Abstract
Concurrent gaps in the Late Devonian/Mississippian fossil records of insects and tetrapods (i.e. Romer's Gap) have been attributed to physiological suppression by low atmospheric pO2. Here, updated stable isotope inputs inform a reconstruction of Phanerozoic oxygen levels that contradicts the low oxygen hypothesis (and contradicts the purported role of oxygen in the evolution of gigantic insects during the late Palaeozoic), but reconciles isotope-based calculations with other proxies, like charcoal. Furthermore, statistical analysis demonstrates that the gap between the first Devonian insect and earliest diverse insect assemblages of the Pennsylvanian (Bashkirian Stage) requires no special explanation if insects were neither diverse nor abundant prior to the evolution of wings. Rather than tracking physiological constraint, the fossil record may accurately record the transformative evolutionary impact of insect flight.
Keywords: atmosphere, insect, oxygen, Palaeozoic, tetrapod, wings
1. Introduction
Various Devonian fossils have been attributed to the Insecta; however, all but one of these have been subsequently challenged [1,2]. This depauperate Devonian fossil record of insects, which does not contain any direct evidence of wings, is followed by a 62-million-year gap that is completely devoid of insect material. This interval, called the ‘Hexapod Gap’, encompasses the entirety of the Late Devonian (383–359 Ma) and the Mississippian (359–323 Ma). In the Pennsylvanian (323–299 Ma), following the Hexapod Gap, winged insects are abundant and diverse: the earliest Pennsylvanian insects represent Palaeoptera and Neoptera, and holometabolous insects appear shortly thereafter [3–6]. The tetrapod fossil record contains a similar gap, also in the Mississippian. The few early tetrapods of the Devonian, including the iconic Eusthenopteron, Tiktaalik and Ichthyostega, were all aquatic, whereas the diverse tetrapods of the later Mississippian and Pennsylvanian include fully terrestrial forms, amniotes and even such structural diversity as secondarily derived limblessness [7]. Many of the intervening evolutionary transitions are unknown due to Romer's Gap, a 15-million-year interval corresponding roughly to the Tournaisian Stage of the Mississippian (360–345 Ma), from which few terrestrial vertebrate fossils are known [7]. The Hexapod Gap and Romer's Gap therefore obscure two of the major events in the history of animal evolution: the origins of insect wings and of fully terrestrial tetrapods. These partly contemporaneous gaps have been attributed to biological constraints induced by low atmospheric oxygen levels [8] or, alternatively, to an insufficiency of terrestrial sedimentary deposits or collecting effort [9].
The link between the hexapod and tetrapod gaps and low atmospheric oxygen levels has been most strongly supported by reconstructions of atmospheric composition [8,10] that use carbon and sulfur isotopes to infer the balance between oxygen production via photosynthesis and oxygen consumption via respiration. The latest iteration of the most influential isotope-based model, GEOCARBSULF, was published nearly a decade ago [11], but the amount of available isotope data has increased substantially in the years since. Here, we used an updated stable isotope dataset to determine whether improved sampling of the geochemical record yields atmospheric reconstructions that are still consistent with the hypothesis that Romer's Gap was caused by low oxygen. We also used stratigraphic and palaeobiological data to test hypothesized explanations for the dearth of insect fossils during the Hexapod Gap involving insufficient rock availability or sampling effort.
2. Results
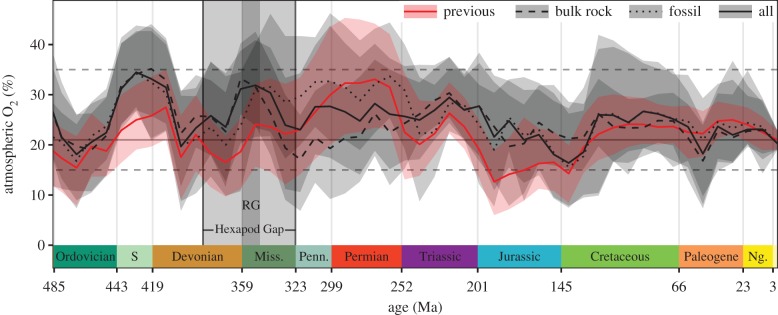
Based on updated input values, the GEOCARBSULF model output does not show the Late Devonian to be a low oxygen interval; furthermore, the Tournaisian—which encompasses Romer's Gap and a large part of the Hexapod Gap—is reconstructed as a high-oxygen interval (figure 1).
Figure 1.
Phanerozoic atmospheric oxygen reconstructed by the GEOCARBSULF model [12]. The light grey box corresponds to the Hexapod Gap and the dark grey box corresponds to Romer's Gap. The shaded region for each curve represents the 95% confidence interval. The dashed lines represent the minimum and maximum plausible oxygen levels, based on the charcoal record [13], and the solid line represents the current atmospheric oxygen level. Abbreviations: S, Silurian; Miss., Mississippian; Penn., Pennsylvanian; Ng., Neogene.
Overall, using the updated isotope dataset as input to GEOCARBSULF yields more muted extremes than the previous iteration. The increased isotopic sampling also resolves a major challenge to the model: the ubiquity of charcoal from the Late Palaeozoic through to the recent suggests that atmospheric oxygen levels have remained above 15% [13], but the original GEOCARBSULF output recovered atmospheric oxygen levels that dropped below this threshold in both the Mississippian and Jurassic (201–145 Ma). In the update presented here, the mean estimate for atmospheric oxygen never drops below 15%, thus reconciling previous inconsistencies between empirical constraints and the model-based reconstruction. This result is broadly consistent with recent studies of Corg : P ratios [14], of charcoal [7,15,16], of cerium [17] and of sediment accumulation [18], none of which have concluded that Romer's Gap was characterized by low atmospheric oxygen levels. Furthermore, the updated isotope input yields predicted carbon dioxide levels through time that are nearly identical to those suggested by the previous iteration of the GEOCARBSULF model (electronic supplementary material, figure S6), reinforcing the plausibility of the updated model output by remaining compatible with other proxies [10,12,13].
