Abstract
Photoreception and vision are fundamental aspects of animal sensory biology and ecology, but important gaps remain in our understanding of these processes in many species. The colour-changing brittle star Ophiocoma wendtii is iconic in vision research, speculatively possessing a unique whole-body visual system that incorporates information from nerve bundles underlying thousands of crystalline ‘microlenses’. The hypothesis that these might form a sophisticated compound eye-like system regulated by chromatophores has been extensively reiterated, with investigations into biomimetic optics and similar supposedly ‘visual’ structures in living and fossil taxa. However, no photoreceptors or visual behaviours have ever been identified. We present the first evidence of photoreceptor networks in three Ophiocoma species, both with and without microlenses and colour-changing behaviour. High-resolution microscopy, immunohistochemistry and synchrotron tomography demonstrate that putative photoreceptors cover the animals' oral, lateral and aboral surfaces, but are absent at the hypothesized focal points of the microlenses. The structural optics of these crystal ‘lenses’ are an exaptation and do not fulfil any apparent visual role. This contradicts previous studies, yet the photoreceptor network in Ophiocoma appears even more widespread than previously anticipated, both taxonomically and anatomically.
Keywords: extraocular photoreception, vision, ophiuroids, photoreceptors, sensory biology
1. Background
The ability to sense light without eyes, extraocular photoreception (EOP), is being discovered across an increasingly diverse range of animal groups at an accelerating rate [1–4]. EOP generally confers behaviours such as circadian rhythms, phototaxis, reflexes and colour change, but not spatial resolution [1,3,5]. Controversially, it has been proposed that some echinoderms may be able to consolidate extraocular information to facilitate image-forming vision [6–9], placing them in a position of exceptional research interest [5]. Understanding the functional model and limits of integration in dispersed photoreceptor systems that may provide spatial resolution will have profound implications for neurobiology, visual evolution and biomimetic design [1,10], but despite considerable research effort these remain elusive [5].
The brittle star Ophiocoma wendtii first attracted attention for its charismatic colour-changing behaviour and extreme sensitivity to illumination [11]. Animals undergo a striking transformation from black-brown during the day to beige-grey with dark bands at night, which can be artificially induced by changing their light environment, and strongly prefer shade to light exposure, including moonlight [11]. Morphological studies reported nerve bundles beneath expanded, highly regular calcite hemispheres on the dorsal arm plates (enlarged peripheral trabeculae, EPTs) [11,12]. The EPTs were speculatively interpreted as potential ‘microlenses’, proposed to focus light onto putative photoreceptors within or associated with the nerve bundles, with the passage of incoming light regulated by the activity of surrounding ‘pupillary’ chromatophores [5,9,11–13]. This proposal remains unexplored and no photoreceptors have been identified to date; however, many subsequent studies interpreted new data in the context of this hypothesis being accepted. The architecture, distribution and optical properties of the arm plates in Ophiocoma are fundamental to the hypothesis that they focus light onto underlying photoreceptor elements [9,11,12], which has also contributed to interpretations of skeletal involvement in echinoid photoreception, yet the EPTs have only been presented in the literature from removed and chemically treated plates [9,12,14,15].
The repeated-unit nature and apparent optical sophistication of this system even led to the speculative suggestion of a compound eye-like function across the dorsal surface of the animal, as has also been proposed in echinoids [7,16,17], facilitating its apparent ability to detect shadows and navigate towards dark shelters from a distance [7,9]. The hypothesis that the EPTs, chromatophores and underlying nerves could form an advanced visual system has been extensively reiterated by other authors [1,5,7,15,18–26], with resultant investigations into biomimetic optics [9,10,19,20], and vision in both living [22,25] and fossil taxa [14,27]. However, there is no morphological or behavioural evidence to support this idea, and no candidates for the necessary neural integration centres that might be required by such a system (though the precise nature of such centres remains unclear) [28].
Since the last morphological investigations of O. wendtii, numerous opsins—key components of most photosensitive pigments—were identified in the genome of the sea urchin Strongylocentrotus purpuratus [29]. This facilitated the discovery of the first opsin-expressing cells in urchins, brittle stars and sea stars, using antibodies subsequently raised against Sp-Op targets [16,26,30], as well as many more opsin sequences in other echinoderms [25,26,31]. Brittle stars, like other echinoderms, possess both rhabdomeric (r-) and ciliary (c-) visual opsins as well as multiple non-visual classes [25,26,32], but exhibit multiple duplications of the rhabdomeric class (closest to Sp-Op4) [26]. These are considered non-visual in most deuterostomes, but are strongly implicated in visual behaviour in both urchins and sea stars [16,33], and sequencing of arm transcriptomes in two brittle stars demonstrated detectable levels of expression of r-opsins similar to Sp-Op4, but not c-opsins, though these were detected at low levels by immunolabelling against Sp-Op1 [25].
We established multiple lines of evidence to investigate the presence and location of photoreceptors, determine their arrangement in relation to putative microlenses in situ, and compare Ophiocoma wendtii with two ecologically co-occurring congeners, one lacking EPTs and colour change behaviour [11]. Immunohistochemistry, scanning electron microscopy (SEM), synchrotron tomography and histology were supplemented with exploratory behavioural experiments (electronic supplementary material) in order to finally locate putative photoreceptors and compare their distribution and structure across Ophiocoma.
2. Material and methods
(a). Specimens
Specimens of Ophiocoma wendtii, O. echinata and O. pumila were collected from shallow reef rubble at Punta Hospital, Isla Solarte, Bocas del Toro, Panama (9°19'44.4″ N, 82°12'21.6″ W, 0–3 m), and housed in outdoor flow-through unfiltered seawater aquaria under a natural 12 L : 12 D cycle at the Smithsonian Tropical Research Institute, Bocas del Toro, Panama. Animals were photographed, measured and identified by disc diameter and longest arm length, and allowed 3 days recovery between collection and experiments. Animals that autotomized arms during or following collection were excluded from trials. Specimens were collected under ARAP permit 2014-52b and exported under ARAP export permit 2015-2.
