days 3, 7, 11 and 14); in these instances all samples were collected after birds had been exposed to experimental temperatures for several hours (14.00–16.00). Following sample collection on day 14, birds were returned to baseline conditions for 12 days (day 14–26), and sperm collected and cloacal temperature measured on day 26. We chose a 12-day recovery period because it approximates the duration of a spermatogenic cycle in birds and we observed a qualitative improvement in sperm quality at this time. While the exact duration of spermatogenesis is unknown for the zebra finch, studies of Japanese quail (Coturnix coturnix) suggest that spermatogenesis (from initial division of spermatogonia to spermiation) takes 12.77 days [22,26], and there is some (albeit limited) evidence that spermatogenesis occurs more rapidly in passerine species [27].
On day 26, we repeated the experiment, exposing birds to a second heat exposure period in order to test the impact of prior exposure to environmental temperatures. During this second period, sample collection and environmental conditions were identical to those described above, but this time birds were exposed to the alternate temperature treatment (i.e. birds that experienced 40°C in the first exposure were subject to conditions of 30°C in the second exposure period and vice versa). The one exception to this is that we collected samples on day 21, 7 days after birds were returned to baseline conditions. This additional sampling point was included because we observed a recovery of sperm quality after 12 days at baseline conditions during the first experimental period, and thus we aimed to gain additional information in the second experimental period in order to understand how quickly sperm quality recovered from potential temperature-dependent damage.
(b). Sperm quality analyses
Sperm samples were obtained by cloacal massage [21], and sperm swimming speed was quantified immediately using standard methods [28]. Briefly, fresh sperm were collected and immediately diluted in a small volume (approx. 50 µl) of pre-heated (40°C) Dulbecco's Modified Eagle Medium (DMEM, Invitrogen Ltd); though samples visibly contaminated with faecal matter were discarded. Following this dilution, we then immediately loaded 6 µl into a pre-heated slide chamber (depth 20 µm; Leja, Netherlands) and sperm videos were captured at 400× magnification using a phase contrast scope (CX41, Olympus, Japan) fitted with a heated stage plate (TP-S, Tokai Hit, Shizuoka, Japan) and connected to a digital camera (Legria HF G25, Canon, Japan). The media, heat stage plate and counting chambers were all maintained at 40°C (the approximate physiological temperature of zebra finches [16]). For each sample, we standardized recordings by capturing six unique fields of view for 5 s, for a total recording time of 30 s.
To quantify sperm swimming speed and the proportion of motile sperm, sperm videos were analysed at a later date using computer-assisted sperm analysis (CASA; Sperm Class Analyzer 5.4.0.0, SCA Motility, Microptic, Barcelona, Spain). Sperm were tracked for 0.5 s in each field of view (frame rate 50 frames s−1). To control for the effects of drift, sperm cells with an average path velocity of less than 30 µm s−1 or a straight line velocity of less than 25 µm s−1 were considered immotile. In addition, sperm tracked for less than 10 frames were excluded and we set a minimum cell detection size of 10 µm2. We also visually inspected each analysis in order to delete rare cases where two sperm crossed paths and CASA switched sperm mid-track. Similarly, when a sperm track was interrupted, and thus two non-independent tracks were recorded, the earlier track was deleted (the remaining track was still required to fit the above criteria). Finally, this visual inspection of sperm videos also allowed us to identify debris (i.e. non-sperm particles, such as the occasional red blood cell or faecal particle) incorrectly identified as a sperm, so we could exclude them from the analysis. These analyses were performed blindly with respect to treatment group by CSM.
Using only sperm tracks that passed these criteria, we quantified sperm swimming speed as curvilinear velocity (VCL; see [28] for VCL use justification) and the proportion of motile sperm (i.e. number of motile sperm tracks divided by the total number of cells). For VCL, samples with less than 10 motile sperm tracks were excluded from all analyses of sperm swimming speed (see electronic supplementary material, S2 for cut-off justification). We calculated the mean VCL for each sample for statistical analysis. Finally, a small aliquot of the sperm suspension was fixed in 5% buffered formaldehyde solution to quantify the proportion of morphologically normal sperm. To do this, we assessed 100 randomly chosen sperm on each of two replicate slide smears (200 sperm cells examined in total) and scored sperm as having either normal (i.e. no obvious damage to the entire sperm cell or visible morphological abnormalities) or abnormal morphology (see electronic supplementary material, S2). All scoring of sperm morphology was done blind to experimental treatment by L.L.H.
(c). Statistical analysis
Statistical analyses were run using R (v. 3.3.2, R Core Team), and all proportion data (i.e. proportion of motile sperm and morphologically normal sperm) were normalized by logit transformation. We tested for differences between the two treatment groups at the start of the experiment (day 1), using a two-sample t-test, in the following traits: body mass, body condition (i.e. the residuals from the regression of body mass on tarsus length, both log-transformed), cloacal temperature, sperm swimming speed (VCL), and both the proportion of motile sperm and normal sperm.
Investigation of how the effect of temperature was influenced by both the duration of exposure (i.e. number of days in heat treatment) and previous exposure to elevated ambient temperatures was done using linear mixed models (LMMs). For these analyses, the change in trait value (i.e. value at dayx – value at day 1, where x is day 3, 7, 11 or 14) was the dependent variable. Thus, our models considered the effect of temperature at 30°C and 40°C relative to trait values measured under baseline conditions (i.e. 23°C) for the following traits: cloacal temperature, VCL, proportion motile sperm and proportion normal sperm. In these models, temperature (30°C versus 40°C), experimental exposure period (1 versus 2) and experimental day (hereafter referred to as exp-day; i.e. day 3, 7, 11, 14), together with their three-way interaction (and all constituent pairwise interactions), and body condition were included as fixed effects. For the three sperm traits, we also ran models including cloacal temperature as a fixed effect covariate, but in all cases sperm quality was not influenced by the individual's cloacal temperature (all p > 0.17). The results of the final models were similar (data not shown), and thus cloacal temperature was not included in the final analysis. Male identity was included as a random effect in all models. We also ran models that included cage number and mother's identity (to control for the potential impact of genetic background given the inclusion of four sets of siblings, accounting for nine birds in total) as random effects, and compared these models with those with the single random effect (male identity) with likelihood ratio tests using maximum likelihood estimation. In all instances, these additional random effects did not significantly improve the models, and thus were not included in the final analysis.