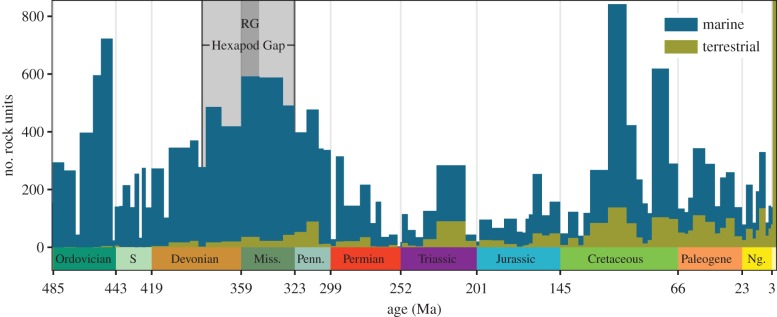
Another proposed explanation for these gaps in the fossil record is a lack of sedimentary deposits from terrestrial environments during this interval. Sea-level rose during the Late Devonian and again during the Tournaisian [19], which could have led to a lack of preserved non-marine sediments. The dearth of plant fossils during Romer's Gap [20] is far more consistent with a lack of non-marine rock record than with a biotic crisis caused by low atmospheric oxygen levels. To determine whether a lack of non-marine sedimentary deposits could be responsible for Romer's Gap and the Hexapod Gap, we examined the presence of non-marine and marine rock units in the Macrostrat database (macrostrat.org). These data do not show a decrease in the amount of non-marine rock during the Late Devonian and Mississippian (figure 2). The Wilcoxon–Mann–Whitney test recovered no significant difference between the number of non-marine sediment deposits per stage from the Hexapod Gap and from subsequent Palaeozoic stages (p = 0.13). These quantitative observations, based on data from North America, are consistent with qualitative observations from other regions. Perhaps the most famous example is that of Scotland, where Tournaisian non-marine sediments are noted for containing vertebrate fossils [16]. Therefore, there is no support for the hypothesis that Romer's Gap and the Hexapod Gap are characterized by an atypical lack of terrestrial sedimentary deposits.
Figure 2.
The number of marine and terrestrial sedimentary rock units identified from North America from each geologic stage, from the Macrostrat database. See figure 1 caption for geologic period abbreviations.
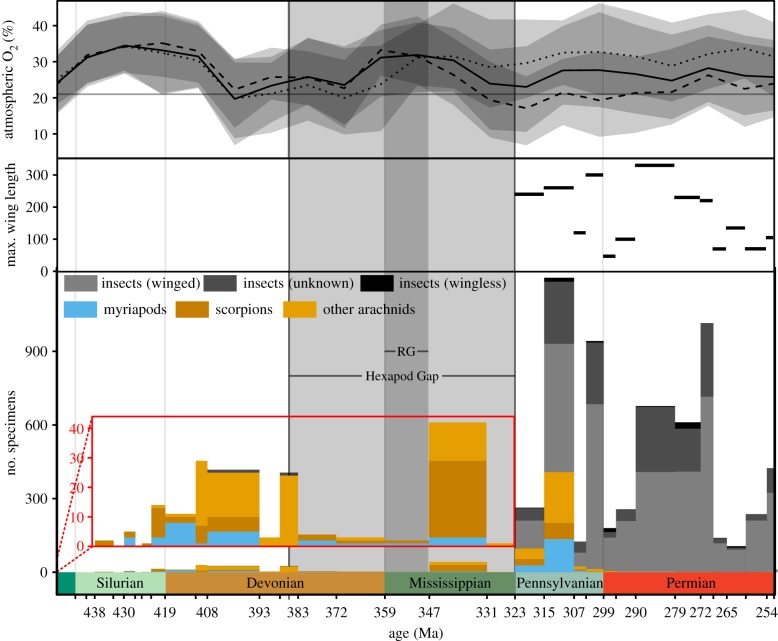
A third potential explanation for the fossil occurrence gaps is that the gaps reflect insufficient collecting effort rather than a true absence of fossils. To test whether the observed gaps are any longer than would be expected given the overall frequency of fossil occurrences, we used arthropod fossil occurrences in the Paleobiology Database. During all Palaeozoic stages that contain fossil insects, an average of 75.9% of all fossil insect occurrences include wings, an average of 1.7% do not include wings and for the remaining 22.4% the presence of wings is unknown (figure 3). Both the arachnid and myriapod fossil records date back to the Silurian, are continuous through the Devonian, and include numerous occurrences in the Tournaisian and Viséan stages of the Mississippian (figure 3). The Bashkirian terrestrial arthropod fauna contains a significantly higher proportion of insects than the Hexapod Gap (bootstrapped p < 0.001), whereas the interval encompassing the Emsian through Givetian stages of the Devonian does not (bootstrapped p = 0.14). The lack of insect fossils during the Hexapod Gap is therefore consistent with the dearth of insect fossils in the preceding geologic intervals. In other words, the Hexapod Gap does not require a special explanation in terms of sampling. Available data do not indicate that the fossil record has failed to capture a diverse and abundant arthropod fauna due to insufficient sampling; the Hexapod Gap is simply the later part of a long Palaeozoic interval in which insects were extremely rare.
Figure 3.
The updated oxygen curves, from figure 1, cropped to show the latest Ordovician through Permian; maximum insect wing length during the Palaeozoic, reproduced from a recent contribution [21] and from the Paleobiology Database; number and timing of Palaeozoic occurrences of myriapods, arachnids and insects from the Paleobiology Database.
3. Discussion
(a). Atmospheric oxygen as a driver of Paleozoic evolution
Because the two updated isotopic datasets yield conflicting estimates of oxygen concentrations for much of the Phanerozoic (figure 1), neither curve can be accepted as entirely correct. However, it can be said definitively that GEOCARBSULF no longer supports low atmospheric oxygen as the cause of Romer's Gap and the Hexapod Gap. This result necessitates a reevaluation of the expectation that variations in atmospheric oxygen levels played a formative role in the evolution of the Palaeozoic terrestrial fauna.
Arguments have been made that low oxygen would have suppressed terrestrial animal life through this interval [8]; however, this suggestion is not consistent with physiological considerations. With the diffusion of oxygen 10 000 times faster in air than in water [22], the terrestrial fauna should not have been specifically disadvantaged relative to the aquatic fauna, regardless of oxygen concentrations. Furthermore, animal life may be broadly insensitive to oxygen fluctuations over the range of concentrations in question (e.g. [23]); meta-analyses have not found consistent trends in insect abundance [24] or body size [25–27] as a function of altitude. Finally, suppression by low oxygen provides no mechanism through which insects specifically would be impacted while other terrestrial arthropods, such as arachnids and myriapods [20,28], continued unaffected through the Hexapod Gap (figure 3).