(b). Synchrotron tomography
Arm segments were fixed in 4% glutaraldehyde in a sodium cacodylate buffer (0.1 M, pH 7.4) in their daylight state and stored in sodium cacodylate buffer. Segments were rinsed in buffer and serially dehydrated in acetone before drying with hexamethyldisilazane (HMDS) and mounting on stubs.
Three samples from Ophiocoma wendtii (three arm segments), Ophiocoma pumila (two arm segments and a pair of arm spines) and Ophiocoma echinata (two arm segments and one arm spine) were studied with non-destructive synchrotron tomography. Synchrotron radiation X-ray tomographic microscopy was performed at the TOMCAT beamline (Swiss Light Source, Paul Scherrer Institut, Villigen, Switzerland). Samples were scanned using an X-ray energy of 20 keV, 1501 projections and an exposure time of 250 ms. This gave tomographic datasets with a voxel size of 1.75 µm (x, y and z), which were digitally reconstructed as three-dimensional virtual models (electronic supplementary material) using SPIERS [34] and AMIRA (FEI Visualization Science Group).
(c). Histology and scanning electron microscopy
Whole specimens and excised arm segments from Ophiocoma wendtii, O. echinata and O. pumila were fixed in glutaraldehyde as above and stored in sodium cacodylate buffer (pH 7.4). For histology, arm segments were post-fixed in 1% osmium tetroxide, decalcified in 2% ascorbic acid in 0.15 M sodium chloride solution for 72 h [16] and dehydrated in an acetone series before embedding in Epon epoxy resin (Agar Scientific). Blocks were sectioned at 1 µm on a Leica RM2255 automated microtome with a diamond knife (HistoJumbo, 8 mm, DiATOME, Switzerland) and stained with Richardson's solution. Sections were photographed using an Olympus E-600 digital camera mounted on an Olympus BX41 microscope.
For SEM, glutaraldehyde-fixed arm segments from Ophiocoma wendtii were washed in dilute cacodylate buffer, serially dehydrated in acetone, chemically dried overnight with HMDS, mounted on stubs and visualized on an FEI Quanta FEG scanning electron microscope at 15 kV.
(d). Immunohistochemistry
Light-adapted arm segments from Ophiocoma wendtii, O. echinata and O. pumila were tested for reactivity to sea urchin ciliary (Sp-Op1) and rhabdomeric (Sp-Op4) opsins [31]. Segments were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 30 min at room temperature before washing in PBS and decalcifying in 2% ascorbic acid in 0.15 M sodium chloride solution for 72 h (adapted from [15]). Samples were rinsed in PBS and stored in 0.05% sodium azide in PBS. Tissue used for sectioning was rinsed in PBS for 20 min before embedding in 4% agarose gel. Thick sections (150 µm) were taken using a Leica VT 1200S vibratome. Arm segments and sections were washed in PBS and 0.1% Triton X (PBS-T) and blocked in PBST and 0.5% normal goat serum for 1 h before incubation with anti-acetylated tubulin (1 : 200) and either anti-Sp-Opsin4 or anti-Sp-Opsin1 (Strongylocentrotus purpuratus, 1 : 50) [16] overnight, all at room temperature. These antibodies bind to and exhibit high sequence similarity to discovered homologues in brittle stars [25,26]. Specimens were then washed in PBST and incubated with either Alexa Fluor 633 goat anti-mouse (1 : 500) or Alexa Fluor 488 goat anti-rabbit (1 : 500) for at least 3 h at room temperature, rinsed with PBST and visualized on a Leica TCS SPE confocal laser scanning microscope. Images and image stacks were captured using Leica Application Suite Advanced Fluorescence v. 2.6.3 and prepared in Fiji [35].
3. Results
(a). Arm plate structure
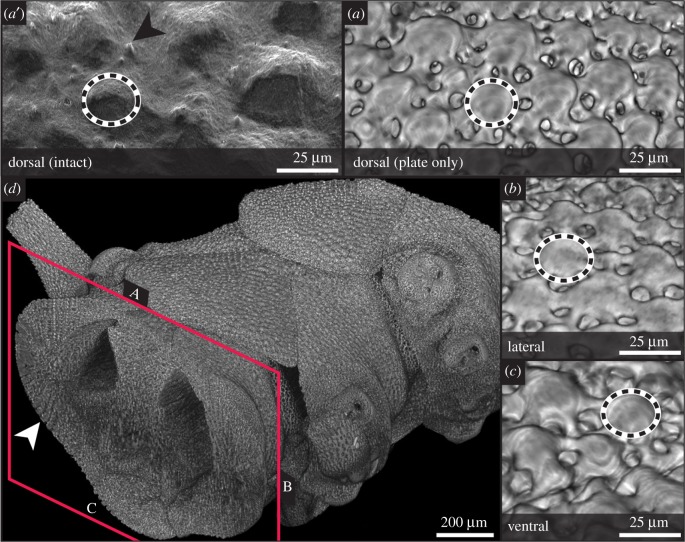
High-resolution synchrotron tomography and SEM visualized expanded peripheral trabeculae (EPTs, putative microlenses) in situ without disrupting soft tissue. Regular, near-hemispherical EPTs, 30–40 µm in diameter, cover the dorsal (aboral) arm plates, but also the ventral (oral) arm plates and the dorsal and ventral margins of the lateral plates in Ophiocoma wendtii (figure 1a–c), contrary to previous reports that they are restricted to the dorsal plates and dorsal margins of the lateral plates [9,12]. In cross-section, EPTs often appear to be at the distal face of an uninterrupted calcite core projecting through the plate (figure 1d; electronic supplementary material, figures S1 and S4), leaving little or no room beneath the centre of the EPT for soft tissue. In vivo, the plates are covered by a fine dermal cuticle that is highly sensitive to chemical treatment [13] (figure 1a′). EPTs are interspersed by the projection of short ciliary tufts through the cuticle (figure 1a′) that may represent receptors described as Stäbchen [36,37].