We also assessed whether or not cloacal temperature and sperm quality recovered from the potential effects of heat exposure when birds were returned to baseline conditions (23°C) using LMMs. For these models, we included exp-day (1 versus 26), exposure period (1 versus 2), and heat treatment (30°C versus 40°C), together with their three-way interaction (and all constituent pairwise interactions), as fixed effects, and male identity as a random effect. Finally, comparisons of trait values at just two time points were performed using paired t-tests.
For all LMMs, non-significant interaction terms were removed in a backwards-stepwise fashion, beginning with the highest-order interaction [29], and models compared using likelihood ratio tests. For post hoc testing, we re-ran models and iteratively changed the reference level for variables that were in significant interactions or that had significant main effects. All final models were fitted with REML, and we summarized results using an ANOVA (Type III sum of squares). All LMMs were run using ‘lme4' [30], graphs were constructed using ‘ggplot2’ [31], and modelling assumptions (normality and heterogeneity of variance of residuals) were assessed visually (following [29]). All tests were two-tailed and considered significant at α < 0.05.
3. Results
There were no significant differences in body mass or condition between the treatment groups before the experiment began (mass: t18 = −0.59, p = 0.56; body condition: t18 = −0.61, p = 0.55). Similarly, groups did not differ with respect to sperm quality traits (sperm swimming speed: t17 = 1.01, p = 0.33; proportion motile sperm: t18 = 0.92, p = 0.37; proportion normal sperm: t18 = 1.65, p = 0.12) or cloacal temperature (t18 = 0.78, p = 0.44).
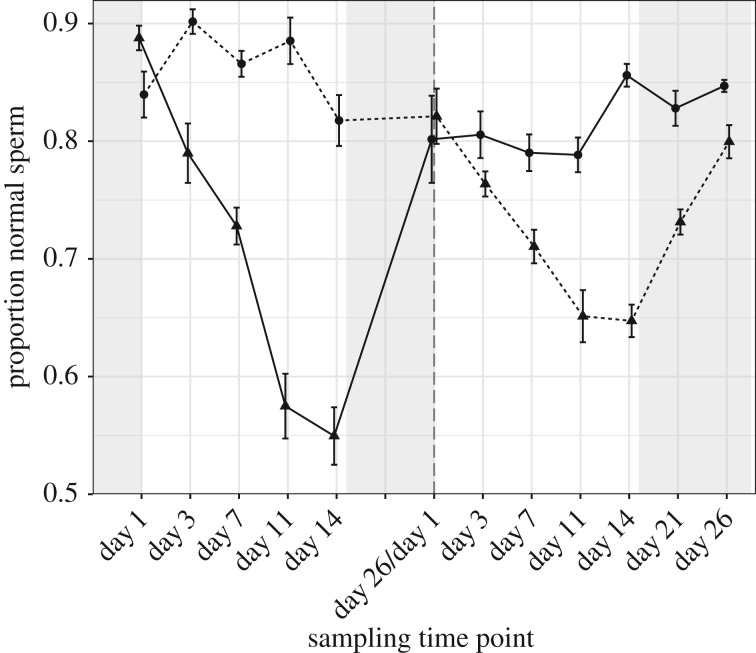
Experimental ambient temperatures strongly affected the proportion of normal sperm (table 1 and figure 1; electronic supplementary material, S4). Patterns of change in the proportion of sperm with normal morphology differed between the two treatment groups, resulting in a significant temperature by exp-day interaction (table 1). During the first exposure period, 40°C birds showed a significant decline in the proportion of normal sperm with increasing duration of heat exposure (t134.0 = −9.97, p < 0.001). Indeed, the proportion of normal sperm declined quickly; at day 3 the proportion of normal sperm was already significantly lower than that measured prior to heat treatment (t9 = 4.38, p = 0.002). There was also a significant, negative relationship between the proportion of normal sperm and exp-day in birds exposed to 30°C (t131.0 = −2.81, p = 0.006; electronic supplementary material, table S6, figure S4); however, this was immediately preceded by a slight increase in the proportion of normal sperm on day 3 relative to pre-experimental levels (mean ± s.e.; 0.90 ± 0.01 versus 0.84 ± 0.02; t9 = −1.92, p = 0.09) and even at their lowest levels (i.e. day 14) the proportion of normal sperm did not differ from values obtained under baseline conditions (mean ± s.e.; 0.82 ± 0.02 versus 0.84 ± 0.02; t9 = 0.92, p = 0.38). Moreover, the impact of heat was significantly greater in the 40°C group (i.e. the relationship was significantly more negative; electronic supplementary material, tables S6 and S7). At the end of the first exposure period and following the 12-day recovery period, the proportion of normal sperm in a sample for birds exposed to 30°C did not differ significantly from values collected prior to the experiment (t54.7 = −1.14, p = 0.26). By contrast, birds in the 40°C group had a significantly lower proportion of normal sperm relative to pre-experiment samples (t53.1 = −3.58, p < 0.001), despite the 12-day recovery period. During the second exposure period, the treatment groups again showed significant responses to heat exposure (figure 1; electronic supplementary material, tables S8 and S9). Specifically, while birds held at 40°C showed a significantly negative relationship between change in proportion normal sperm and exp-day (t131.0 = −4.79, p < 0.001), birds held at 30°C exhibited a positive relationship between these variables (t133.5 = 2.27, p = 0.03). As before, the effect of temperature on the proportion of normal sperm was already apparent at day 3 of the experimental period (t9 = 2.42, p = 0.04). In addition, there was a significant interaction between exp-day and exposure period (table 1), showing that the response to heat exposure was significantly stronger during the first exposure period (figure 1; electronic supplementary material, tables S6–S9). At the end of the second exposure period, birds held at 40°C appeared to recover from heat treatment as the proportion of normal sperm in samples returned to baseline levels following the 12-day recovery period (t54.7 = −1.11, p = 0.27), and even showed partial recovery of sperm on day 21 (i.e. 7 days into the recovery period; figure 1). Similarly, in the 30°C birds there was no significant difference in the proportion of normal sperm in samples collected at the beginning of the second exposure period and samples following the 12-day recovery period (t53.1 = 1.28, p = 0.21). However, in this case the proportion of normal sperm remained significantly lower relative to pre-experimental levels (t9 = 3.64, p = 0.005). Finally, body condition was negatively associated with the change in the proportion of normal sperm (table 1), though we acknowledge that relative body mass estimates of condition must be interpreted with caution.