The new reconstructions of atmospheric oxygen presented here also call into question the canonical interpretation of Palaeozoic insect body size as being largely determined by the diffusional limits imposed by atmospheric oxygen levels [21,29]. The late Palaeozoic maximum in oxygen concentrations has been a celebrated feature of the previous GEOCARBSULF curve, and of other reconstructions of pO2 that use similar carbon isotope data as inputs [14] or were validated with similar carbon isotope data [30]. These models were based on isotope datasets that are not only outdated, but also incomplete: for example, one such model had substantial isotopic inputs from the Quaternary, but data from only three Mississippian, three Pennsylvanian, two Permian and two pre-Quaternary Cenozoic formations [14].
The elevated oxygen levels seen in these reconstructions have been taken as a causal explanation for the coeval increase in insect size, but this peak does not occur with our updated datasets, and elevated oxygen should not be considered a sufficient explanation in any case. During the Palaeozoic alone, maximum wing length increased from 11 to 330 mm [5,6,31]. Even if most of this 30-fold increase is thought to reflect the initial evolution of flight at the small end of the permissible range of body sizes, oxygen is doubtful as the determinant of the upper limit of that range. If body size was limited by oxygen diffusion, then maximum thoracic diameter (setting the maximal diffusional path length) should increase at most linearly with pO2 and would be further limited by oxygen demand increasing with volume, which will scale with the cube of linear dimension [32]. Thoracic diameter has been shown to increase roughly linearly with wing length [33]. Thus, maximum possible insect wing length should scale no more than linearly with pO2. However, the largest known Palaeozoic insect had a wing length of approximately 330 mm [31], whereas the largest extant insect has a wing length of approximately 135 mm [34], and many other extant insect groups have a maximum wing length around 100 mm [35]; oxygen levels during the Permian could not have reached even twice the current level, yet maximum insect wing length during the Permian was more than double the current maximum length. It therefore appears that atmospheric oxygen is not the primary constraint on insect body size. Consequently, other explanations must be considered, such as ecological interactions with predators [21].
(b). The cause of the Hexapod Gap
A new explanation for the Hexapod Gap emerges from ecological considerations. The vast majority of fossilized insects have wings (figure 3), but there is no direct evidence for wings before or during the Hexapod Gap. As soon as there is direct evidence for winged insects in the fossil record, insect fossils become far more abundant than arachnids or myriapods (figure 3). There are two reasons why wings may be associated with a more robust fossil record. First, wings made available new ecologies and facilitated habitat-specific insect diversification modes that most likely led to increased insect abundance [36]. Second, wings are the insect body parts that are most easily preserved in compression fossils [37]. The vast majority of insect holotypes from the Palaeozoic are isolated wings [37], suggesting that it is unlikely that ambiguous remains of pterygote insects, such as cuticle or legs, would fossilize in the absence of wings—more unlikely still would be a scenario in which the cuticle or legs of pterygote insects, but not wings, would fossilize over time spans comparable to the Hexapod Gap.
The fossil record of arachnids and myriapods is variable during the Hexapod Gap (figure 3), but regardless of whether these groups are abundant or scarce within a given stage, insects are always absent. It appears that fossil insects are rare during the Hexapod Gap for three related reasons: arachnids and myriapods were the dominant terrestrial arthropods until the Pennsylvanian, winged insects had not yet diversified during the Hexapod Gap, and fewer terrestrial fossils have been collected from the Devonian and Mississippian than from the Pennsylvanian and Permian. Thus, the Hexapod Gap requires no special physiological or ecological explanation; it is precisely the type of gap that should be expected due to stochasticity in the preservation of rare taxa. The fossil record may accurately record the transformative impact of the evolution of insect flight.
This perspective, along with a recent reexamination of the oldest purported fossil insect [2], has the potential to resolve previous conflicts between the insect fossil record and molecular estimates of the origin of winged insects. Molecular methods have been used to estimate that winged insects originated slightly over 400 Ma [38], an age that corresponds to the fossil mandible that had been interpreted to represent a winged insect [39], but which has now been reinterpreted as representing a myriapod [2]. Though the interpretation presented here contradicts molecular estimates [38], it is not contradicted by any direct evidence in that there are no fossil wings known from the Hexapod Gap or from any preceding intervals.
Wings and holometaboly are the two innovations traditionally credited with facilitating insects' tremendous diversification, and the direct evidence for both of these originations extends back to the Carboniferous [36]. But unlike the presence of wings, holometaboly was not a prerequisite for insect abundance during the Palaeozoic, and in fact is not characteristic of most Palaeozoic insect occurrences. Palaeodictyopteroids—palaeopterous insects that are known not to have been holometabolous, because nymphs with wing buds are preserved [40]—account for approximately half of all described insect species from the Palaeozoic [41], thus demonstrating that wings alone are sufficient to facilitate a tremendous increase in insect diversity. Holometabolous insects were present during the Palaeozoic, but were relatively rare [41].
(c). The cause of Romer's Gap
When Romer first called attention to a gap in the tetrapod fossil record, it spanned the Tournaisian and Viséan stages, lasting roughly 30 Myr [42]. Subsequent discoveries of Viséan tetrapods shortened the gap from 30 to 15 Myr [43], and recent studies have greatly increased the number of tetrapods and tetrapod-bearing localities known from the Tournaisian Stage [7,16]. Two previously known localities from Romer's Gap were recently revealed to contain tetrapod fossils [16], demonstrating that evidence of tetrapods from this interval had previously been overlooked. These purported gaps appear to be similar in nature to the famed ‘three-metre gap’ of the latest Cretaceous, which initially appeared to separate the youngest dinosaur fossils from the impact clay later. This dinosaur gap was later filled with additional fossil material following increased collecting effort [44]. Thus, as with the insects—albeit for different reasons—low oxygen is no longer a plausible explanation of the early Mississippian fossil record of tetrapods, but it may be the case that no special explanation is needed.