Figure 1.
Expanded peripheral trabeculae (EPTs), skeletal structures in Ophiocoma wendtii. Synchrotron X-ray tomography (a–d) and scanning electron microscopy (a') images of arm segments. Hemispherical calcite structures previously characterized as lenses (dashed outlines) on the dorsal (a, a′), lateral (b) and, to a lesser extent, ventral (c) arm plates. In vivo, arm plates are covered by the cuticle, which obscures the regular form of the EPTs, and is interspersed by ciliary projections (arrowhead) (a′). In cross section (d), the continuous nature of the EPTs with the rest of the stereom is visible, particularly in the lateral regions (arrowhead). See electronic supplementary material (S1) for reconstructed model. (Online version in colour.)
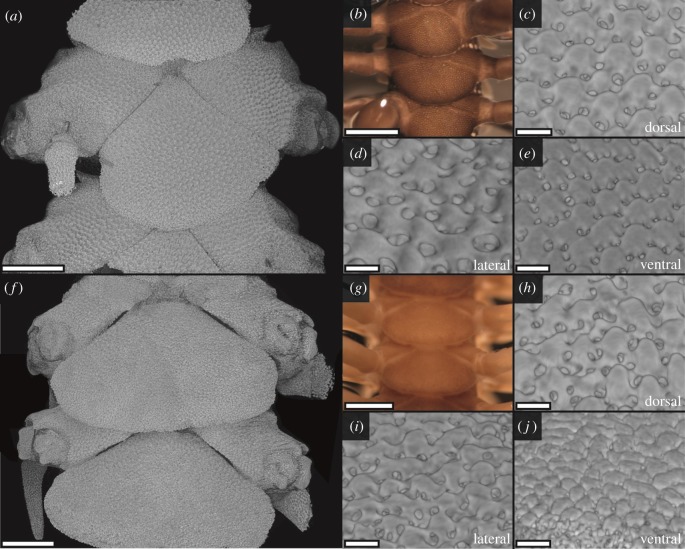
Ophiocoma echinata and O. pumila are sympatric with O. wendtii and were included in the original study of colour change and light sensitivity in the latter [11]. Whereas O. echinata exhibits a similar day-to-night colour change to O. wendtii, O. pumila does not [11], and the EPTs found in both O. wendtii and O. echinata were apparently lacking in O. pumila [9,12]. However, synchrotron scans of Ophiocoma echinata and O. pumila showed similarities between all three species. Ophiocoma echinata have slightly smaller (diameter 20–30 µm) EPTs than O. wendtii, again present on the dorsal, ventral and lateral arm plates and highly regular in shape (figure 2a–e and electronic supplementary material, figure S2). The dorsal, ventral and dorsoventral margins of the lateral arm plates in O. pumila also bear EPT-like structures, in contrast to previous findings from chemically treated plates [9,12] (figure 2f–j). These structures are smaller (diameter 20–25 µm), particularly on the ventral arm plates (diameter 15–20 µm), and more irregular yet anatomically similar to the EPTs observed in the other two species (figures 1 and 2 and electronic supplementary material, figures S1–S3).
Figure 2.
Calcite elements on the arm plates in Ophiocoma echinata and O. pumila visualized by synchrotron X-ray tomography (a, c–f, h–j) and photography (b,g). Ophiocoma echinata (a–e) is covered with very regular, hemispherical EPTs on the dorsal arm plates (a–c), ventral arm plates (d), and the dorsal and ventral regions of the lateral (a,e) arm plates. The EPTs are surrounded by pigmented chromatophores giving a dark colour (b). Ophiocoma pumila (f–j) lacks chromatophores and appears much paler (g). The skeletal elements are less regular than the EPTs observed in O. wendtii (figure 1) and O. echinata (a–c), but EPT-like hemispheres are present across the dorsal arm plates (f,h), margins of the lateral arm plates (i) and ventral arm plates (j). See the electronic supplementary material (S2–S3) for reconstructed models. Scale bars: (a,f) 250 µm; (b,g) 500 µm; (c–e, h–j) 25 µm. (Online version in colour.)