Table 1.
Reduced LMM examining the change in the proportion of sperm with normal morphology with heat treatment, exposure period, experimental day (exp-day) and male body condition as fixed factors (see electronic supplementary material, S3 for full models).
| predictor | Fdf | p |
|---|---|---|
| temperature | F1,132.7 = 2.44 | 0.12 |
| exposure period | F1,132.6 = 5.53 | 0.02 |
| experimental day | F1,131.0 = 44.44 | <0.0001 |
| body condition | F1,34.6 = 5.78 | 0.02 |
| temp×exp-day | F1,133.2 = 38.18 | <0.0001 |
| temp×exposure period | F1,17.5 = 6.31 | 0.02 |
| exposure period×exp-day | F1,132.9 = 18.97 | <0.0001 |
Figure 1.
Proportion of normal sperm. Mean ± s.e. of the proportion of normal sperm on given experimental day (exp-day) over two experimental heat exposure periods (separated by vertical hatched line). Spacing between time points is related to sampling on exp-day, not continuous time. Day 1 reflects measurements taken under baseline conditions immediately prior to heat exposure. Males exposed first to 40°C in part 1 of experiment then to 30°C in part 2 (solid line), or 30°C then 40°C (dashed line). Experimental heat exposure: 30°C (circles) or 40°C (triangles). During experimental treatment birds were exposed to heat for 8 h a day and returned to 23°C for 16 h (white background). During acclimation and recovery periods birds were kept continuously at 23°C (shaded background).
Change in sperm swimming speed (VCL) was significantly affected by heat treatment (F1,99.5 = 5.85, p = 0.017) and exposure period (F1,99.1 = 20.92, p < 0.001). However, these effects were related to the differential response of birds exposed to 30°C versus 40°C (electronic supplementary material, table S10). Specifically, in the first exposure period and relative to samples collected under baseline (23°C) conditions, birds exposed to 40°C conditions showed a non-significant tendency towards a reduction in swimming speed (electronic supplementary material, figure S5), whereas birds at 30°C showed a non-significant tendency towards an increase in sperm speed (electronic supplementary material, figure S5) resulting in a significant difference between groups in terms of the change in sperm swimming speed (electronic supplementary material, tables S12 and S13; figure S5). Interestingly, following the 12-day recovery period, birds in both groups showed an increase in sperm swimming speed relative to pre-experimental levels; while this increase was significant in the 30°C (t11 = −2.57, p = 0.03), it was not significant for 40°C birds (t10.8 = −0.48, p = 0.64). For both treatment groups, sperm swimming speed showed a significantly greater decline in the second heat exposure period relative to the first (electronic supplementary material, tables S14 and S15; figure S5). Moreover, during the second exposure period, the intercept for the 40°C birds differed significantly from 0 (t86.6 = −3.16, p = 0.002); suggesting that in birds exposed to 40°C conditions, sperm swimming speed decreased significantly compared to sperm samples collected under baseline (23°C) conditions prior to the second heat exposure (electronic supplementary material, table S15). However, following the 12-day recovery period at 23°C, all birds showed recovery of sperm swimming speed to baseline levels (i.e. no difference between day 1 and 26 in second exposure period: 30°C: t10.7 = −0.15, p = 0.89; 40°C: t12.1 = −0.12, p = 0.91). The change in sperm swimming speed was not influenced by the duration of exposure (i.e. exp-day number; F1,88.4 = 2.15, p = 0.15) or body condition (F1,19.9 = 2.15, p = 0.52) and all interaction terms were non-significant and removed from the model (electronic supplementary material, table S11).
The change in the proportion of motile sperm was also significantly affected by temperature (F1,130.1 = 7.95, p = 0.006: electronic supplementary material, table S16). Birds in the two treatment groups showed significantly different initial responses to elevated temperatures; birds exposed to 30°C temperatures showed a slight tendency towards an increase in the proportion of motile sperm, whereas birds in the 40°C treatment showed a tendency towards a slight decrease in the proportion of motile sperm (electronic supplementary material, tables S18–S21; figure S6). However, neither group differed significantly from samples collected under baseline (23°C) conditions in either the first (electronic supplementary material, tables S18–S19) or second (electronic supplementary material, tables S20–S21) exposure period. The change in the proportion of motile sperm was not affected by exp-day number (F1,121.9 = 1.06, p = 0.31), exposure period (F1,126.2 = 3.21, p = 0.08) or body condition (F1,28.0 = 0.01, p = 0.92) (all interaction terms were non-significant and removed from the model; electronic supplementary material, table S17)).
