Abstract
The defence of a society often requires that some specialized members coordinate to repel a threat at personal risk. This is especially true for honey bee guards, which defend the hive and may sacrifice their lives upon stinging. Central to this cooperative defensive response is the sting alarm pheromone, which has isoamyl acetate (IAA) as its main component. Although this defensive behaviour has been well described, the neural mechanisms triggered by IAA to coordinate stinging have long remained unknown. Here we show that IAA upregulates brain levels of serotonin and dopamine, thereby increasing the likelihood of an individual bee to attack and sting. Pharmacological enhancement of the levels of both amines induces higher defensive responsiveness, while decreasing them via antagonists decreases stinging. Our results thus uncover the neural mechanism by which an alarm pheromone recruits individuals to attack and repel a threat, and suggest that the alarm pheromone of honey bees acts on their response threshold rather than as a direct trigger.
Keywords: honey bee, defence, alarm pheromone, serotonin, dopamine
1. Introduction
The evolutionary transition to social life is associated with significant benefits, such as increased resources thanks to division of labour and effective cooperative defence [1]. However, greater resources and grouping of individuals typically attract more predators and parasites [2]. At the extreme end of the sociality spectrum, eusocial insects evolved specialized defenders as an efficient way to cope with this dilemma, including soldier castes which protect the colony at personal risk [3,4]. Honey bees, one of the most remarkable cases of animal social evolution, are a perfect example of this situation. A specific caste, the guards, alerts and defends the colony by attacking any potential threat [5]. Defence may cost the bee its own life as death follows stinging when the sting tears loose from the abdomen [3]. To prevent unnecessary losses, defensive responses are under the control of an alarm pheromone released by alerted guards, which triggers coordinated attacks and stinging [6] only when potential threats become compelling [7].
The alarm pheromone of honey bees is chemically well characterized and includes isoamyl acetate (IAA) as a major component, which is sufficient to elicit most of the bees' defensive response [8–10]. However, the proximate causes underlying this remarkable altruistic behaviour remained relatively unexplored [5]. The lack of controlled protocols to elicit honey bee aggression in the laboratory has precluded advances in characterizing the neural mechanisms triggered by this alarm signal, but this deficit has been recently overcome thanks to the development of an arena-based assay that reliably elicits stinging responses in individual honey bees [11]. We took advantage of this methodology to examine the interplay between alarm pheromone, brain neurochemistry and the initiation of threat-response behaviour in bees. We focused on biogenic amines, which are small, ubiquitous molecules synthetized by the nervous system. Among the most important biogenic amines found in invertebrate brains are octopamine (OA), dopamine (DA) and serotonin (5HT). Their functions are extremely diverse, ranging from classical neurotransmitters to neuromodulators and neurohormones circulating both at the periphery and at the central level [12]. These molecules have a highly conserved function across animal phyla as regulators of aggressive behaviour [13–21] and are thus good candidates for modulating defensive responses in honey bees. We tested this hypothesis and report here that the main component of the alarm pheromone of the honey bee modifies the levels of 5HT and DA in the bee brain and increases, thereby, responsiveness to threat. We thus present the first mechanistic account of honey bee social defence.
2. Material and methods
(a). Honey bees
Four hives housing unrelated Italian honey bee colonies (Apis mellifera ligustica) were used in the experiments investigating brain biogenic amine levels. These colonies were located at the Queensland Brain Institute, Brisbane, Australia. Honey bees involved in colony defence were selectively collected using a black feather waived at the hive entrance. All bees were collected and handled (chilling anaesthesia followed by at least 15 min recovery with sugar, see [11]) in exactly the same way before being either sacrificed without testing or tested in the behavioural assay as described below.
Pharmacological experiments were performed at the University Paul Sabatier, Toulouse, France. The honey bees were collected at the hive entrance with a black feather as before. To ensure that our observations were not specific to a single genotype, two to three unrelated colonies participated equally in each experiment, with a total of five colonies used. The colonies participating in the antagonistic treatments were chosen for being quite reactive to potential threats while those participating in the agonistic treatments were known to be gentler. This was done to increase our chances of measuring the expected effect of the amines and their antagonists, and explains why the sting responsiveness of control groups differ between some of the experiments.
(b). Sting-responsiveness assay
The behavioural assay for testing sting responsiveness in honey bees has been described in detail previously [11]. In this assay, pairs of honey bees are confronted with a rotating dummy in a cylindrical arena, which they can choose to sting or not. The frequency at which at least one of the bees stung the dummy and the latency to sting (i.e. the time between the introduction of the bees in the arena and the occurrence of the first stinging response, if it happened) were recorded. Each pair of bees was exposed to either triethyl citrate (TEC, solvent, Sigma-Aldrich) or IAA (10% vol/vol, main active compound of the honey bee alarm pheromone, Sigma-Aldrich), carried through an airflow, during the whole length of the trial (3 min). Importantly, if a bee exhibited locomotor defects (clumsy walk or inability to hold upside down), the whole pair was excluded from further analysis. The pharmacological treatments did not affect the number of bees being excluded (χ2, p = 0.787). The sample sizes are 26 pairs of bees per group in experiments assaying colony defensiveness, and 32 pairs of bees per group in all pharmacological experiments.
For HPLC (high performance liquid chromatography) measurements, honey bees were classified into two groups according to their behaviour: ‘responsive’ bees that stung the dummy, and ‘non-responsive’ bees that did not. If both bees in the assay chamber displayed stinging behaviour, only the first responding bee was used for further analysis, because the second bee might have been responding to additional cues and signals from the first responder.