(b). Nerves and opsin reactivity
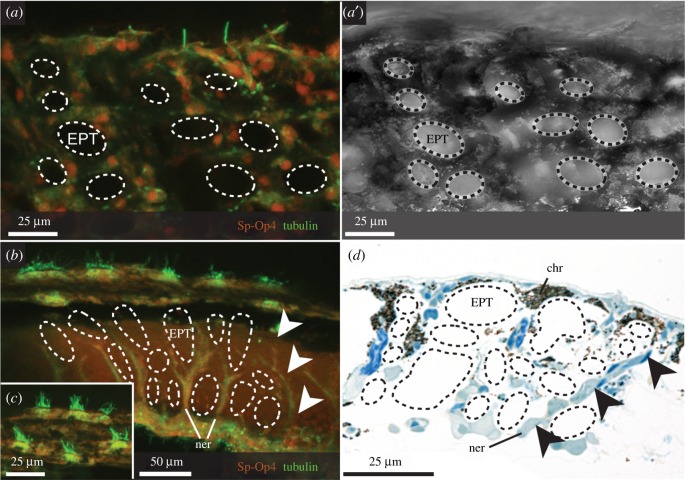
Immunohistochemistry allowed us to specifically target nerve fibres and cells reactive to sea urchin opsins, where photoreceptors have proved elusive using classical methods [12]. In all three Ophiocoma spp., a branching nerve net covers the proximal faces of the arm plates, extending laterally from the midline and emitting branching nerve bundles distally into the plate (figures 3a,b and 4a and electronic supplementary material, figure S5a). These originate in the radial nerve cord at the oral side and a smaller medial nerve at the aboral side (figures 3b and 4a electronic supplementary material, figure S4 and S5a). Crucially, the bundles innervating the arm plates do not terminate at the proposed focal point of the EPTs according to Aizenberg et al. [9], instead projecting between them towards the outer surface of the arm (figures 3b,d and 4c). Ovoid cells (soma approx. 10 µm) associated with these nerves surround the EPTs and react to r-opsin antibody Sp-Op4 (figures 3a and 4d and electronic supplementary material, figure S5b,c; see electronic supplementary material, figure S6 for controls). Cell bodies are located just above the midline of the EPTs, project towards the surface of the arm and bear rounded terminal expansions that react strongly to the r-opsin antibody (figure 4d). These cells are notably absent at the putative focal point of the EPTs, where photoreceptors had been predicted [9,11,12]. They appear to lack specialized membrane structures and are reminiscent of the general receptors described in Ophioderma longicauda [38], though a short cilium is not always visible (e.g. figure 4d); however, they do not resemble those reported in Ophiura ophiura [39], which are more akin to the Stäbchen. The opsin-reactive cells are regularly arranged over the aboral, lateral and oral sides of the arm, as well as some at the surface of the spines, in O. wendtii, O. echinata and O. pumila. They sometimes appear associated with ciliated cells potentially corresponding to those in Ophionereis schayeri [40]. Single and multiciliary tufts protrude between the EPTs (figure 3).
Figure 3.
Opsin-reactive cells are arranged between the EPTs in Ophiocoma wendtii. (a, a′): Cells reactive to a sea urchin rhabdomeric opsin (Sp-Op4, red) and acetylated tubulin (green) are arranged around the distal part of the EPTs (dashed outlines) on the DAP. Dorsal view of arm plate, with stack reaching slightly beneath plate surface. (b–d) Stacked images of transverse sections through the DAP show the distal projection of nerves between EPTs towards the surface of the arm (b, d, arrowheads), originating from an underlying lateral nerve (b) and terminating in multiciliary bundles at the surface (c). Proximal side of the plate is at the bottom of the image. Note that images in both planes show no opsin-reactive cells present at the focal point of the EPTs as predicted by Aizenberg et al. [9]. Chr, chromatophore; EPT, expanded peripheral trabecula; ner, nerve.
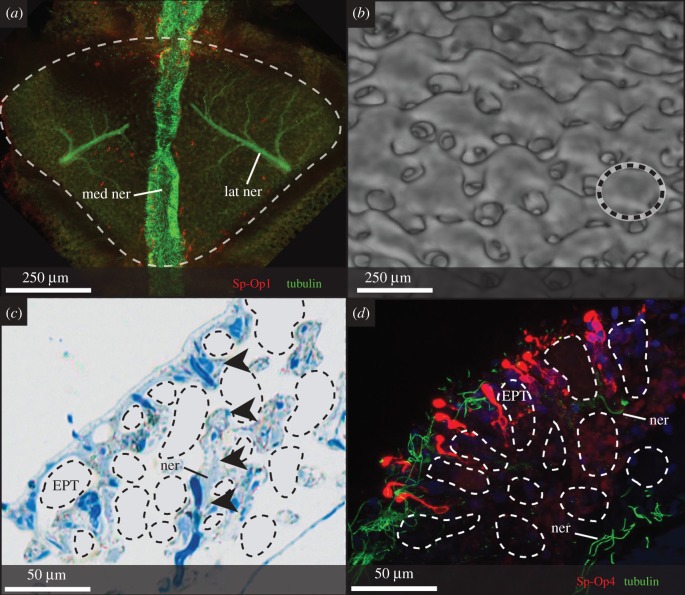
Figure 4.
An expansive system of opsin-reactive cells and ‘lens’-like skeletal structures is also present in Ophiocoma pumila. (a) Horizontal section through DAP (dashed outline) in O. pumila demonstrates the same innervation as O. wendtii, with a median nerve and paired, branching nerves (acetylated tubulin, green) extending laterally. Reactivity to the c-opsin Sp-Op1 is visible inconsistently across the plate surface and within the median nerve. Dorsal view. (b) Surface of DAP reconstructed from synchrotron scan, with EPT-like structures (dashed outline) among more irregularly shaped stereom elements. Dorsal view. (c,d) Transverse sections through the arm plate show projections from the lateral nerve (arrowheads) to opsin-reactive cells and ciliary tufts at the surface, between the EPT-like structures. Chr, chromatophore; EPT, expanded peripheral trabecula; lat ner, lateral nerve; med ner, median nerve; ner, nerve bundles.
There are also scattered Sp-Op1-reactive cells of similar size (figure 4a), but these were less consistently observed and so are not further discussed here other than to highlight their presence. We also observed some reactivity to both opsins within the medial and lateral nerves and the radial nerve cord (figures 3b and 4a and electronic supplementary material, figure S5c), of which the latter has been reported to exhibit intrinsic photosensitivity and opsin expression [2,26,31].
Potential nerve connections between Sp-Op4-reactive cells, both laterally at the surface and in convergent innervating bundles (figure 3a,b and electronic supplementary material, figure S5c), could indicate integration or summation between them. However, we found no unusual or concentrated area of neuropil as might be expected for integrating visual information across such an expansive network.