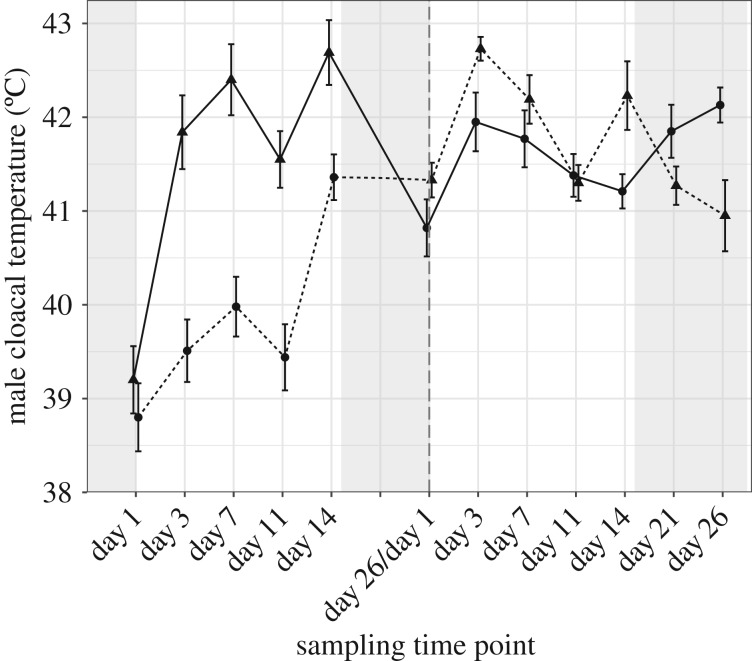
High ambient temperature also affected male cloacal temperature (table 2 and figure 2; electronic supplementary material, S22). In the first exposure period, birds in both treatment groups (30°C and 40°C) showed an increase in cloacal temperature, though this increase was significantly greater in the 40°C group relative to birds at 30°C (t32.5 = 4.55, p < 0.001; electronic supplementary material, tables S24 and S25). In the second exposure period, birds in both the 30°C and 40°C treatment groups again showed an initial rise in cloacal temperature, though in this instance there was no significant difference between the groups (figure 2; electronic supplementary material, tables S26 and S27). Change in cloacal temperature varied significantly with exp-day (electronic supplementary material, tables S24–S27; figure S7), though the pattern of change over exp-day differed by exposure period resulting in a significant exposure period by exp-day interaction (table 2). During the first exposure period, the change in cloacal temperature increased significantly over exp-day for both 30°C and 40°C treatment groups (electronic supplementary material, tables S24 and S25), indicating a continuous rise in cloacal temperature with continued heat exposure (figure 2). Cloacal temperatures did not return to pre-experimental levels at the end of the first exposure period, but remained elevated even though birds were held at 23°C for 12 days (30°C: t55 = 8.11, p < 0.001; 40°C: t55 = 4.24, p < 0.001). During the second exposure period, the change in cloacal temperature was significantly negatively associated with exp-day (electronic supplementary material, tables S26 and S23, figure S7). Thus, after an initial increase in cloacal temperature upon secondary heat exposure, cloacal temperature for all birds began to stabilize and decrease towards values obtained under 23°C conditions immediately prior to the second heat period (figure 2). However, cloacal temperature of the birds exposed to 30°C in the second exposure period again remained elevated relative to baseline conditions following a 12-day recovery period at 23°C (t55 = 3.32, p = 0.002). Moreover, while cloacal temperatures of birds exposed to 40°C in the second exposure period returned to levels at the beginning of this period (t55 = −0.55, p = 0.58), cloacal temperature following the 12-day recovery period was significantly greater than those recorded pre-experiment (t9 = −3.84, p = 0.004).
Table 2.
Reduced LMM examining the change in male cloacal temperature with heat treatment, exposure period, experimental day (exp-day) and male body condition as fixed factors (see electronic supplementary material, S3 for full models).
| predictor | Fdf | p |
|---|---|---|
| temperature | F1,136.0 = 19.323 | <0.0001 |
| exposure period | F1,136.8 = 0.004 | 0.95 |
| experimental day | F1,136.0 = 0.082 | 0.78 |
| body condition | F1,25.8 = 0.013 | 0.91 |
| temp × exposure period | F1,17.3 = 6.931 | 0.017 |
| exposure period × exp-day | F1,137.3 = 11.291 | 0.001 |
Figure 2.
Male cloacal temperature. Mean ± s.e. of cloacal temperature on given experimental day over two experimental heat exposure periods (separated by vertical hatched line). Spacing between time points is related to sampling on experimental day, not continuous time. Day 1 reflects measurements taken under baseline conditions immediately prior to heat exposure. Males exposed first to 40°C in part 1 of experiment then to 30°C in part 2 (solid line), or 30°C then 40°C (dashed line). Experimental heat exposure: 30°C (circles) or 40°C (triangles). During experimental treatment birds were exposed to heat for 8 h a day and returned to 23°C for 16 h (white background). During acclimation and recovery periods birds were kept continuously at 23°C (shaded background).