(d). The importance of Phanerozoic pO2
In the light of the revised Phanerozoic oxygen curve presented here, other proposed associations between atmospheric oxygen and the history of animal life now appear to be similarly tenuous. For example, the extinction events at the ends of the Devonian, Permian and Triassic periods were previously believed to have been followed by intervals of low atmospheric oxygen, and the physiological constraints exerted by low oxygen were hypothesized to have led to the evolution of new body plans [45]. However, increased isotopic sampling yields curves that show far less severe drops in atmospheric oxygen levels during these intervals (figure 1). For example, low atmospheric oxygen levels during the Triassic were hypothesized to have exerted selective pressures that led to the origin of archosaur air-sacs [45], but all three revised oxygen curves presented here recovered higher oxygen levels than the previous model output (figure 1). The results presented here also contradict previous hypotheses that rising atmospheric oxygen levels over the past 205 Myr enabled the evolution and diversification of large placental mammals [46]. From animal extinction to diversification to physiology, the dramatically different oxygen curves presented here add to the case for reexamination of the importance of oxygen in the history of terrestrial animal life.
4. Material and methods
(a). The GEOCARBSULF model
Atmospheric oxygen was reconstructed with the GEOCARBSULFvolc model [8], which for convenience is referred to as GEOCARBSULF [47], using previously published code [47] implemented in the computing application R [48]. All figures were produced with the R package ggplot2 [49].
The isotope inputs for the model were updated based on recent compilations of isotope data (electronic supplementary material, figure S1). A single compilation exists for sulfur and for strontium [50]. For carbon, two compilations are available, one based on measurements from fossils [50] and one based on measurements from bulk-rock samples [51]. The two carbon isotope compilations, here called the ‘fossil samples’ and ‘bulk-rock samples’ datasets (see ‘Sensitivity tests’), were evaluated separately and were also combined into a single dataset, here called the ‘all samples’ dataset. For each time bin, the mean value of the updated isotope data from that interval was used as the model input. The GEOCARBSULF model was originally run with 10 Myr bins [10,47]. Residence times of strontium and carbon are far less than 10 Myr [52,53], but the residence time of sulfur is not [52], and so the original 10 Myr bin length was retained for the present study.
When the GEOCARB model was first implemented, the Phanerozoic was believed to have begun 570 Ma and so the model spanned the last 570 Myr [54,55]. However, the base of the Cambrian is now dated to 541 Myr before the present [56]. When later iterations of the GEOCARB model were published, it was known that the Phanerozoic spanned less than 570 Myr but the model was still run for the same length of time [57]. Here, the model is run from 570 Ma to the present—and therefore includes Neoproterozoic isotope data from 570 to 541 Ma—but results are only presented from the Ordovician onwards because the oldest sulfur isotope data are from the Mid-Cambrian and the applicability to the issues in this report begin in the Early Devonian.
5. Sensitivity tests
To test the effects of the residence time of sulfur on the model output, the model was run with two additional datasets. In the first dataset, the bins of sulfur data extended 5 Myr below the bin boundary, such that the bin centred on 500 Ma included all sulfur isotope values from the interval between 510 and 495 Ma. In the second dataset, the bins of sulfur data extended 10 Myr below the bin boundary, such that the bin centred on 500 Ma included all sulfur isotope values from the interval between 515 and 495 Ma. The decay function for marine sulfur is expected to be exponential but is not known, and so for the purposes of these two naive examples, no decay was assumed and so all isotope values were weighted equally. The model output was not notably different regardless of the sulfur residence time assumed (electronic supplementary material, figure S2), and so all results are presented here with the original 10 Myr bin length.
The current implementation of the GEOCARBSULF model [47] can be run on one of two sets of time arrays: the original time arrays [10] or the newer ‘Goddéris’ arrays [58]. With the two sets of time arrays, as with the three bin lengths for sulfur, the model output was not notably different depending on the time arrays used (electronic supplementary material, figure S3). All results presented here are based on the Goddéris time arrays.
The two compilations of carbon isotope data, ‘bulk-rock’ and ‘fossil,’ should theoretically reflect the same underlying changes in carbon isotope ratios through time. However, the two datasets are based on measurements from different sources. The ‘bulk-rock’ dataset has a comparatively high temporal resolution but greater potential to be influenced by diagenetic resetting, whereas the ‘fossil’ dataset has a comparatively low temporal resolution but is less likely to be biased by diagenetic effects, because fossils were selected for inclusion in the dataset based on quality of preservation.
The ‘bulk-rock’ dataset is based on measurements from carbonate rocks [51]. This dataset is a composite of measured sections from around the world. Each interval can be represented by as little as one section, so the spatial coverage of the dataset is, in some cases, low. This low spatial coverage may be problematic because carbon isotope values are known to vary throughout the ocean due to factors such as depth and latitude [51]. Another potential limitation of this dataset is that the amount of diagenetic alteration for each sample is unknown.
The ‘fossil’ dataset is based on measurements from fossil shells that are believed to have undergone minimal diagenetic alteration, primarily brachiopods and belemnites [50]. However, diagenetic alteration of fossils does not always leave clear petrographic or geochemical evidence (e.g. [59]), and so some fossils used for this dataset may have been altered diagenetically to a greater extent than originally expected. Furthermore, different species fractionate isotopes to different degrees (e.g. [60]); these ‘vital’ effects are more easily taken into account in smaller-scale studies than at Phanerozoic timescales spanning measurements of many species. Within each 10 Myr bin, the representation of smaller time intervals may be biased by factors such as change in sea level, which would cause overrepresentation of isotope measurements from warmer periods. For this dataset, stratigraphic resolution is low because the fossils were not collected bed-by-bed, so the temporal resolution of each measurement is known, at best, to the level of its biostratigraphic zone. However, because the data are lumped into 10 Myr bins for the present study, this relatively low temporal resolution is unlikely to cause any issues for the GEOCARBSULF model.
A generalized least-squares analysis was conducted on the mean value per 10 Myr bin from the two carbon isotope datasets, and shows that the two datasets are highly correlated (p < 0.001). However, because the two datasets produce notably different reconstructions of atmospheric oxygen across time, both are included in the results presented here.
To visualize each isotope's contribution to the changes in the model output, we ran the model with the original sulfur and strontium datasets and updated carbon, and vice versa (electronic supplementary material, figures S4 and S5).