(c). Brain collection and dissection
Honey bees from all four colonies participated in the HPLC experiments. They were snap-frozen in liquid nitrogen either just before the start of the behavioural test (but after handling and recovery) for the bees allocated to the colony assessment, or quickly re-caught in a 50 ml Falcon tube after the behavioural test for the other bees. The Falcon tube was drilled with four holes (3–4 mm) at its base and two holes (1–2 mm) on its lid to ensure the quick flow of liquid nitrogen inside. The honey bees were then put on dry ice until the end of this day's trials and then stored at −80°C. The delay between the end of the trial and the bees being put on dry ice was 54.3 s on average thus ensuring only minimal changes in biogenic amine levels could occur after the end of the behavioural trial. Brains were then partially freeze-dried (55 min, 600 mTorr, −40°C) and dissected on dry ice. Intact brains were separated into three regions: the optic lobes, the sub-oesophageal zone and the remaining central brain, which contained all the structures involved in olfactory processing such as the antennal lobes, mushroom bodies and lateral horns. Incomplete or thawed brains were discarded. The final sample size was 27 bees per group in all HPLC experiments.
(d). Biogenic amine quantification
Biogenic amine levels were measured using HPLC at Macquarie University, Sydney, Australia. The HPLC system was an Agilent 1200 series (Agilent Technologies, Santa Clare, CA, USA) coupled to an electrochemical detector (ESA coulechem III) connected to a dual electrode analytical cell (ESA, Chelmsford, MA, USA). Just before analysis, samples were taken out of the −80°C freezer, slowly thawed on ice and sonicated in a solution of 0.2 mol l−1 perchloric acid and 10 pg µl−1 DHBA (internal standard). All brain regions were analysed individually. Central brains and optic lobes were extracted in 40 µl of this solution, while sub-oesophageal zones were extracted in 20 µl. After sonication, the samples were incubated for 20 min on ice in the dark, then centrifuged (14 min, 13.2 r.p.m., 0°C), and 18 µl of the supernatant of all samples were loaded in the autosampler, which then injected 10 µl. Seven-point standard curves of the external standards of OA, DA, tyramine and 5HT and the internal standard DHBA (all from Sigma-Aldrich) were included before and after each run. Each run consisted of 24 samples, representing all three brains regions from an individual in each group (four colonies and four individual-test groups). Biogenic amine amounts in each sample were calculated from the peak area for each biogenic amine normalized to the size of the DHBA peak within each sample, and quantified relative to the average of the two standard curves bracketing each run. Although a standard for tyramine was included, tyramine levels very seldom reached the detection threshold in our samples. Hence, the data for this amine could not be analysed. As honey bees vary very little in size and a previous study found no effects of age, source colony or task allocation on brain protein amounts, the data were not normalized [22].
(e). Pharmacological manipulations
Pharmacological treatments consisted of topical applications of 1 µl of solution on the thorax of the bees, 15–50 min before the aggression assay. Topical application on the thorax was chosen for being non-invasive and for yielding, nevertheless, a significant effect in terms of drug penetration [23]. Four sets of experiments were performed during which the bees were treated with 5HT hydrochloride, DA hydrochloride, cyproheptadine hydrochloride sesquihydrate (CYP, 5HT antagonist) or cis-(Z)-flupentixol dihydrochloride (FLU, DA antagonist) dissolved in dimethylformamide (dMF). Both antagonists have repeatedly proved to inhibit their respective aminergic signalling system in honey bees [24–26]. All chemicals were obtained from Sigma-Aldrich. Control bees received pure dMF, while treated bees received 0.2 mg ml−1, 2 mg ml−1 or 20 mg ml−1 of the active compounds. The highest concentration corresponds to 113 mM of 5HT, 130 mM of DA, 62 mM of CYP and 39 mM of FLU. Drug concentrations were chosen based on previous work on the modulation of aversive responsiveness in honey bees [25]. Both bees in a test pair received the same treatment.
(f). Statistical analysis
Behavioural results were analysed using a generalized linear model (GLM) set-up with a logit function appropriate for binomial data. The main effects were then computed using an ANOVA with a χ2-test. No interaction could be detected between the presence/absence of alarm pheromone and the pharmacological treatment in any of the four datasets collected after pharmacological manipulations (GLM, all p > 0.2), so we used an additive model for further pairwise testing. The HPLC data were first checked for the presence of outliers using the method described in Hoaglin & Iglewicz [27]. Among 1941 data points (from 215 bees), 17 outliers were removed, with a maximum of two in the same test group. To study the effect of the colony of origin, the results were analysed with Kruskal–Wallis tests. For the study at the individual level, the results were analysed with a two-way ANOVA taking as factors odour and behaviour. For displaying the overall effect of pharmacological treatments, the data were normalized with respect to the dMF-TEC control groups, and the two dMF-IAA groups averaged after normalization.
3. Results
(a). Biogenic amines in colony defensiveness
We first studied the relationship between the defensive response of a colony and biogenic amine levels in the brain of its workers (figure 1a). Colony defensiveness was assessed by measuring the frequency at which pairs of defensive bees, enclosed in a circular arena, stung a rotating dummy (n = 26 pairs of bees/group). This revealed significant differences in the stinging behaviour of workers from the four different source colonies (figure 1b, GLM, LRT(3) = 32.379, p < 0.001). Post hoc pairwise comparisons showed that bees from colonies 3 and 4 stung the dummy more often than bees from colonies 1 and 2 (t tests, all corrected p values < 0.01). Thus, colonies 3 and 4 can be described as highly defensive while colonies 1 and 2 were comparatively gentle. These results reflect accurately the obvious differences in general defensiveness of these colonies observed when collecting the bees or during routine beekeeping inspections.
Figure 1.