4. Discussion
We found that extreme environmental temperatures resulted in decreased sperm quality and increased cloacal temperature in male zebra finches. Most notably, when birds were exposed to 40°C temperatures, we observed a strong decline in the proportion of sperm with normal morphology, and these proportions declined with continued exposure to 40°C conditions. Birds exposed to 40°C during the first exposure period followed by 30°C during the second exposure period showed incomplete recovery from heat exposure, as the proportion of sperm with normal morphology was significantly lower at the end of the experiment relative to pre-experimental levels, even after 12-day recovery at 23°C. One explanation for this pattern is that exposure to extreme heat conditions (40°C) may have long-lasting effects on sperm, as has been observed in some mammalian species [32]. Interestingly, however, prior exposure to high temperatures (i.e. birds exposed to 30°C conditions during the first exposure period) appeared to somewhat mitigate the negative impact of 40°C temperatures on the proportion of normal sperm. Experimental 30°C and 40°C temperatures also lead to an approximately 2°C rise in cloacal temperature, which is consistent with studies in poultry and other passerine species [12,33]. In this study, however, we noted that the increase in cloacal temperature persisted even 12 days after the last heat exposure, which may partially explain the lasting effects of our temperature treatment on sperm morphology. In the current study, we were unable to determine whether sperm damage was the direct result of high temperatures (i.e. temperature induced) or the indirect result of systemic physiological stress induced by high temperatures (i.e. temperature associated). Future studies are required to tease out these options and to further investigate both the long-term effects of heat on sperm and the potential for acclimation to elevated environmental temperatures. Nonetheless, our findings indicate that ecologically relevant, extreme temperatures have the potential to impact sperm quality and function.
We observed a decline in the proportion of sperm with normal morphology across the duration of each heat exposure treatment. During the first 7 days of heat treatment, the proportion of normal sperm was reduced to a similar level in both exposure periods, whereas further decline in the proportion of normal sperm on day 11 and 14 was considerably stronger for birds subjected to 40°C temperatures during the first heat exposure. In birds, spermatogenesis is divided into three major phases: (1) the spermatogonial stage, involving mitotic cell division; (2) the spermatocyte stage, involving cell division via meiosis; and (3) the spermatid stage, involving differentiation to produce mature spermatozoa [22]. In Japanese quail, the duration of spermatogenesis has been estimated at 12.77 days, with each spermatogenic phase lasting roughly 4–4.5 days [26]. In other non-passerine species, such as the domestic fowl and Barbary drake, the duration from the onset of meiosis to spermiation (phases 2–3) has been estimated at 11–12 days [22]. Sperm are then transported along the ductus deferens to the seminal glomera, a process that takes approximately 1 day [22,34]. Studies of passerines are generally lacking; though one study of the yellow-throated sparrow Gymnoris xanthocollis suggests that spermatogenesis may occur more rapidly in passerines relative to non-passerine taxa [27]. This may offer some clue as to the nature of sperm damage in our study. One explanation for the patterns of sperm damage observed in this study is that sperm stored in the seminal glomera are damaged by our treatment, with the continued decline in the proportion of morphologically normal sperm with prolonged heat exposure resulting from the accumulation of damaged sperm. However, an alternative explanation is that sperm damage may, at least in part, result from temperature effects on developing sperm cells during spermatogenesis. In mammals, the primary spermatocytes (especially pachytene and diplotene) of phase 2 and early spermatids of phase 3 appear particularly susceptible to heat stress [35]. Thus, it is possible that the observed reduction in the proportion of normal sperm early in the exposure period (e.g. exp-day 3) reflects damage to sperm populations in the seminal glomera and ductus deferens, while the further decline in sperm quality observed as the treatment continued reflects the cumulative effects of damage to these same sperm populations combined with damage to developing sperm cells. We found that the proportion of sperm with normal morphology was at its lowest at experimental days 11 and 14, and suggest this may reflect damage inflicted on these cells while undergoing meiosis (e.g. pachytene and diplotene spermatocytes in phase 2) and spermatid development (spermatids in phase 3). Such a pattern fits well with the 11–12 days taken for sperm to transition from primary spermatocytes to mature spermatids (see above) with an additional day for sperm to be transported along the ductus deferens.
Interestingly, the fact that we did not observe as strong a decline in the proportion of normal sperm in birds exposed to 40°C temperatures during the second exposure period suggests that these birds may have become acclimated to higher ambient temperatures via prior exposure to 30°C conditions, and that this may allow them to minimize defects to spermatogonia, spermatocytes and developing spermatids. During spermatogenesis, organisms produce a number of highly conserved proteins, known as heat shock proteins (HSPs), which play a role in protein folding and transport under physiological conditions [36]. HSPs are induced in response to elevated ambient temperatures, a cellular response referred to as the heat shock response [37,38], and in response to a range of other stressors, including oxidative stress and infection [39]. HSPs function as molecular chaperones to mitigate damage by binding to proteins and preventing protein denaturation and incorrect folding [35]. In mice, acute heat stress has been shown to increase the expression levels of genes belonging to the HSP family in testis tissue, attenuating the heat-induced damage to sperm [40]. In chickens Gallus gallus domesticus high environmental temperatures elevate the expression of several HSPs (e.g. HSP25, HSPA2 [38]). In the current study, we observed a sustained increase in male cloacal temperature. While we have no information on testis gene expression in our study, we suggest it is plausible that this rise in cloacal temperature led to the upregulation of genes related to the heat shock response, which would explain the diminished response to elevated temperatures we observed during the second exposure period.