6. Sedimentological and palaeontological data
To compare the amount of terrestrial and marine rock per interval, occurrences of terrestrial and marine rock units were downloaded from the Macrostrat database (macrostrat.org). Rock units that are entirely igneous and/or metamorphic were excluded. At present, the only Palaeozoic rock units included in Macrostrat are those from North America and the Caribbean. The midpoint of each unit was assigned to a geologic stage, and the total terrestrial and marine rock units were summed for each stage. The Wilcoxon–Matt–Whitney test, performed with the base-R function wilcox.test, was used to compare the number of available terrestrial sedimentary rock units between the hexapod gap and the subsequent Palaeozoic stages.
Insect, arachnid and myriapod occurrence data, and insect measurement data, were downloaded from the Paleobiology Database on 13 March 2017. A total of 5628 insect occurrences are known from the Palaeozoic, with associated measurement data for 4070 of these. An insect specimen was considered to be winged if at least one measurement from that specimen is of a wing, or of a homologous structure such as a nymphal wing pad, an elytron or a tegmen. An insect specimen was considered not to be ‘winged’ if at least one of its body parts was measured, but no wing measurements are available. Such specimens were not necessarily wingless in life, but were preserved without wings. The goal of this procedure is to evaluate the impact of wings on fossilization potential for each specimen, and so the focus here is on whether individual insects were preserved with wings, not whether they belong to pterygote lineages.
A bootstrapping procedure was used to evaluate the extent to which the Hexapod Gap (the Frasnian through Serpukhovian stages) resembles the previous interval that contains fossil insects (the Emsian through Givetian stages) and the subsequent interval that contains fossil insects (the Bashkirian Stage). Fifty-two terrestrial arthropod occurrences are known from the Hexapod Gap, and so 52 terrestrial arthropod occurrences were randomly sampled with replacement from the Emsian through Givetian stages (the interval that spans the occurrence of the oldest putative fossil insect through the lower bound of the Hexapod Gap) and from the Bashkirian Stage (the interval that immediately follows the Hexapod Gap). This procedure was repeated for 10 000 iterations. The fraction of iterations in which no insect occurrences were sampled is used here as a p-value that reflects whether these two intervals are significantly different from the Hexapod Gap.
As discussed below, the true affinities of putative fossil insects from the Devonian remain controversial; for the bootstrapping analysis, the Emsian through Givetian stages were assumed to contain two true fossil insects. We consider this to be the most conservative possible assumption. The latest Devonian fossil insect material, from Gilboa, New York [1], whose affinities have never been challenged, is considered here to represent a true insect, and we assume that one of the following also represents a true insect: either the bristletail described from the Devonian of Quebec [61], which was subsequently proposed to be a recent contaminant by authors who did not examine the original fossil material [62]; or Rhyniognatha hirsti Tillyard, 1928 from the Rhynie Chert, a taxon known primarily from its mandibles that was described as the oldest insect [39] but was subsequently reassigned to the Myriapoda upon further examination [2]. Here, Strudiella devonica Garrouste et al. [63], whose affinities have been challenged [64], is not considered to be an insect.
There has been some controversy regarding whether Palaeozoic scorpions were aquatic [65], but recent studies have concluded that they were terrestrial [66,67]. Only one scorpion, Eramoscorpius brucensis Waddington et al. [68] from the mid-Silurian, has been demonstrated to have been aquatic. This species is believed to represent a phase of scorpions' original transition from aquatic to terrestrial habitats [68]. Here, all scorpions except Eramoscorpius brucensis are considered to be terrestrial and are therefore included in all relevant figures and analyses, but are represented in a slightly darker colour in the arthropod occurrence figure.
Owing to recent updates to the geologic time scale, the original stages given for the insects on both sides of the Hexapod Gap have shifted in some cases [69]. Since the redescription of the earliest putative insect, Rhyniognatha hirsti from the Rhynie Chert [39], two dates have been proposed for this deposit. The spore assemblages beneath the Rhynie Chert have been dated to the early Pragian to early Emsian [70] and the andesite beneath the Rhynie Chert has been dated to 411 Ma [70,71], though the temporal relationship between the andesite and the chert is subject to controversy [72,73]. Here, Rhyniognatha hirsti is considered to be approximately 407 Myr in age. This corresponds to the earliest Emsian stage, which was used as a minimum constraint in a recent contribution [69]. The insect that marks the lower boundary of the Hexapod Gap, from Gilboa, New York, was originally assigned to the Givetian [1] and this assignment has not changed [9]. No absolute age has been assigned to this fossil, so here it is assumed to occur at the midpoint of the Givetian. This fossil insect from Gilboa is the only proposed fossil insect from the Devonian or Mississippian whose affinities have not subsequently been challenged [2], and so it is considered here to represent a definitive lower bound for the Hexapod Gap.
The range of Delitzschala bitterfeldensis Brauckmann & Schneider, 1996, the second-oldest known winged insect, was found to begin in the very latest Mississippian and end at the boundary between the Mississippian and Pennsylvanian [74], suggesting that the two oldest known winged insects occur very close to the Serpukhovian–Bashkirian boundary. This stratigraphic assignment is supported by recent radiometric dates [75]. In recent reexaminations of the early fossil record of insects, the oldest winged insect was assigned an earliest Pennsylvanian age of 318 Ma [9], corresponding to the Bashkirian stage and to a Mississippian age of approximately 325 Ma [76], corresponding to the Serpukhovian stage. Because of the uncertainty regarding whether the two oldest winged insects are Serpukhovian or Bashkirian, they are treated here as having occurred exactly on the boundary between these two stages at 323 Ma [77], and are not included in either bin for the analyses conducted here. This decision does not have any impact on the results of the analyses.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Matthew Clapham for his contributions of insect data to the Paleobiology Database and for discussion. We also thank Aviv Bachan, Daniel E. Ibarra, Tyler J. Kukla, C. Page Chamberlain, Noel Heim and Samantha R. Ritzer for discussion. Feedback from two anonymous reviewers improved this manuscript. This is Paleobiology Database contribution no. 300. This is contribution 325 of the Evolution of Terrestrial Ecosystems consortium at the National Museum of Natural History.
Data accessibility
All palaeontological occurrence data were downloaded from the Paleobiology Database, all isotope data come from previously published sources cited in the manuscript, and the model output underlying our figures are available as electronic supplementary material.
Authors' contributions
S.R.S., J.L.P. and C.K.B. conceived the study; M.R.S. and B.D.C. contributed isotope data; S.R.S. analysed data and drafted the initial version of the manuscript; all authors contributed to writing and editing the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
S.R.S. is funded by National Science Foundation Graduate Research Fellowship grant no. DGE-1125191.