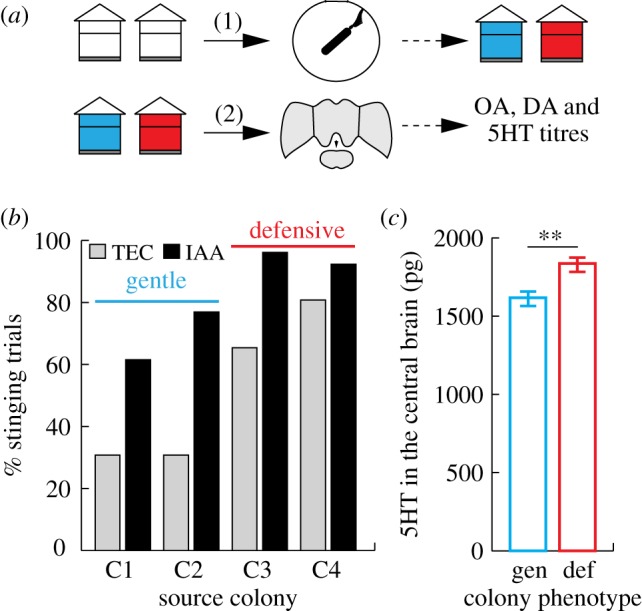
Correlation between colony-level behaviour and biogenic amine levels. (a) Experimental design: (1) colony-level threat responsiveness was assessed by testing bees in the arena assay. (2) Brains were collected from naive (untested) bees from these colonies and biogenic amine levels measured. (b) Percentage of trials in which bees stung, depending on the odour present (TEC, solvent; IAA, alarm pheromone) and the source colony, n = 26 pairs of bees/group. The difference between gentle and defensive colonies is significant (GLM + post hoc pairwise comparisons). (c) Mean ± s.e.m amount of 5HT measured in the central brain of bees from defensive (‘def’, C3 and C4) versus gentle (‘gen’, C1 and C2) colonies. Kruskal–Wallis, **p < 0.01, n = 54 bees/colony type. (Online version in colour.)
We then examined the biogenic amine levels in the brains of workers from these colonies. Defensive bees were again collected, and their brains were dissected into three regions for HPLC (figure 1a, n = 25–27 bees per colony after removal of outliers). OA and DA levels did not vary significantly between colonies in any of the brain regions (Kruskal–Wallis, p > 0.05 in all cases). However, differences in 5HT levels between source colonies were detected in the central brains and optic lobes of these bees (Kruskal–Wallis, central brain: H(3) = 15.218, p = 0.002, optic lobes: H(3) = 8.968, p = 0.035). When the data were analysed according to the classification established previously (defensive versus gentle colonies), no differences were detected in the optic lobes. However, 5HT levels in the central brain were significantly higher in bees from the defensive colonies (figure 1c; Kruskal–Wallis, H(1) = 9.251, p = 0.002). Although analyses on a higher number of colonies should confirm these results, this is the first hint that 5HT may play a role in the regulation of the defensive behaviour of honey bees.
(b). Biogenic amines in individual threat responses
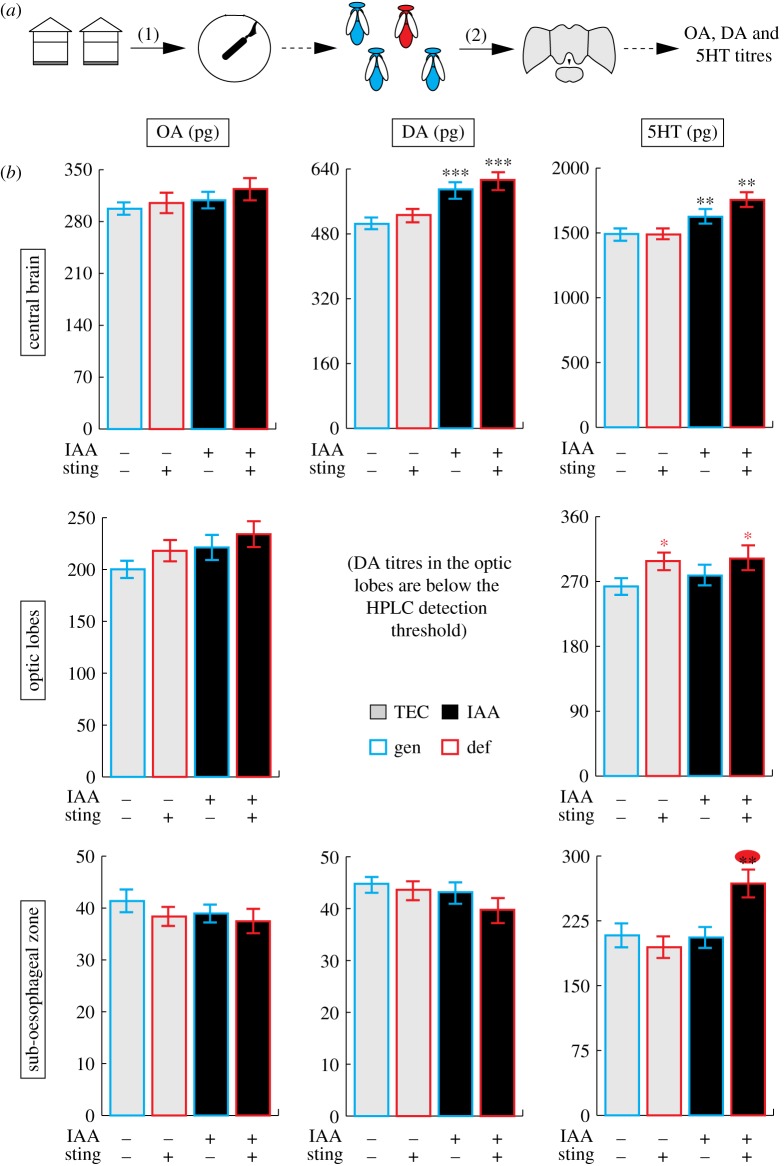
To verify the existence of a link between 5HT and threat responsiveness at the individual level, we tested bees in the arena, classified them according to their behaviour (responsive/stinging bees and non-responsive bees, which did not sting) and analysed the biogenic amine contents of their brains using HPLC. This was done within each of the two odour groups (bees exposed to TEC or to IAA), thus resulting in four groups in total for each of the brain structures considered (n = 25–27 bees per group after removal of outliers; figure 2a). Statistical results are summarized in table 1.