4. Discussion
The putative photoreceptor system in Ophiocoma wendtii, O. echinata and O. pumila is extensive; our findings revealed a much larger network than previously posited, which is present across almost the complete body surface in all three species. The morphology, reactivity and arrangement of Sp-Op4-reactive cells support their candidacy as photoreceptors; past work indicates that r-opsins homologous to Sp-Op4 are involved in brittle star photoreception, and that they are likely expressed at higher levels than c-opsin homologues to Sp-Op1 [25,26], in line with our findings. Critically, the nerve bundles proposed to act as photoreceptors project past the EPTs towards the opsin-reactive cells. Contrary to expectations, these putative photoreceptors appear to be entirely independent of the EPTs; their anatomical configuration relative to the EPTs demonstrates no support for an optical role as ‘microlenses’ (figures 3a,b and 4d).
The three Ophiocoma species possess vast networks of putative dermal photoreceptors covering their dorsal, ventral and lateral arm plates. This is a considerable expansion on the system hypothesized to exist beneath the EPTs [9,12], both anatomically and taxonomically, and may represent one of the largest dispersed photoreceptor systems described to date, thanks to the ability to monitor expression of molecular markers. These findings complement proposed dermal photoreceptor networks in other echinoderms, most notably sea urchins [6,7], but turn the tables on previous theories about Ophiocoma wendtii [9,11]. We anticipate that future researchers will find similarly large extraocular systems in other taxa.
The optical involvement of the EPTs in a photoreceptor system is problematic for several reasons. The EPTs are present on the oral (ventral) and lateral surfaces (figures 1 and 2) as well as the dorsal arm plates. The lateral plates would be a complex surface for integrated photoreception, let alone vision and the oral surfaces would be largely redundant; although some brittle stars expose the ventral arm during feeding, Ophiocoma does not [41]. Second, the sheer number of EPTs is enormous; we found an average of 510 EPTs per dorsal arm plate (DAP) in Ophiocoma wendtii, with around 75 plates per arm (mean length 112 mm). Rough calculations indicate that an average-sized individual would possess over 300 000 EPTs. However, they apparently lack any further organization of the photoreceptors into discrete units, as seen in other distributed visual systems [18,42,43], or a processing centre beyond the radial nerve cords, providing no indication of potential integration mechanisms for such an enormous network. Additionally, the acceptance angle of each receptor between the EPTs would be too large to enable high resolution. Indeed, Ophiocoma wendtii exhibits limited visual behaviour according to preliminary tests herein (electronic supplementary material, figure S7). As a third, independent, argument against an optical role for the EPTs, the cuticle, chromatophores and other biological material also occlude their rounded shape and surface in vivo and may interfere with the passage of light (figure 1). Expanded chromatophores cover the EPTs completely, with no aperture to indicate pupillary function [5,11,12] (figure 3). Conversely, contracted chromatophores appear to lie beneath as well as between the EPTs [12], further shielding peripheral nerve elements from incoming light in dark-adapted animals.
Finally, and most importantly, the presence of photoreceptive elements is primarily detected in between and not beneath the EPTs. No opsin-reactive cells were observed at the reported focal point beneath the EPTs, and the nerve bundles that were implicated as primary photoreceptors [12] not only lack reactivity to the tested opsins, but project past the EPTs towards the plate surface. Visual photoreceptors in other taxa are not universally located at the optical focal point [42,44], but these opsin-reactive cells are within the dermal layer and apparently far from any potential optical effect of the EPTs; their projection and expansion above, the EPTs also negate channelling or light-gathering roles. An identical pattern of anti-Sp-Op4 reactivity is present in O. pumila, which lacks highly regular EPTs and colour change (figure 3). The optical properties of the EPTs may be an exaptation relevant to materials science [9,10], but they do not appear to perform any optical role in Ophiocoma.
Although our findings contest the interpretation of the EPTs as microlenses in Ophiocoma, they are still compatible with the electrophysiological studies of Cobb & Hendler [13]. They demonstrated increasing photosensitivity correlating with increasing loss of arm tissue, bleaching EPTs and dermal tissue, including chromatophores, until the nerve bundles beneath each EPT were affected. They argued that this demonstrated these nerve bundles are the primary photoreceptors. However, their findings that the receptors were located beneath the epidermis, regulated in their sensitivity by chromatophores, and became more sensitive with the removal of overlying tissue, are also compatible with the data presented here. The authors acknowledge that other unrecognized cell types could be responsible; given the resemblance of the r-opsin-reactive cells to generalized dermal receptors, it appears that they were indeed overlooked.
Of course, we too cannot eliminate the possibility that additional cells at the base of the EPTs were not detected in this (or any other) study, and echinoderms [31] including brittle stars [26] demonstrate high opsin diversity. Identifying a complete suite of opsin candidates in Ophiocoma will help detect other opsin-expressing (or cryptochrome-expressing [45]) tissues underlying the EPTs, if present, although transcriptomic studies in other brittle stars support a key role for Sp-Op4 homologues [25,26]. In addition, functions besides photoreception have now been described for several r-opsins in some arthropods and vertebrates [46]. However, the Sp-Op4-reactive cells we interpret as photoreceptor candidates conform to previous descriptions of receptor morphology and r-opsin expression in other ophiuroids, are positioned within the EPT-chromatophore layer in line with Hendler & Cobb [13], are highly numerous, and represent the only candidates identified in any study in over 30 years. We propose it is highly likely that they are responsible for photosensitivity and corresponding behaviours in Ophiocoma.