In contrast to sperm morphology, we found that the motile performance of sperm (i.e. sperm swimming speed/proportion of motile sperm) was relatively unaffected by high ambient temperatures. Although we did observe an initial decline in sperm swimming speed in 40°C conditions during the second exposure period, both within-male (across exp-day) and between-male variability was high and no clear effects of temperature were apparent, which was also the case for the proportion of motile sperm in a sample. Although measures of sperm performance under in vitro conditions should be interpreted with some caution, sperm motile performance generally exhibits low within-male repeatability in passerine birds [41,42]. In domestic fowl, sperm swimming speed is influenced by seminal fluid proteins [43] and the presence of extracellular ions (e.g. calcium and sodium [44]). While it is unknown how quickly components of seminal fluid may change in birds, plasticity in seminal fluid production and composition has been reported in rodents [45] and Drosophila [46], and in fowl sperm swimming speed is capable of rapid change (i.e. within days) in response to shifts in social competitiveness [47]. As such, sperm motile performance may be a relatively plastic trait, and somewhat buffered from the negative effects of temperature via rapid changes in the chemical and protein milieu of seminal fluid. Finally, a potentially important methodological consideration is that sperm performance was measured using standard temperature conditions (i.e. the microscope set-up and media were maintained at a constant 40°C). While this approach is typical of studies in birds (e.g. [28,44]) and other taxa (e.g. [45]), it is possible that measurements obtained under conditions perfectly matched to the individual's body temperature would provide different results.
Reductions in sperm quality (e.g. sperm motility, viability) due to elevated body temperatures have been linked to infertility in poultry [12,48] and a range of mammalian species [32]. Our findings suggest extreme environmental temperatures may result in a limited supply of functional sperm, and thus may also impact male fertility in passerine birds. Only morphologically normal sperm appear to be able to enter the sperm storage tubules (SSTs) of females [49], and therefore a reduction in the proportion of morphologically normal sperm is likely to impact the total number of sperm available for fertilizing ova. In zebra finches, there appears to be a minimum number of sperm required at the site of fertilization to ensure successful embryonic development [50]. Thus, it is plausible that natural selection may act on males to protect sperm from the detrimental effects of elevated temperatures in order to maintain sufficient sperm numbers to ensure reproductive success. In a general sense, our results might help to explain, at least partially, the recent finding of constrained avian breeding activity across the hotter parts of Australia, during the hottest parts of the year [20], a finding that has yet to be explored in other regions of the world. More importantly, functional infertility may be an important selective pressure in systems where females mate with multiple males. Under conditions of sperm competition, sperm numbers are an important determinant of fertilization success [51]. Thus, males that are best able to mitigate sperm damage resulting from extreme temperatures are likely to be superior competitors for the fertilization of ova during hot conditions. Selection may therefore drive changes in gene expression and sequence evolution of proteins linked to spermatogenesis (e.g. HSPs), the composition and plasticity of seminal fluid or male behaviours (e.g. shade seeking or other thermoregulatory behaviours) in response to heatwaves. Male functional infertility may also generate selection on females to seek extra-pair copulations to avoid the potential costs of infertile social mates [52,53]. Thus, heatwaves may have profound ecological and evolutionary consequences for the reproductive biology and behaviour of birds.
An important next step will be to determine if the negative effects of temperature have consequences for male fitness and whether similar results are observed in wild populations under natural heatwave conditions. In our experiments, we found that females also exhibited an increase in cloacal temperature qualitatively similar to that reported for males, but how this relates to sperm performance within the female reproductive tract is unknown. Thus, the effect of elevated temperature on female processes also warrants investigation; for example, whether sperm are prone to temperature-induced damage while residing in female SSTs, and the implications of this for female reproductive success. In addition, it would be especially interesting to known if temperature-induced sperm damage is likely in avian species experiencing high levels of sperm competition for which sperm quality is particularly important to fertilization success. We found an effect of 40°C, but not 30°C conditions in this study, and indeed globally numerous species will be regularly exposed to such high temperatures [7]. However, it remains to be determined if sperm function in temperate species or species breeding at high latitudes are similarly affected by increases in maximum environmental temperatures. Indeed, species in temperate regions may be expected to show even greater reductions in sperm quality due to elevated temperatures if arid living is associated with adaptations to high temperatures. In conclusion, we suggest that temperature-induced or temperature-associated reductions in sperm quality may be an important biological consequence of the anthropocene as global temperatures rise and the frequency of extreme heat events increase.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Becky Cramer for useful discussion and two anonymous reviewers for useful comments on earlier versions of the manuscript.
Ethics
All work was conducted according to national and international guidelines with approval of Macquarie University Animal Ethics Committee (Animal Research Authority 2015/028).
Data accessibility
Datasets are available from Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g7765) [54]: (i) Fowlers Gap 2014–2017 temperature data; (ii) Sperm quality and cloacal temperature analyses.
Authors' contributions
L.L.H., S.C.G. and M.R. conceived and designed the experiment. L.L.H., M.R. and S.C.G. wrote the manuscript, with contributions from other authors. L.L.H. and C.S.M. conducted empirical work. C.R.F. and C.S.M. optimized CASA system. M.R. and L.L.H. conducted statistical analysis. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by Australian Research Commission grant DP130100417 to S.C.G. M.R. was funded by a Young Research Talent grant from the Research Council of Norway (230434/F20).