Figure 2.
Correlation between individual behaviour and biogenic amine levels. (a) Experimental design: (1) individual sting response was assessed by testing bees in the arena assay. (2) The brains of these bees were then collected and biogenic amine levels measured. (b) OA, DA and 5HT titres (mean ± s.e.m.) in different brain regions of bees as a function of their behaviour (sting+ or −) and the odour they were exposed to during the arena assay (IAA+ or −). GLM, *p < 0.05, **p < 0.01 and ***p < 0.001, the colour of the stars indicates the main effect represented—black: odour, red: behaviour, both: interaction, n = 25–27 samples/group. (Online version in colour.)
Table 1.
Summary of ANOVA analysis of the brain biogenic amines content of individually tested bees. *p < 0.05, **p < 0.01 and ***p < 0.001.
| region | amine | factor | d.f. | F | p-value |
|---|---|---|---|---|---|
| central brain | OA | odour | 1 | 0.596 | 0.442 |
| behaviour | 1 | 0.276 | 0.6 | ||
| od × beh | 1 | 0.228 | 0.634 | ||
| DA | odour | 1 | 18.932 | <0.001*** | |
| behaviour | 1 | 0.689 | 0.409 | ||
| od × beh | 1 | 0.077 | 0.783 | ||
| 5HT | odour | 1 | 16.529 | <0.001*** | |
| behaviour | 1 | 3.084 | 0.082 | ||
| od × beh | 1 | 0.162 | 0.688 | ||
| optic lobes | OA | odour | 1 | 0.333 | 0.565 |
| behaviour | 1 | 0.903 | 0.344 | ||
| od × beh | 1 | 0.525 | 0.47 | ||
| 5HT | odour | 1 | 0.274 | 0.602 | |
| behaviour | 1 | 5.616 | 0.020* | ||
| od × beh | 1 | 0.056 | 0.814 | ||
| sub-oesophageal zone | OA | odour | 1 | 0.882 | 0.35 |
| behaviour | 1 | 1.094 | 0.298 | ||
| od × beh | 1 | 0.057 | 0.812 | ||
| DA | odour | 1 | 1.55 | 0.216 | |
| behaviour | 1 | 1.076 | 0.302 | ||
| od × beh | 1 | 0.269 | 0.605 | ||
| 5HT | odour | 1 | 6.91 | 0.010* | |
| behaviour | 1 | 6.931 | 0.010* | ||
| od × beh | 1 | 10.616 | 0.002** |
In the central brain (figure 2b, upper row), OA levels did not vary significantly between the groups tested. However, there was a significant increase in the levels of DA and 5HT (p < 0.001 in both cases) following exposure to IAA, thus revealing for the first time that the major component of the alarm pheromone acts on neuromodulators in the bee brain. In the optic lobes (figure 2b, middle row), again OA levels did not show any significant variation between the groups tested. In this region, 5HT levels clearly reflected the likelihood that the bee would sting, as they were higher in responsive bees irrespective of the odour to which they were exposed (p < 0.05). DA levels were below the detection threshold of the HPLC and could not be analysed. Finally, in the sub-oesophageal zone (figure 2b, lower row), neither OA nor DA levels varied significantly according to any of the factors tested (table 1). Analysis of the 5HT dataset revealed a significant interaction between odour and behaviour (p < 0.01). This effect was driven by an increase in 5HT levels in the sub-oesophageal zone of bees that were exposed to IAA and stung. This result shows the robustness of the relationship between alarm pheromone exposure, elevated brain 5HT levels and increased sting response.
(c). High 5HT levels shorten the latency to sting
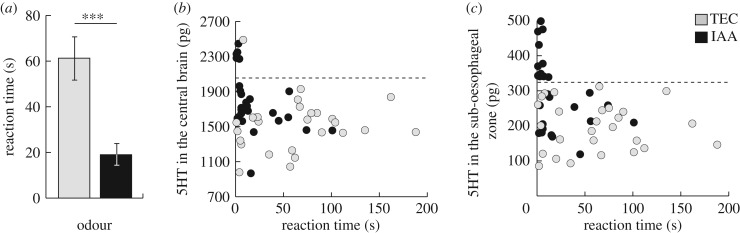
The stinging response defined in our bioassays is an all-or-none measure, which can be transformed into a more continuous variable for bees that stung by measuring the stinging latency. This variable, which has often been used in studies of honey bee-defensive behaviour [28,29], revealed that bees exposed to IAA stung much faster than control bees (figure 3a, Mann–Whitney, W(1) = 569.5, p < 0.001, n = 27 bees per condition). Plotting the 5HT content of the brain regions of each aggressive bee against its latency to sting revealed a striking pattern: within a certain range of 5HT amounts (figure 3b,c: up to 2000 pg in the central brain, and up to around 300 pg in the sub-oesophageal zone), there was no correlation between both variables (Spearman, central brain: ρ = −0.074, p = 0.595; sub-oesophageal zone: ρ = −0.070, p = 0.662). Above these thresholds, however, all bees reacted extremely quickly to the dummy, stinging it within the first 10 s. A similar although less obvious trend was found for 5HT in the optic lobes, but not for any other amine/brain region combinations (electronic supplementary material, figure S1). These results suggest again that high 5HT levels, such as those observed in the brain following exposure to IAA, are sufficient to induce elevated threat responsiveness in honey bees.