Concerning visual ability, and especially the compound eye model suggested by several authors, we cannot support it based on our findings. Ophiocoma wendtii certainly exhibits high sensitivity to light [11] and strong shade-seeking responses (electronic supplementary material, figure S7). Our preliminary behavioural experiments showed that Ophiocoma wendtii could be capable of basic image formation, as indicated by its ability to detect large, high-contrast targets (electronic supplementary material, figure S7). However, response to targets of 35–57° is coarse even in comparison to other echinoderms, including urchins using a dermal photoreceptor system where skeletal structures have also been implicated in spatial resolution [6,7]. The detection and location of large, dark, high-contrast targets from short distances also do not necessarily equate to spatial resolution rather than phototaxis (owing to lower overall light intensity in the region of the target), so we hesitate to unequivocally support visual capability. It is not yet clear precisely how the abilities of O. echinata and O. pumila compare to O. wendtii beyond their lesser sensitivity [11]; in the light of their relatively distant phylogenetic positions in the genus [47], further comparisons will be of great interest in the context of wider photosensitivity in the taxon. A compound eye requires that each repeated optical unit represents, or scales to, a unit of resolution, a pixel. We find no evidence that the EPTs act as lenses in ommatidium-like optical units, so the photoreceptors could theoretically represent these themselves. If it acts as a compound eye sensu stricto, the vast photoreceptor network in Ophiocoma should confer fine resolution [48], but this is not supported by behavioural data (electronic supplementary material, figure S7).
Local signal integration and spatial summation could explain high sensitivity and low spatial resolution (if any; electronic supplementary material, figure S7) in O. wendtii [49]. However, the innervation networks do not show any organizational structure that would presumably be a prerequisite for complex signal integration in a compound-type eye, and synapses are known to be relatively rare in ophiuroid nervous systems [28]. Photoresponsive behaviours may instead function through reflex activity within arms or arm segments. Thus, even basic directional light/dark perception could guide non-visual phototactic shelter-seeking behaviour in complex environments with high light intensity and low turbidity [50].
5. Conclusion
The correlation between increasing responsiveness, EPT distribution and colour change formerly contributed a key piece of indirect evidence that EPTs are integral to photoreception [9,12]. The joint absence of EPTs and colour change in Ophiocoma pumila was interpreted as evidence for the involvement of the EPTs in light sensing [9,11], but it may still indicate their function. Colour change in Ophiocoma depends on the expansion and retraction of chromatophores over and around the EPTs [11]. Chromatophore activity is likely to be autonomous and does not appear to be associated with nervous or muscular accessories [12]. We therefore propose that the large, regular EPTs found on the arm plates in O. wendtii and O. echinata could be a structural adaptation relating to chromatophore activity. By maximizing separation of chromatophores in their contracted state, the distinction between contracted and expanded states is amplified, producing a more dramatic colour change. The chromatophore activity likely affects photoreceptor sensitivity by altering the amount of screening pigment surrounding them, in line with increased sensitivity in dark-adapted arms [13], but not by controlling the amount of light reaching the EPTs. Thus, the EPTs may have an accessory role in photoreception, through their potential role in colour change, but there is no optical focusing. This is dramatically at odds with the published literature and the popular status of O. wendtii as an advanced visual species [5,9].
Our findings also caution against interpretations of complex photoreceptor systems from skeletal evidence alone in living and fossil echinoderms [14,22,27]. For example, some asteroids with visual optic cushions also have EPTs [22,33,51]; these skeletal structures that have optical properties (in the physical sense) are likely irrelevant to the organism's sensory biology. We propose that the placement, concentration and connectivity of dermal photoreceptors confer high photosensitivity across the body, resulting in sensitive directional EOP and not vision per se in Ophiocoma wendtii. This more accurate model, without requiring focusing lenses, marks a significant advance in understanding the capabilities of EOP.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank Arcadio Castillo, Anders Hansen, Deyvis Gonzalez, Carly Otis (STRI), Stefanie Blaue (MfN) and Dan Sykes (NHM) for help with specimen collection and preliminary work; Rachel Collin, Bill Wcislo, Plinio Gondola and Paola Galgani for their support at STRI; and Todd Oakley (UC Santa Barbara), David Lindberg (UC Berkeley) and reviewers for helpful comments on the manuscript. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland for provision of synchrotron radiation beamtime on the Swiss Light Source TOMCAT beamline and we thank Pablo Villanueva for assistance.
Ethics
Specimens were collected under ARAP permit 2014-52b and exported under ARAP export permit 2015-2.
Data accessibility
All data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.kc041 [52].
Authors' contributions
L.S.R. designed the study, collected animals, performed histology, SEM, and behavioural experiments, and analysed the data, assisted and supervised by J.D.S. L.S.R. and E.U.L. performed immunohistochemistry and interpreted results. I.A.R. scanned specimens at the synchrotron, and L.S.R. and I.A.R. processed scan data. L.S.R. and J.D.S. wrote the manuscript, and all authors contributed editorial input and gave their approval for submission.
Competing interests
The authors have no competing interests.
Funding
This research was funded by the Smithsonian Tropical Research Institute, American Microscopical Society, government of Northern Ireland (Department of Employment and Learning), DAAD-Leibniz Fellowship scheme, European Commission (award H2020-MSCA-IF-2014-655661), 1851 Commission and German Research Foundation (DFG grant no. UL 428/2-1).