References
- 1.Shear WA, Bonamo PM, Grierson JD, Rolfe WD, Smith EL, Norton RA. 1984. Early land animals in North America: evidence from Devonian age arthropods from Gilboa, New York. Science 224, 492–494. ( 10.1126/science.224.4648.492) [DOI] [PubMed] [Google Scholar]
- 2.Haug C, Haug JT. 2017. The presumed oldest flying insect: more likely a myriapod? PeerJ 5, e3402 ( 10.7717/peerj.3402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, et al. 2013. The earliest known holometabolous insects. Nature 503, 257–261. ( 10.1038/nature12629) [DOI] [PubMed] [Google Scholar]
- 4.Haug JT, Labandeira CC, Santiago-Blay JA, Haug C, Brown S. 2015. Life habits, hox genes, and affinities of a 311-million-year-old holometabolan larva. BMC Evol. Biol. 15, 208 ( 10.1186/s12862-015-0428-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauckmann C, Schneider J. 1996. Ein unter-karbonisches Insekt aus dem Raum Bitterfeld/Delitzsch (Pterygota, Arnsbergium, Deutschland). A Lower Carboniferous insect from the Bitterfeld/Delitzsch area (Pterygota, Arnsbegian, Germany). Neues Jahrb. Geol. Palaontol. Monatsh. 1996, 17–30. [Google Scholar]
- 6.Prokop J, Nel A, Hoch I. 2005. Discovery of the oldest known Pterygota in the Lower Carboniferous of the upper Silesian basin in the Czech Republic (Insecta: Archaeorthoptera). Geobios 38, 383–387. ( 10.1016/j.geobios.2003.11.006) [DOI] [Google Scholar]
- 7.Clack JA, et al. 2016. Phylogenetic and environmental context of a Tournaisian tetrapod fauna. Nat. Ecol. Evol. 1, 2 ( 10.1038/s41559-016-0002) [DOI] [PubMed] [Google Scholar]
- 8.Ward P, Labandeira C, Laurin M, Berner RA. 2006. Confirmation of Romer's gap as a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization. Proc. Natl Acad. Sci. USA 103, 16 818–16 822. ( 10.1073/pnas.0607824103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel MS, Davis SR, Prokop J. 2013. Insect wings: the evolutionary development of nature's first flyers. In Arthropod biology and evolution (eds Minelli A, Boxshall G, Fusco G), pp. 269–298. Berlin, Germany: Springer. [Google Scholar]
- 10.Berner RA. 2006. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664. ( 10.1016/j.gca.2005.11.032) [DOI] [Google Scholar]
- 11.Berner RA. 2009. Phanerozoic atmospheric oxygen: new results using the GEOCARBSULF model. Am. J. Sci. 309, 603–606. ( 10.2475/07.2009.03) [DOI] [Google Scholar]
- 12.Berner RA. 2006. Inclusion of the weathering of volcanic rocks in the GEOCARBSULF model. Am. J. Sci. 306, 295–302. ( 10.2475/05.2006.01) [DOI] [Google Scholar]
- 13.Belcher CM, McElwain JC. 2008. Limits for combustion in low O2 redefine paleoatmospheric predictions for the Mesozoic. Science 321, 1197–1200. ( 10.1126/science.1160978) [DOI] [PubMed] [Google Scholar]
- 14.Algeo TJ, Ingall E. 2007. Sedimentary Corg:P ratios, Paleocean ventilation, and Phanerozoic atmospheric pO2. Palaeogeogr. Palaeoclimatol. Palaeoecol. 256, 130–155. ( 10.1016/j.palaeo.2007.02.029) [DOI] [Google Scholar]
- 15.Glasspool IJ, Scott AC, Waltham D, Pronina N, Shao L. 2015. The impact of fire on the late Paleozoic earth system. Front. Plant Sci. 6, 1–13. ( 10.3389/fpls.2015.00756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smithson TR, Wood SP, Marshall JEA, Clack JA. 2012. Earliest Carboniferous tetrapod and arthropod faunas from Scotland populate Romer's Gap. Proc. Natl Acad. Sci. USA 109, 4532–4537. ( 10.1073/pnas.1117332109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace MW, Hood A vS, Shuster A, Greig A, Planavsky NJ, Reed CP. 2017. Oxygenation history of the Neoproterozoic to early Phanerozoic and the rise of land plants. Earth Planet. Sci. Lett. 466, 12–19. ( 10.1016/j.epsl.2017.02.046) [DOI] [Google Scholar]
- 18.Husson JM, Peters SE. 2017. Atmospheric oxygenation driven by unsteady growth of the continental sedimentary reservoir. Earth Planet. Sci. Lett. 460, 68–75. ( 10.1016/j.epsl.2016.12.012) [DOI] [Google Scholar]
- 19.Kaiser SI, Steuber T, Becker RT. 2008. Environmental change during the Late Famennian and Early Tournaisian (Late Devonian–Early Carboniferous): implications from stable isotopes and conodont biofacies in southern Europe. Geol. J. 43, 241–260. ( 10.1002/gj.1111) [DOI] [Google Scholar]
- 20.Lerner AJ, Mansky C, Lucas SG. 2013. A possible diplopod from the Lower Mississippian (Tournaisian) Horton Bluff Formation, Blue Beach, Nova Scotia, Canada. New Mex. Museum Nat. Hist. Sci. Bull. 60, 212–213. [Google Scholar]
- 21.Clapham ME, Karr JA. 2012. Environmental and biotic controls on the evolutionary history of insect body size. Proc. Natl Acad. Sci. USA 109, 10 927–10 930. ( 10.1073/pnas.1204026109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel S. 1994. Life in moving fluids: the physical biology of flow. Princeton, NJ: Princeton University Press. [Google Scholar]
- 23.Sperling EA, Frieder CA, Raman AV, Girguis PR, Levin LA, Knoll AH. 2013. Oxygen, ecology, and the Cambrian radiation of animals. Proc. Natl Acad. Sci. USA 110, 13 446–13 451. ( 10.1073/pnas.1312778110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodkinson ID. 2005. Terrestrial insects along elevation gradients: species and community responses to altitude. Biol. Rev. 80, 489–513. ( 10.1017/S1464793105006767) [DOI] [PubMed] [Google Scholar]