Figure 3.
Correlation between 5HT levels and latency to sting. (a) Latency to sting of IAA- or TEC-exposed threat-responsive bees. ***: Mann–Whitney, p < 0.001. (b) Latency to sting of each bee plotted against the amount of 5HT measured in its central brain. All bees with over approximately 2000 pg (dotted line) of 5HT reacted extremely quickly. (c) Latency to sting of each bee plotted against the amount of 5HT measured in its sub-oesophageal zone. All bees with over approximately 300 pg (dotted line) of 5HT reacted extremely quickly. n = 27 bees/odour group.
(d). Causal link between 5HT/DA levels in the bee brain and threat response
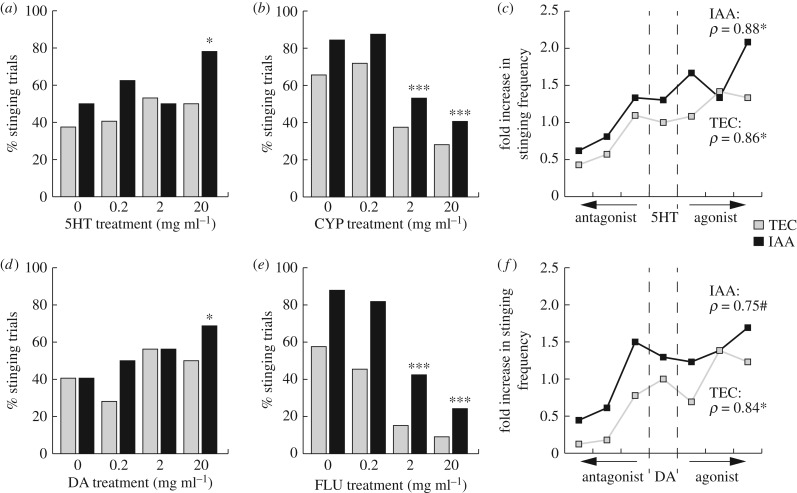
To demonstrate a causal link between sting responsiveness and 5HT and DA levels in the bee brain, we treated bees with a topical thoracic application of a solution containing one of four molecules at a range of concentrations: 5HT, CYP (5HT antagonist), DA or FLU (DA antagonist). These drugs were selected in order to downregulate (FLU, CYP) or upregulate (DA, 5HT) the levels of DA and 5HT present in the bee brain. After this treatment, bees were again tested in the arena assay, either in the presence of IAA or of the solvent TEC (n = 32 pairs of bees in all test groups).
The presence of IAA always induced higher stinging levels, except in the DA dataset where it was non-significant (figure 4; GLM, odour, 5HT: LRT(1) = 6.119, p = 0.013, DA: LRT(1) = 2.825, p = 0.093, CYP: LRT(1) = 8.066, p = 0.004, FLU: LRT(1) = 24.374, p < 0.001). The pharmacological treatment was also a significant factor influencing the bees' behaviour in all experiments except in the case of 5HT treatments (figure 4; GLM, treatment, 5HT: LRT(3) = 5.962, p = 0.113, DA: LRT(3) = 8.721, p = 0.033, CYP: LRT(3) = 41.799, p < 0.001, FLU: LRT(3) = 66.174, p < 0.001). Pairwise comparisons revealed, however, that bees treated with the highest concentration of 5HT stung more than the controls (figure 4a; GLM, 0 versus 20 mg ml−1, z = 2.313, p = 0.021). Similar results were obtained for the group of bees treated with the highest concentration of DA (figure 4d; GLM, 0 versus 20 mg ml−1, z = 2.12, p = 0.034).
Figure 4.
Pharmacological manipulations of 5HT and DA brain levels. Overall, IAA induced significantly more bees to sting, and there was no interaction between odour and pharmacological treatment (GLM). (a) Honey bee stinging likelihood after 5HT treatments. (b) Honey bee stinging likelihood after 5HT blockade through CYP treatments. (c) Overall effect of manipulating 5HT levels. (d) Honey bee stinging likelihood after DA treatments. (e) Honey bee stinging likelihood after DA blockade through FLU treatments. (f) Overall effect of manipulating DA levels. (a,b,d,e) GLM, *: treatment significant at threshold 0.05; ***: treatment significant at threshold 0.001. (c,f) Spearman ρ, *p < 0.05, #p = 0.066. n = 32 pairs of bees in all test groups.
By contrast, the two highest concentrations of both antagonists, CYP and FLU, significantly decreased honey bee stinging (figure 4b,e; GLM, z < −3, p < 0.001 for all four comparisons). Normalizing the controls and ordering the bee groups from the lowest to the highest expected brain level of 5HT (figure 4c) or DA (figure 4f) highlights the monotonic relationship between biogenic amine levels and sting reactivity (Spearman, 5HT: ρ = 0.86, p = 0.024 for bees exposed to TEC and ρ = 0.88, p = 0.015 for bees exposed to IAA, DA: ρ = 0.84, p = 0.024 for TEC-exposed groups and ρ = 0.75, p = 0.066 for IAA-exposed groups). Thus, manipulating the brain contents of 5HT or DA directly affects the stinging behaviour of honey bees: elevating them produces high sting responsiveness, while decreasing them reduces sting responsiveness.
4. Discussion
Our results provide the first neural account of social defence of a honey bee colony. IAA, the main component of the alarm pheromone, increases the likelihood of a stinging attack via an increase of 5HT, and to a lesser extent of DA, in the bee brain. Topical application of these amines confirmed that this increase enhances the stinging responsiveness of the bees. By contrast, antagonists of 5HT and DA receptors decrease stinging responses. Previous studies investigated the effect of IAA exposure on electric-shock responsiveness [30] and appetitive learning [31] while other studies looked at the effect of DA/5HT brain injections on the same behaviours [25,32]. Integrating these results into the framework of our findings reveals a consistent scenario: exposure to IAA modifies the bees’ behaviour in exactly the same way as DA and 5HT injections do, thus confirming the interactions between alarm pheromone, biogenic amines and behaviour found in our work.