References
- 1.Pizzari T, Parker GA. 2009. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 207–245. New York, NY: Academic Press. [Google Scholar]
- 2.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt K, Dobler R, Abbott J. 2015. An ecology of sperm: sperm diversification by natural selection. Annu. Rev. Ecol. Evol. Syst. 46, 435–459. ( 10.1146/annurev-ecolsys-120213-091611) [DOI] [Google Scholar]
- 4.McKechnie AE, Hockey PAR, Wolf BO. 2012. Feeling the heat: Australian landbirds and climate change. Emu 112, i–vii. ( 10.1071/MUv112n2_ED) [DOI] [Google Scholar]
- 5.Visser ME, Holleman LJ, Caro SP. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331. ( 10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nord A, Sandell MI, Nilsson JA. 2010. Female zebra finches compromise clutch temperature in energetically demanding incubation conditions. Funct. Ecol. 24, 1031–1036. ( 10.1111/j.1365-2435.2010.01719.x) [DOI] [Google Scholar]
- 7.Naef-Daenzer B, Luterbacher J, Nuber M, Rutishauser T, Winkel W. 2012. Cascading climate effects and related ecological consequences during past centuries. Clim. Past. 8, 1527–1540. ( 10.5194/cp-8-1527-2012) [DOI] [Google Scholar]
- 8.Hansen PJ. 2009. Effects of heat stress on mammalian reproduction. Phil. Trans. R. Soc. B 364, 3341–3350. ( 10.1098/rstb.2009.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wechalekar H, Setchell BP, Peirce EJ, Ricci M, Leigh C, Breed WG. 2010. Whole-body heat exposure induces membrane changes in spermatozoa from the cauda epididymidis of laboratory mice. Asian. J. Androl. 12, 591–598. ( 10.1038/aja.2010.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaca AG, Parker HM, Yeatman JB, McDaniel CD. 2002. The effects of heat stress and sperm quality classification on broiler breeder male fertility and semen ion concentrations. Br. Poult. Sci. 43, 621–628. ( 10.1080/0007166022000004552) [DOI] [PubMed] [Google Scholar]
- 11.McDaniel CD, Bramwell RK, Wilson JL, Howarth JB. 1995. Fertility of male and female broiler breeders following exposure to elevated ambient temperatures. Poult. Sci. 74, 1029–1038. ( 10.3382/ps.0741029) [DOI] [PubMed] [Google Scholar]
- 12.McDaniel CD, Bramwell RK, Howarth B Jr. 1996. The male contribution to broiler breeder heat-induced infertility as determined by sperm-egg penetration and sperm storage within the hen's oviduct. Poult. Sci. 75, 1546–1554. ( 10.3382/ps.0751546) [DOI] [PubMed] [Google Scholar]
- 13.Birkhead TR. 2010. Post-copulatory sexual selection and the zebra finch. Emu 110, 189–198. ( 10.1071/MU09086) [DOI] [Google Scholar]
- 14.Zann RA. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Griffith SC, Mainwaring MC, Sorato E, Beckmann C. 2016. High atmospheric temperatures and ‘ambient incubation’ drive embryonic development and lead to earlier hatching in a passerine bird. R. Soc. open sci. 3, 150371 ( 10.1098/rsos.150371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder WA. 1964. Gaseous metabolism and water relations of the zebra finch, Taeniopygia castanotis. Physiol. Zool. 37, 400–413. ( 10.1086/physzool.37.4.30152758) [DOI] [Google Scholar]
- 17.Griffith SC, Pryke SR, Mariette M. 2008. Use of nest-boxes by the zebra finch (Taeniopygia guttata): implications for reproductive success and research. Emu 108, 311–319. ( 10.1071/MU08033) [DOI] [Google Scholar]
- 18.Perkins SE, Alexander LV. 2013. On the measurement of heat waves. J. Climate 26, 4500–4517. ( 10.1175/jcli-d-12-00383.1) [DOI] [Google Scholar]
- 19.McKechnie AE, Gerson AR, McWhorter TJ, Smith EK, Talbot WA, Wolf BO. 2017. Avian thermoregulation in the heat: evaporative cooling in five australian passerines reveals within-order biogeographic variation in heat tolerance. J. Exp. Biol. 220, 2436–2444. ( 10.1242/jeb.155507) [DOI] [PubMed] [Google Scholar]
- 20.Duursma DE, Gallagher RV, Griffith SC. 2017. Characterizing opportunistic breeding at a continental scale using all available sources of phenological data; an assessment of 337 species across the australian continent. Auk 134, 509–519. ( 10.1642/AUK-16-243.1) [DOI] [Google Scholar]
- 21.Wolfson A. 1952. The cloacal protuberance: a means for determining breeding condition in live male passerines. Bird-banding 23, 159–165. ( 10.2307/4510381) [DOI] [Google Scholar]
- 22.Jones RC, Lin M. 1993. Spermatogenesis in birds. Oxf. Rev. Reprod. Biol. 15, 233–264. [PubMed] [Google Scholar]
- 23.Phalen DN, Mitchell ME, Cavazos-Martinez ML. 1996. Evaluation of three heat sources for their ability to maintain core body temperature in the anesthetized avian patient. J. Avian Med. Surg. 10, 174–178. [Google Scholar]
- 24.Beaupré CE, Tressler CJ, Beaupré SJ, Morgan JL, Bottje WG, Kirby JD. 1997. Determination of testis temperature rhythms and effects of constant light on testicular function in the domestic fowl (Gallus domesticus). Biol. Reprod. 56, 1570–1575. ( 10.1095/biolreprod56.6.1570) [DOI] [PubMed] [Google Scholar]
- 25.Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997. ( 10.1126/science.1098704) [DOI] [PubMed] [Google Scholar]
- 26.Lin M, Jones RC. 1992. Renewal and proliferation of spermatogonia during spermatogenesis in the japanese quail, Coturnix coturnix japonica. Cell Tissue Res. 267, 591–601. ( 10.1007/BF00319382) [DOI] [PubMed] [Google Scholar]
- 27.Bhat G, Maiti BR. 1988. Study of spermatogenesis in a wild bird the yellow throated sparrow Petronia xanthocollis burton. Zool. Anz. 221, 430–434. [Google Scholar]