- 25.Dillon ME, Frazier MR, Dudley R. 2006. Into thin air: physiology and evolution of alpine insects. Integr. Comp. Biol. 46, 49–61. ( 10.1093/icb/icj007) [DOI] [PubMed] [Google Scholar]
- 26.Chown SL, Gaston KJ. 2010. Body size variation in insects: a macroecological perspective. Biol. Rev. 85, 139–169. ( 10.1111/j.1469-185X.2009.00097.x) [DOI] [PubMed] [Google Scholar]
- 27.Shelomi M. 2012. Where are we now? Bergmann's rule sensu lato in insects. Am. Nat. 180, 511–519. ( 10.1086/667595) [DOI] [PubMed] [Google Scholar]
- 28.Dunlop JA, Penney D, Jekel D. 2015. A summary list of fossil spiders and their relatives. In World spider catalog (ed. NI Platnick), p. 258 Bern, Switzerland: Natural History Museum Bern. [Google Scholar]
- 29.Dudley R. 1998. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J. Exp. Biol. 201, 1043–1050. [DOI] [PubMed] [Google Scholar]
- 30.Bergman NM, Lenton TM, Watson AJ. 2004. COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 304, 397–437. ( 10.2475/ajs.304.5.397) [DOI] [Google Scholar]
- 31.Carpenter FM. 1939. The Lower Permian insects of Kansas. Part 8: additional Megasecoptera, Protodonata, Odonata, Homoptera, Psocoptera, Protelytroptera, Plectoptera, and Protoperlaria. Proc. Am. Acad. Arts Sci. 73, 29–70. ( 10.2307/25130151) [DOI] [Google Scholar]
- 32.Alexander RM. 1971. Size and shape. London, UK: Edward Arnold. [Google Scholar]
- 33.May M. 1981. Allometric analysis of the body and wing dimensions of male Anisoptera. Odonatologica 10, 279–291. [Google Scholar]
- 34.Simonsen TJ, Kristensen NP. 2003. Scale length/wing length correlation in Lepidoptera (Insecta). J. Nat. Hist. 37, 673–679. ( 10.1080/00222930110096735) [DOI] [Google Scholar]
- 35.Kästner A. 1973. Lehrbuch der Speziellen Zoologie. Band I. Wirbellose. 3 Teil. Insecta. B. Speziellen Teil. Stuttgart, Germany: Gustav Fischer. [Google Scholar]
- 36.Nicholson DB, Ross AJ, Mayhew PJ. 2014. Fossil evidence for key innovations in the evolution of insect diversity. Proc. R. Soc. B 281, 20141823 ( 10.1098/rspb.2014.1823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karr JA, Clapham ME. 2015. Taphonomic biases in the insect fossil record: shifts in articulation over geologic time. Paleobiology 41, 16–32. ( 10.1017/pab.2014.3) [DOI] [Google Scholar]
- 38.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 39.Engel MS, Grimaldi DA. 2004. New light shed on the oldest insect. Nature 427, 627–630. ( 10.1038/nature02334.1.) [DOI] [PubMed] [Google Scholar]
- 40.Haug JT, Haug C, Garwood RJ. 2016. Evolution of insect wings and development - new details from Palaeozoic nymphs. Biol. Rev. 91, 53–69. ( 10.1111/brv.12159) [DOI] [PubMed] [Google Scholar]
- 41.Grimaldi D, Engel MS. 2005. Evolution of the insects. New York, NY: Cambridge University Press. [Google Scholar]
- 42.Romer AS. 1956. The early evolution of land vertebrates. Proc. Am. Philos. Soc. 100, 157–167. [Google Scholar]
- 43.Paton RL, Smithson TR, Clack JA. 1999. An amniote-like skeleton from the Early Carboniferous of Scotland. Nature 398, 508–513. ( 10.1038/19071) [DOI] [Google Scholar]
- 44.Sheehan PM, Fastovsky DE, Barreto C, Hoffmann RG. 2000. Dinosaur abundance was not declining in a ‘3 m gap’ at the top of the Hell Creek Formation, Montana and North Dakota. Geology 28, 523–526. ( 10.1130/0091-7613(2000)28%3C523:DAWNDI%3E2.0.CO;2) [DOI] [Google Scholar]
- 45.Berner RA, VandenBrooks JM, Ward PD. 2007. Oxygen and evolution. Science 316, 557–558. ( 10.1126/science.1140273) [DOI] [PubMed] [Google Scholar]
- 46.Falkowski P, Katz M, Milligan A, Fennel K, Cramer B, Aubry M, Berner R, Novacek MJ, Zapol W. 2005. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 309, 2202–2204. ( 10.1126/science.1116047) [DOI] [PubMed] [Google Scholar]
- 47.Royer DL, Donnadieu Y, Park J, Kowalczyk J, Goddéris Y. 2014. Error analysis of CO2 and O2 estimates from the long-term geochemical model GEOCARBSULF. Am. J. Sci. 314, 1259–1283. ( 10.2475/09.2014.01) [DOI] [Google Scholar]
- 48.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Berlin, Germany: Springer. [Google Scholar]
- 50.Prokoph A, Shields GA, Veizer J. 2008. Compilation and time-series analysis of a marine carbonate δ18O, δ13C, 87Sr/86Sr and δ34S database through Earth history. Earth-Science Rev. 87, 113–133. ( 10.1016/j.earscirev.2007.12.003) [DOI] [Google Scholar]
- 51.Saltzman MR, Thomas E. 2012. Carbon isotope stratigraphy. Geol. Time Scale 1–2, 207–232. ( 10.1016/B978-0-444-59425-9.00011-1) [DOI] [Google Scholar]
- 52.Walker JCG. 1986. Global geochemical cycles of carbon, sulfur and oxygen. Mar. Geol. 70, 159–174. ( 10.1016/0025-3227(86)90093-9) [DOI] [PubMed] [Google Scholar]
- 53.Veizer J. 1989. Strontium isotopes in seawater through time. Annu. Rev. Earth Planet. Sci. 17, 14167 ( 10.1146/annurev.ea.17.050189.001041) [DOI] [Google Scholar]
- 54.Berner RA. 1991. A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 291, 339–376. ( 10.2475/ajs.291.4.339) [DOI] [PubMed] [Google Scholar]
- 55.Berner RA. 1994. GEOCARB II: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 294, 56–91. ( 10.2475/ajs.294.1.56) [DOI] [Google Scholar]