Our data underline the importance of 5HT for the social regulation of colony defence in bees. This biogenic amine is known to play a central role in regulating aggression levels in many species of both mammals [18,21,33] and arthropods [15,17,19,34,35]. We show that in bees, IAA increases brain levels of 5HT to the point where workers are very likely to attack and sting. We hypothesize, therefore, that in the evolution of colony defence, 5HT systems regulating individual aggression have become activated by a social signal, the sting alarm pheromone. The result of this process is both a social coordination of the defence response, and a strong motivation of some individuals to attack a threat to the colony despite the risk to their own life.
We also found that DA levels in the central brain were increased after exposure to the alarm pheromone. Populations of dopaminergic neurons have been postulated as a gain control system, generally suppressing responsiveness to a large variety of stimuli [25]. In doing so, such a network could allow selective attention processes to take place [36], focusing the attack on the noxious target. Furthermore, in fruit flies single dopaminergic neurons modulating aggression have been identified, with putative pre-synaptic targets in the central complex [14]. This structure, which was included in our broad ‘central brain’ region, is also strongly innervated by dopaminergic fibres in honey bees [37], hence it would be a good candidate for further studies of the neural substrates underlying honey bee aggression.
OA was not involved in aggression in our study. This observation is surprising given that OA plays a crucial role during fighting in male fruit flies [14,38] and termites [39]. Yet, it is consistent with results obtained in ants where it does not account for aggressive behaviour [40,41]. Thus, this likely reflects differences between these species in terms of OA involvement in aggressive interactions. Indeed, in honey bees, OA has been repeatedly shown to signal appetitive experiences with no relation to aversive or aggressive contexts [42,43].
While our work did not identify the exact cells or networks implicated in the regulation of aggressive responses, our HPLC results do allow for some spatial dissection of the underlying circuitry. In the optic lobes, 5HT levels were higher in all stinging bees and, as could be expected, the odour context had no influence. Injections of 5HT in the optic lobes reduce spontaneous neural activity and the specificity of motion-sensitive neurons [44], suggesting a possible function of this amine in the detection of moving targets. IAA increased DA and 5HT levels in the central brain, which is consistent with the fact that this region contained all the structures involved in olfactory processing (antennal lobes, lateral horns and mushroom bodies). Finally, 5HT levels in the sub-oesophageal zone were specifically higher in threat-responsive bees triggered by IAA. It is interesting to note that the primary olfactory centres, the antennal lobes, are innervated by a single serotoninergic interneuron, the deutocerebral giant cell [45]. This neuron connects the antennal lobes, the lateral protocerebrum (which is believed to play an important role in linking odorants to their intrinsic biological values [46,47]) and the sub-oesophageal zone before descending along the nerve cord, possibly towards motor centres. Future studies could focus on the role of this neuron in honey bee threat responses and stinging attacks. Furthermore, it would be interesting to study the link between biogenic amines and other aggression-regulating mechanisms triggered by the alarm pheromone, such as altered gene expression [48] and metabolism [49] in the brain.
We, recently, proposed a new model for the decision-making process underlying the honey bee-defensive response, in which a defensive score resulting from the integration of the surrounding stimuli is weighted against an internal threshold to determine if the bee engages in colony defence [11]. The present study improves this model by indicating that 5HT and DA levels may set this threshold for responsiveness in honey bees (figure 5). Furthermore, because exposure to IAA also increases the amount of 5HT and DA in the central brain (figure 2b), we propose that the alarm pheromone may not operate as a stimulus itself, but rather changes the likelihood that a bee initiates a stinging attack. Reinterpreting the action of the alarm pheromone from a signal of danger to a fine regulator of honey bee threat responsiveness is an important conceptual change, and may account for the variability in responsiveness to IAA observed across time, nest-mates and colonies [5]. This view sheds new light on previous studies of honey bee aggression and is fundamental for future attempts at understanding and managing this conspicuous behaviour.
Figure 5.
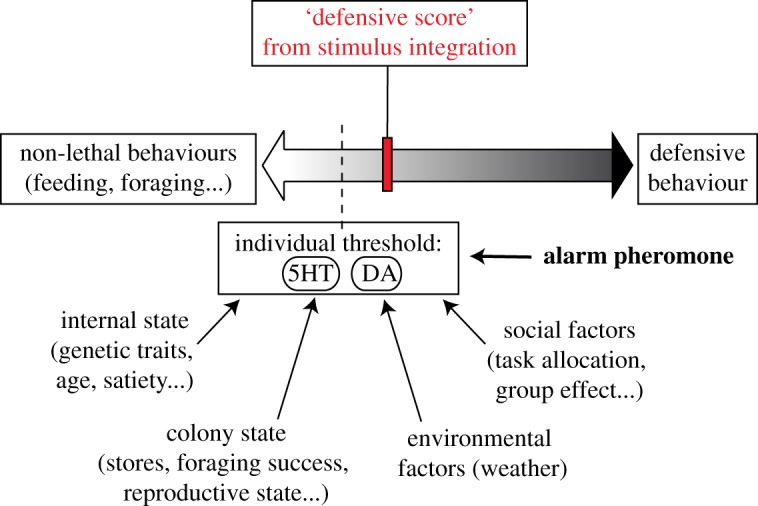
Refined model for the decision-making process underlying honey bee aggression. Adapted from Nouvian et al. [11]. This model postulates that the honey bee brain computes a defensive score from stimuli integration, which is compared to an individual threshold in order to determine the behavioural outcome. In agreement with our new results, this threshold may be represented by the levels of 5HT and DA in the bee brain. Furthermore, we suggest that the alarm pheromone is not integrated as a stimulus, but rather modifies this individual threshold by increasing the levels of these biogenic amines. (Online version in colour.)