- 28.Rowe M, Griffith SC, Hofgaard A, Lifjeld JT. 2015. Subspecific variation in sperm morphology and performance in the long-tailed finch (Poephila acuticauda). Avian Res. 6, 1–10. ( 10.1186/S40657-015-0032-Z) [DOI] [Google Scholar]
- 29.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 30.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 31.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 32.Setchell BP. 1998. The parkes lecture: Heat and the testis. J. Reprod. Fertil. 114, 179–194. ( 10.1530/jrf.0.1140179) [DOI] [PubMed] [Google Scholar]
- 33.King JR. 1964. Oxygen consumption and body temperature in relation to ambient temperature in the white-crowned sparrow. Comp. Biochem. Physiol. 12, 13–24. ( 10.1016/0010-406X(64)90044-1) [DOI] [PubMed] [Google Scholar]
- 34.Clulow J, Jones RC. 1982. Production, transport, maturation, storage and survival of spermatozoa in the male japanese quail, Coturnix coturnix. J. Reprod. Fertil. 64, 259–266. ( 10.1530/jrf.0.0640259) [DOI] [PubMed] [Google Scholar]
- 35.Durairajanayagam D, Agarwal A, Ong C. 2015. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 30, 14–27. ( 10.1016/j.rbmo.2014.09.018) [DOI] [PubMed] [Google Scholar]
- 36.Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. 2000. The role of heat shock proteins in reproduction. Hum. Reprod. Update 6, 149–159. ( 10.1093/humupd/6.2.149) [DOI] [PubMed] [Google Scholar]
- 37.Pei Y, Wu Y, Qin Y. 2012. Effects of chronic heat stress on the expressions of heat shock proteins 60, 70, 90, a2, and hsc70 in the rabbit testis. Cell Stress Chaperones 17, 81–87. ( 10.1007/s12192-011-0287-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SH, Cheng CY, Tang PC, Chen CF, Chen HH, Lee YP, Huang SY. 2015. Acute heat stress induces differential gene expressions in the testes of a broiler-type strain of Taiwan country chickens. PLoS ONE 10, e0125816 ( 10.1371/journal.pone.0125816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 40.Zaprjanova S, Rashev P, Zasheva D, Martinova Y, Mollova M. 2013. Electrophoretic and immunocytochemical analysis of hsp72 and hsp73 expression in heat-stressed mouse testis and epididymis. Eur. J. Obstet. Gynecol. Reprod. Biol. 168, 54–59. ( 10.1016/j.ejogrb.2012.12.021) [DOI] [PubMed] [Google Scholar]
- 41.Birkhead TR, Fletcher F. 1995. Male phenotype and ejaculate quality in the zebra finch Taeniopygia guttata. Proc. R. Soc. Lond. B 262, 329–334. ( 10.1098/rspb.1995.0213) [DOI] [PubMed] [Google Scholar]
- 42.Cramer ER, Laskemoen T, Stensrud E, Rowe M, Haas F, Lifjeld JT, Saetre GP, Johnsen A. 2015. Morphology-function relationships and repeatability in the sperm of passer sparrows. J. Morphol. 276, 370–377. ( 10.1002/jmor.20346) [DOI] [PubMed] [Google Scholar]
- 43.Borziak K, Alvarez-Fernandez A, Karr TL, Pizzari T, Dorus S. 2016. The seminal fluid proteome of the polyandrous red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Sci. Rep. 6, 35864 ( 10.1038/srep35864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Froman DP, Feltmann AJ. 2005. Fowl (Gallus domesticus) sperm motility depends upon mitochondrial calcium cycling driven by extracellular sodium. Biol. Reprod. 72, 97–101. ( 10.1095/biolreprod.104.033209) [DOI] [PubMed] [Google Scholar]
- 45.Ramm SA, Edward DA, Claydon AJ, Hammond DE, Brownridge P, Hurst JL, Beynon RJ, Stockley P. 2015. Sperm competition risk drives plasticity in seminal fluid composition. BMC Biol. 13, 87 ( 10.1186/s12915-015-0197-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fedorka KM, Winterhalter WE, Ware B. 2011. Perceived sperm competition intensity influences seminal fluid protein production prior to courtship and mating. Evolution 65, 584–590. ( 10.1111/j.1558-5646.2010.01141.x) [DOI] [PubMed] [Google Scholar]
- 47.Pizzari T, Cornwallis CK, Froman DP. 2007. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc. R. Soc. B 274, 853–860. ( 10.1098/rspb.2006.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karaca AG, Parker HM, McDaniel CD. 2002. Elevated body temperature directly contributes to heat stress infertility of broiler breeder males. Poult. Sci. 81, 1892–1897. ( 10.1093/ps/81.12.1892) [DOI] [PubMed] [Google Scholar]
- 49.Lake PE. 1975. Gamete production and the fertile period with particular reference to domesticated birds. Symp. Zool. Soc. Lond. 35, 225–244. [Google Scholar]
- 50.Hemmings N, Birkhead TR. 2015. Polyspermy in birds: sperm numbers and embryo survival. Proc. R. Soc. B 282, 20151682 ( 10.1098/rspb.2015.1682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273. ( 10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 52.Sheldon BC. 1994. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. Lond. B 257, 25–30. ( 10.1098/rspb.1994.0089) [DOI] [Google Scholar]
- 53.Wetton JH, Parkin DT. 1991. An association between fertility and cuckoldry in the house sparrow, Passer domesticus. Proc. R. Soc. Lond. B 245, 227–233. ( 10.1098/rspb.1991.0114) [DOI] [Google Scholar]
- 54.Hurley LL, McDiarmid CS, Friesen CR, Griffith SC, Rowe M. 2018. Data from: Experimental heatwaves negatively impact sperm quality in the zebra finch Dryad Digital Repository. ( 10.5061/dryad.g7765) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hurley LL, McDiarmid CS, Friesen CR, Griffith SC, Rowe M. 2018. Data from: Experimental heatwaves negatively impact sperm quality in the zebra finch Dryad Digital Repository. ( 10.5061/dryad.g7765) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets are available from Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.g7765) [54]: (i) Fowlers Gap 2014–2017 temperature data; (ii) Sperm quality and cloacal temperature analyses.