References
- 1.Ramirez MD, Speiser DI, Pankey SM, Oakley TH. 2011. Understanding the dermal light sense in the context of integrative photoreceptor cell biology. Vis. Neurosci. 28, 265–279. ( 10.1017/S0952523811000150) [DOI] [PubMed] [Google Scholar]
- 2.Millott N, Yoshida M. 1959. The shadow reaction of Diadema antillarum Philippi I. The spine response and its relation to the stimulus. J. Exp. Biol. 37, 363–375. [Google Scholar]
- 3.Yoshida M. 1979. Extraocular photoreception. In Handbook of sensory physiology. Volume VII/6A: vision in invertebrates A: invertebrate photoreceptors (ed. Autrum H.), pp. 581–640. Berlin, Germany: Springer. [Google Scholar]
- 4.Bielecki J, Zaharoff AK, Leung NY, Garm A, Oakley TH. 2014. Ocular and extraocular expression of opsins in the rhopalium of Tripedalia cystophora (Cnidaria: Cubozoa). PLoS ONE 9, e98870 ( 10.1371/journal.pone.0098870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendler G. 2005. An echinoderm's eye view of photoreception and vision. In Echinoderms: München: Proceedings of the 11th International Echinoderm Conference (eds Heinzeller T, Nebelsick J), pp. 339–349. München, Germany: Taylor & Francis. [Google Scholar]
- 6.Blevins E, Johnsen S. 2004. Spatial vision in the echinoid genus Echinometra. J. Exp. Biol. 207, 4249–4253. ( 10.1242/jeb.01286) [DOI] [PubMed] [Google Scholar]
- 7.Yerramilli D, Johnsen S. 2010. Spatial vision in the purple sea urchin Strongylocentrotus purpuratus (Echinoidea). J. Exp. Biol. 213, 249–255. ( 10.1242/jeb.033159) [DOI] [PubMed] [Google Scholar]
- 8.Jackson E, Johnsen S. 2011. Orientation to objects in the sea urchin Strongylocentrotus purpuratus depends on apparent and not actual object size. Biol. Bull. 220, 86–88. ( 10.1086/BBLv220n2p86) [DOI] [PubMed] [Google Scholar]
- 9.Aizenberg J, Tkachenko A, Weiner S, Addadi L, Hendler G. 2001. Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature 412, 819–822. ( 10.1038/35090573) [DOI] [PubMed] [Google Scholar]
- 10.Aizenberg J, Hendler G. 2004. Designing efficient microlens arrays: lessons from nature. J. Mater. Chem. 14, 2066 ( 10.1039/b402558j) [DOI] [Google Scholar]
- 11.Hendler G. 1984. Brittlestar color-change and phototaxis (Echinodermata: Ophiuroidea: Ophiocomidae). Mar. Ecol. 5, 379–401. ( 10.1111/j.1439-0485.1984.tb00131.x) [DOI] [Google Scholar]
- 12.Hendler G, Byrne M. 1987. Fine structure of the dorsal arm plate of Ophiocoma wendti: evidence for a photoreceptor system (Echinodermata, Ophiuroidea). Zoomorphology 107, 261–272. ( 10.1007/BF00312172) [DOI] [Google Scholar]
- 13.Cobb JLS, Hendler G. 1990. Neurophysiological characterisation of the photoreceptor system in a brittlestar, Ophiocoma wendtii (Echinodermata: Ophiuroidea). Comp. Biochem. Physiol. A 97, 329–333. ( 10.1016/0300-9629(90)90619-4) [DOI] [Google Scholar]
- 14.Gorzelak P, Salamon MA, Lach R, Loba M, Ferré B. 2014. Microlens arrays in the complex visual system of Cretaceous echinoderms. Nat. Commun. 5, Article number: 3576 ( 10.1038/ncomms4576) [DOI] [PubMed] [Google Scholar]
- 15.Polishchuk I, et al. 2017. Coherently aligned nanoparticles within a biogenic single crystal: a biological prestressing strategy. Science 358, 1294–1298. ( 10.1126/science.aaj2156) [DOI] [PubMed] [Google Scholar]
- 16.Ullrich-Lüter EM, Dupont S, Arboleda E, Hausen H, Arnone MI. 2011. Unique system of photoreceptors in sea urchin tube feet. Proc. Natl Acad. Sci. USA 108, 8367–8372. ( 10.1073/pnas.1018495108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodley JD. 1982. Photosensitivity in Diadema antillarum: does it show scototaxis? In Echinoderms: Tampa Bay: Proceedings of the International Echinoderm Conference (ed. Lawrence JM.), p. 61 Balkema and NH Salem: Rotterdam, The Netherlands. [Google Scholar]
- 18.Speiser DI, Eernisse DJ, Johnsen S. 2011. A chiton uses aragonite lenses to form images. Curr. Biol. 21, 665–670. ( 10.1016/j.cub.2011.03.033) [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Aizenberg J. 2005. Microlens arrays with integrated pores. Nano Today 12, 40–46. [Google Scholar]
- 20.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 21.Mashanov V, Zueva O, Rubilar T, Epherra L, García-Arrarás JE. 2015. Echinodermata. In Structure and evolution of invertebrate nervous systems (eds Schmidt-Rhaesa A, Harzsch S, Purschke G), pp. 665–688. Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Vinogradova E, Ruíz-Zepeda F, Plascencia-Villa G, José-Yacamán M. 2016. Calcitic microlens arrays in Archaster typicus: microstructural evidence for an advanced photoreception system in modern starfish. Zoomorphology 135, 83–87. ( 10.1007/s00435-015-0276-5) [DOI] [Google Scholar]
- 23.Burke RD, et al. 2006. A genomic view of the sea urchin nervous system. Dev. Biol. 300, 434–460. ( 10.1016/j.ydbio.2006.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg R, Lundberg L. 2004. Photoperiodic activity pattern in the brittle star Amphiura filiformis. Mar. Biol. 145, 651–656. [Google Scholar]
- 25.Delroisse J, Mallefet J, Flammang P. 2016. De novo adult transcriptomes of two European brittle stars: spotlight on opsin-based photoreception. PLoS ONE 11, e0152988 ( 10.1371/journal.pone.0152988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delroisse J, Ullrich-Lüter E, Ortega-Martinez O, Dupont S, Arnone M-I, Mallefet J, Flammang P. 2014. High opsin diversity in a non-visual infaunal brittle star. BMC Genomics 15, 1035 ( 10.1186/1471-2164-15-1035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorzelak P, Rahman IA, Zamora S, Gasinski A, Trzcinski J, Brachaniec T, Salamon MA. 2017. Towards a better understanding of the origins of microlens arrays in Mesozoic ophiuroids and asteroids. Evol. Biol. 44, 339–346. ( 10.1007/s11692-017-9411-1) [DOI] [Google Scholar]