Supplementary Material
Acknowledgements
We thank our beekeepers, Trevor Weatherhead, Peter Anderson and Lucie Hotier for their technical support.
Ethics
All honey bees were handled with care and cold-euthanized.
Data accessibility
The raw data (excel files, including full statistical tables) are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.rj10c [50]. Videos of the behavioural tests, too voluminous for the online repository, are available upon request to M.N.
Authors' contributions
M.N., J.R., M.G., A.B.B. and C.C. designed the study. C.J. did some pilot experiments for the pharmacological manipulation and S.M. performed the antagonistic pharmacological experiments. M.N. did all other experiments, analysed the data and wrote the original draft. J.R., P.d.E., M.G., A.B.B. and M.N. edited the paper.
Competing interests
The authors have no competing interests.
Funding
This work was supported by grants from the Australian Research Council (ARC DP120102301 to J.R. and C.C.) and from the French National Research Agency (ANR, Grant Pheromod to P.d.E. and M.G.). C.C. was supported by an ARC Future Fellowship (ARC FT110100292) and M.G. by the Institut Universitaire de France (IUF), the French Research Council (CNRS) and the University Paul Sabatier (UPS) of Toulouse. M.N. was supported by funding from the UPS for binational PhD programmes and by the GSITA of the University of Queensland.
References
- 1.Robinson GE. 1992. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665. ( 10.1146/annurev.en.37.010192.003225) [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel P. 2017. Parasites and their social hosts. Trends Parasitol. 33, 453–462. ( 10.1016/j.pt.2017.01.003) [DOI] [PubMed] [Google Scholar]
- 3.Hermann HR. 1971. Sting autotomy, a defensive mechanism in certain social hymenoptera. Insectes Soc. 2, 111–120. ( 10.1007/BF02223116) [DOI] [Google Scholar]
- 4.Boomsma JJ. 2013. Beyond promiscuity: mate-choice commitments in social breeding. Phil. Trans. R. Soc. B 368, 20120050 ( 10.1098/rstb.2012.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouvian M, Reinhard J, Giurfa M. 2016. The defensive response of the honey bee Apis mellifera. J. Exp. Biol. 219, 3505–3517. ( 10.1242/jeb.143016) [DOI] [PubMed] [Google Scholar]
- 6.Millor J, Pham-Delegue M, Deneubourg JL, Camazine S. 1999. Self-organized defensive behavior in honey bees. Proc. Natl Acad. Sci. USA 96, 12 611–12 615. ( 10.1073/pnas.96.22.12611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Free JB, Simpson J. 1968. The alerting pheromones of the honey bee. Z. vergl. Physiologie 61, 361–365. ( 10.1007/BF00428008) [DOI] [Google Scholar]
- 8.Boch R, Shearer DA, Stone BC. 1962. Identification of isoamyl acetate as an active component in the sting pheromone of the honey bee. Nature 195, 1018–1020. ( 10.1038/1951018b0) [DOI] [PubMed] [Google Scholar]
- 9.Collins AM, Blum SM. 1982. Bioassay of compounds derived from the honey bee sting. J. Chem. Ecol. 8, 463–469. ( 10.1007/BF00987794) [DOI] [PubMed] [Google Scholar]
- 10.Grandperrin D, Cassier P. 1983. Anatomy and ultrastructure of the Koschewnikow's gland of the honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int. J. Insect Morphol. Embryol. 12, 25–42. ( 10.1016/0020-7322(83)90033-8) [DOI] [Google Scholar]
- 11.Nouvian M, Hotier L, Claudianos C, Giurfa M, Reinhard J. 2015. Appetitive floral odours prevent aggression in honey bees. Nat. Commun. 6, 10247 ( 10.1038/ncomms10247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libersat F, Pflueger H-J. 2004. Monoamines and the orchestration of behavior. Bioscience 54, 17–25. ( 10.1641/0006-3568(2004)054%5B0017:MATOOB%5D2.0.CO) [DOI] [Google Scholar]
- 13.Adamo SA, Linn CE, Hoy RR. 1995. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691–1700. [DOI] [PubMed] [Google Scholar]
- 14.Alekseyenko OV, Chan YB, Li R, Kravitz EA. 2013. Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl Acad. Sci. USA 110, 6151–6156. ( 10.1073/pnas.1303446110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dierick HA, Greenspan RJ. 2007. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 39, 678–682. ( 10.1038/ng2029) [DOI] [PubMed] [Google Scholar]
- 16.Jones TC, Akoury TS, Hauser CK, Neblett MF 2nd, Linville BJ, Edge AA, Weber NO. 2011. Octopamine and serotonin have opposite effects on antipredator behavior in the orb-weaving spider, Larinioides cornutus. J. Comp. Physiol. A 197, 819–825. ( 10.1007/s00359-011-0644-7) [DOI] [PubMed] [Google Scholar]
- 17.Kravitz EA, Huber R. 2003. Aggression in invertebrates. Curr. Opin. Neurobiol. 13, 736–743. ( 10.1016/j.conb.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP, Merschdorf U. 2000. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav. Sci. Law 18, 581–604. ( 10.1002/1099-0798(200010)18:5%3C581::AID-BSL411%3E3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- 19.Pedetta S, Kaczer L, Maldonado H. 2010. Individual aggressiveness in the crab Chasmagnathus: influence in fight outcome and modulation by serotonin and octopamine. Physiol. Behav. 101, 438–445. ( 10.1016/j.physbeh.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Rao Y, Rao Y. 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1067. ( 10.1038/nn.2164) [DOI] [PubMed] [Google Scholar]