- 56.Peng S, Babcock LE, Cooper RA. 2012. The Cambrian period. Geol. Time Scale 2, 437–488. ( 10.1016/B978-0-444-59425-9.00019-6) [DOI] [Google Scholar]
- 57.Berner RA, Kothavala Z. 2001. GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 301, 182–204. ( 10.2475/ajs.301.2.182) [DOI] [Google Scholar]
- 58.Goddéris Y, Donnadieu Y, Lefebvre V, Le Hir G, Nardin E. 2012. Tectonic control of continental weathering, atmospheric CO2, and climate over Phanerozoic times. Comptes Rendus Geosci. 344, 652–662. ( 10.1016/j.crte.2012.08.009) [DOI] [Google Scholar]
- 59.Banner JL, Kaufman J. 1994. The isotopic record of ocean chemistry and diagenesis preserved in non-luminescent brachiopods from Mississippian carbonate rocks, Illinois and Missouri. Geol. Soc. Am. Bull. 106, 1074–1082. ( 10.1130/0016-7606(1994)106%3C1074:TIROOC%3E2.3.CO;2) [DOI] [Google Scholar]
- 60.Erez J. 1978. Vital effect on stable-isotope composition seen in Foraminifera and coral skeletons. Nature 273, 199–202. ( 10.1038/273199a0) [DOI] [Google Scholar]
- 61.Labandeira CC, Beall BS, Hueber FM. 1988. Early insect diversification: evidence from a Lower Devonian bristletail from Quebec. Science 242, 913–916. ( 10.1126/science.242.4880.913) [DOI] [Google Scholar]
- 62.Jeram AJ, Selden PA, Edwards D. 1990. Land animals in the Silurian: arachnids and myriapods from Shropshire, England. Science 250, 658 ( 10.1126/science.250.4981.658) [DOI] [PubMed] [Google Scholar]
- 63.Garrouste R, et al. 2012. A complete insect from the Late Devonian period. Nature 488, 82–85. ( 10.1038/nature11281) [DOI] [PubMed] [Google Scholar]
- 64.Hörnschemeyer T, et al. 2013. Is Strudiella a Devonian insect? Nature 494, E3–E4. ( 10.1038/nature11887) [DOI] [PubMed] [Google Scholar]
- 65.Kjellesvig-Waering EN. 1986. A restudy of the fossil Scorpionida of the world. New York, NY: Paleontological Research Institution. [Google Scholar]
- 66.Scholtz G, Kamenz C. 2006. The book lungs of Scorpiones and Tetrapulmonata (Chelicerata, Arachnida): evidence for homology and a single terrestrialisation event of a common arachnid ancestor. Zoology 109, 2–13. ( 10.1016/j.zool.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 67.Kühl G, Bergmann A, Dunlop J, Garwood RJ, Rust J. 2012. Redescription and palaeobiology of Palaeoscorpius devonicus Lehmann, 1944 from the Lower Devonian Hunsrück Slate of Germany. Paleontology 55, 775–787. ( 10.1111/j.1475-4983.2012.01152.x) [DOI] [Google Scholar]
- 68.Waddington J, Rudkin DM, Dunlop JA. 2015. A new mid-Silurian aquatic scorpion: one step closer to land? Biol. Lett. 11, 201408 ( 10.1098/rsbl.2014.0815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfe JM, Daley AC, Legg DA, Edgecombe GD. 2016. Fossil calibrations for the arthropod Tree of Life. Earth-Sci. Rev. 160, 43–110. ( 10.1016/j.earscirev.2016.06.008) [DOI] [Google Scholar]
- 70.Parry SF, Noble SR, Crowley QG, Wellman CH. 2011. A high-precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications. J. Geol. Soc. 168, 863–872. ( 10.1144/0016-76492010-043) [DOI] [Google Scholar]
- 71.Parry SF, Noble SR, Crowley QG, Wellman CH. 2013. Reply to discussion on ‘a high-precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications' Journal, 168, 863–872. J. Geol. Soc. 170, 703–706. ( 10.1144/jgs2012-089) [DOI] [Google Scholar]
- 72.Mark DF, Rice CM, Fallick AE, Trewin NH, Lee MR, Boyce A, Lee JKW. 2011. 40 Ar/39 Ar dating of hydrothermal activity, biota and gold mineralization in the Rhynie hot-spring system, Aberdeenshire, Scotland. Geochim. Cosmochim. Acta 75, 555–569. ( 10.1016/j.gca.2010.10.014) [DOI] [Google Scholar]
- 73.Mark DF, Rice CM, Trewin NH. 2013. Discussion on ‘A high-precision U–Pb age constraint on the Rhynie Chert Konservat-Lagerstätte: time scale and other implications’ Journal, vol. 168, 863–872. J. Geol. Soc. 170, 701–703. ( 10.1144/jgs2011-110) [DOI] [Google Scholar]
- 74.Brauckmann C, Brauckmann B, Gröning E. 1996. The stratigraphical position of the oldest known Pterygota (Insecta. Carboniferous, Namurian). Ann. Soc. géol. Belg. 117, 47–56. [Google Scholar]
- 75.Pointon MA, Chew DM, Ovtcharova M, Sevastopulo GD, Crowley QG. 2012. New high-precision U–Pb dates from western European Carboniferous tuffs; implications for time scale calibration, the periodicity of late Carboniferous cycles and stratigraphical correlation. J. Geol. Soc. 169, 713–721. ( 10.1144/jgs2011-092) [DOI] [Google Scholar]
- 76.Prokop J, Pecharová M, Nel A, Hörnschemeyer T, Krzemińska E, Krzemiński W, Engel MS. 2017. Paleozoic nymphal wing pads support dual model of insect wing origins. Curr. Biol. 27, 263–269. ( 10.1016/j.cub.2016.11.021) [DOI] [PubMed] [Google Scholar]
- 77.Walker JD, Geissman JW, Bowring SA, Babcock LE. 2013. The Geological Society of America Geologic Time Scale. Geol. Soc. Am. Bull. 125, 259–272. ( 10.1130/B30712.1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All palaeontological occurrence data were downloaded from the Paleobiology Database, all isotope data come from previously published sources cited in the manuscript, and the model output underlying our figures are available as electronic supplementary material.