- 28.Cobb JLS, Moore A. 1989. Studies on the integration of sensory information by the nervous system of the brittlestar Ophiura ophiura. Mar. Behav. Physiol. 14, 211–222. ( 10.1080/10236248909378708) [DOI] [Google Scholar]
- 29.Raible F, Tessmar-Raible K, Arboleda E, Kaller T, Bork P, Arendt D, Arnone MI. 2006. Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475. ( 10.1016/j.ydbio.2006.08.070) [DOI] [PubMed] [Google Scholar]
- 30.Lesser MP, Carleton KL, Böttger SA, Barry TM, Walker CW. 2011. Sea urchin tube feet are photosensory organs that express a rhabdomeric-like opsin and PAX6. Proc. R. Soc. B 278, 3371–3379. ( 10.1098/rspb.2011.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Aniello S, et al. 2015. Opsin evolution in the Ambulacraria. Mar. Genomics 24, 177–183. ( 10.1016/j.margen.2015.10.001) [DOI] [PubMed] [Google Scholar]
- 32.Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH. 2016. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol. Evol. 8, 3640–3652. ( 10.1093/gbe/evw135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garm A, Nilsson D-E. 2014. Visual navigation in starfish: first evidence for the use of vision and eyes in starfish. Proc. R. Soc. B 281, 1–8. ( 10.1098/rspb.2013.3011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton MD, Garwood RJ, Siveter DJ, Siveter DJ. 2012. SPIERS and VAXML; a software toolkit for tomographic visualisation and a format for virtual specimen interchange. Paleontol. Electron. 15, 1–15. [Google Scholar]
- 35.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield PJ, Emson RH. 1983. Presumptive ciliated receptors associated with the fibrillar glands of the spines of the echinoderm Amphipholis squamata. Cell Tissue Res. 232, 609–624. ( 10.1007/BF00216433) [DOI] [PubMed] [Google Scholar]
- 37.Reichensperger A. 1908. Die Drüsengebilde der Ophiuren. Zeitschrift für Wissenschaftliche Zool. 91, 304–350. [Google Scholar]
- 38.Märkel K, Röser U. 1985. Comparative morphology of echinoderm calcified tissues: histology and ultrastructure of ophiuroid scales (Echinodermata, Ophiuroida). Zoomorphology 105, 197–207. ( 10.1007/BF00312157) [DOI] [Google Scholar]
- 39.Moore PA, Cobb JLS. 1986. Neurophysiological studies on the detection of mechanical stimuli by Ophiura ophiura (L.). J. Exp. Mar. Bio. Ecol. 104, 125–141. ( 10.1016/0022-0981(86)90100-0) [DOI] [Google Scholar]
- 40.Byrne M. 1994. Ophiuroidea. In Microscopic anatomy of invertebrates, volume 14: echinodermata (eds Harrison FW, Chia FS), pp. 247–343. New York, NY: WileyLiss, Inc. [Google Scholar]
- 41.Sides EM, Woodley JD. 1985. Niche separation in three species of Ophiocoma (Echinodermata: Ophiuroidea) in Jamaica, West Indies. Bull. Mar. Sci. 36, 701–715. [Google Scholar]
- 42.Land MF. 1965. Image formation by a concave reflector in the eye of the scallop, Pecten maximus. J. Physiol. 179, 138–153. ( 10.1113/jphysiol.1965.sp007653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bok MJ, Capa M, Nilsson DE. 2016. Here, there and everywhere: the radiolar eyes of fan worms (Annelida, Sabellidae). Integr. Comp. Biol. 56, 784–795. ( 10.1093/icb/icw089) [DOI] [PubMed] [Google Scholar]
- 44.Nilsson D-E, Gislen L, Coates MM, Skogh C, Garm A. 2005. Advanced optics in a jellyfish eye. Nature 435, 201–205. ( 10.1038/nature03484) [DOI] [PubMed] [Google Scholar]
- 45.Müller WEG, Wang X, Schröder HC, Korzhev M, Grebenjuk VA, Markl JS, Jochum KP, Pisignano D, Wiens M. 2010. A cryptochrome-based photosensory system in the siliceous sponge Suberites domuncula (Demospongiae). FEBS J. 277, 1182–1201. ( 10.1111/j.1742-4658.2009.07552.x) [DOI] [PubMed] [Google Scholar]
- 46.Leung NY, Montell C. 2017. Unconventional roles of opsins. Annu. Rev. Cell Dev. Biol. 33, 241–264. ( 10.1146/annurev-cellbio-100616-060432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Hara TD, Hugall AF, Thuy B, Stöhr S, Martynov AV. 2017. Molecular phylogenetics and evolution restructuring higher taxonomy using broad-scale phylogenomics: the living Ophiuroidea. Mol. Phylogenet. Evol. 107, 415–430. ( 10.1016/j.ympev.2016.12.006) [DOI] [PubMed] [Google Scholar]
- 48.Richter S, et al. 2010. Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front. Zool. 7, 29 ( 10.1186/1742-9994-7-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Land MF, Nilsson D-E. 2012. Animal eyes, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Nilsson D-E. 2009. The evolution of eyes and visually guided behaviour. Phil. Trans. R. Soc. B 364, 2833–2847. ( 10.1098/rstb.2009.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petie R, Garm A, Hall MR. 2016. Crown-of-thorns starfish have true image forming vision. Front. Zool. 13, 41 ( 10.1186/s12983-016-0174-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumner-Rooney L, Rahman I, Sigwart J, Ullrich-Lüter E. 2018. Data from: Whole-body photoreceptor networks are independent of ‘lenses’ in brittle stars Dryad Digital Repository. ( 10.5061/dryad.kc041) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sumner-Rooney L, Rahman I, Sigwart J, Ullrich-Lüter E. 2018. Data from: Whole-body photoreceptor networks are independent of ‘lenses’ in brittle stars Dryad Digital Repository. ( 10.5061/dryad.kc041) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.kc041 [52].