- 21.Niederkofler V, Asher TE, Okaty BW, Rood BD, Narayan A, Hwa LS, Beck SG, Miczek KA, Dymecki SM. 2016. Identification of serotonergic neuronal modules that affect aggressive behavior. Cell Rep 17, 1934–1949. ( 10.1016/j.celrep.2016.10.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz DJ, Robinson GE. 1999. Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 184, 481–488. ( 10.1007/s003590050348) [DOI] [PubMed] [Google Scholar]
- 23.Barron AB, Maleszka J, Vander Meer RK, Robinson GE, Maleszka R. 2007. Comparing injection, feeding and topical application methods for treatment of honey bees with octopamine. J. Insect. Physiol. 53, 187–194. ( 10.1016/j.jinsphys.2006.11.009) [DOI] [PubMed] [Google Scholar]
- 24.Howarth CJ, Prince RI, Dyker H, Lösel PM, Seinsche A, Osborne RH. 2002. Pharmacological characterisation of 5-hydroxytryptamine-induced contractile effects in the isolated gut of the lepidopteran caterpillar Spodoptera frugiperda. J. Insect. Physiol. 48, 43–52. ( 10.1016/s0022-1910(01)00142-1) [DOI] [PubMed] [Google Scholar]
- 25.Tedjakumala SR, Aimable M, Giurfa M. 2014. Pharmacological modulation of aversive responsiveness in honey bees. Front. Behav. Neurosci. 7, 221 ( 10.3389/fnbeh.2013.00221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blenau W, Erber J, Baumann A. 1998. Characterization of a dopamine D1 receptor from Apis mellifera: cloning, functional expression, pharmacology, and mRNA localization in the brain. J. Neurochem. 70, 15–23. ( 10.1046/j.1471-4159.1998.70010015.x) [DOI] [PubMed] [Google Scholar]
- 27.Hoaglin DC, Iglewicz B. 1987. Fine-tuning some resistant rules for outlier labeling. JASA 82, 1147–1149. ( 10.2307/2289392) [DOI] [Google Scholar]
- 28.Guzmán-Novoa E, Prieto-Merlos D, Uribe-Rubio JL, Hunt GJ. 2003. Relative reliability of four field assays to test defensive behaviour of honey bees (Apis mellifera). J. Apic. Res. 42, 42–46. ( 10.1080/00218839.2003.11101088) [DOI] [Google Scholar]
- 29.Kolmes SA, Fergusson-Kolmes LA. 1989. Measurements of stinging behavior in individual worker honey bees (Apis mellifera L). J. Apic. Res. 28, 71–78. ( 10.1080/00218839.1989.11100824) [DOI] [Google Scholar]
- 30.Núñez J, Maldonado H, Miralto A, Balderrama N. 1983. The stinging response of the honey bee: effects of morphine, naloxone and some opiod peptides. Pharmacol. Biochem. Behav. 19, 921–924. ( 10.1016/0091-3057(83)90391-X) [DOI] [PubMed] [Google Scholar]
- 31.Urlacher E, Frances B, Giurfa M, Devaud JM. 2010. An alarm pheromone modulates appetitive olfactory learning in the honey bee (Apis mellifera). Front. Behav. Neurosci. 4, 157 ( 10.3389/fnbeh.2010.00157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercer AR, Menzel R. 1982. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honey bee Apis mellifera. J. Comp. Physiol. A 145, 363–368. ( 10.1007/BF00619340) [DOI] [Google Scholar]
- 33.Miczek KA, Fish EW, De Bold JF, De Almeida RM. 2002. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology 163, 434–458. ( 10.1007/s00213-002-1139-6) [DOI] [PubMed] [Google Scholar]
- 34.Dyakonova VE, Schurmann F, Sakharov DA. 1999. Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 86, 435–437. ( 10.1007/s001140050647) [DOI] [PubMed] [Google Scholar]
- 35.Edwards DH, Kravitz EA. 1997. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 7, 812–819. ( 10.1016/S0959-4388(97)80140-7) [DOI] [PubMed] [Google Scholar]
- 36.Van Swinderen B, Andretic R. 2011. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc. R. Soc. B 278, 906–913. ( 10.1098/rspb.2010.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schurmann FW, Elekes K, Geffard M. 1989. Dopamine-like immunoreactivity in the bee brain. Cell Tissue Res. 256, 399–410. ( 10.1007/BF00218898) [DOI] [Google Scholar]
- 38.Dierick HA. 2008. Fly fighting: octopamine modulates aggression. Curr. Biol. 18, R161–R163. ( 10.1016/j.cub.2007.12.026) [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa Y, Aonuma H, Sasaki K, Miura T. 2016. Tyraminergic and octopaminergic modulation of defensive behavior in termite soldier. PLoS ONE 11, e0154230 ( 10.1371/journal.pone.0154230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohkawara K, Aonuma H. 2016. Changes in the levels of biogenic amines associated with aggressive behavior of queen in the social parasite ant Vollenhovia nipponica. Insectes Soc. 63, 257–264. ( 10.1007/s00040-016-0461-7) [DOI] [Google Scholar]
- 41.Szczuka A, Korczynska J, Wnuk A, Symonowicz B, Gonzalez Szwacka A, Mazurkiewicz P, Kostowski W, Godzinska EJ. 2013. The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta. Neurobiol. Exp. 73, 495–520. [DOI] [PubMed] [Google Scholar]
- 42.Giurfa M, Sandoz JC. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honey bees. Learn Memory 19, 54–66. ( 10.1101/lm.024711.111) [DOI] [PubMed] [Google Scholar]
- 43.Hammer M, Menzel R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honey bees. Learn Memory 5, 146–156. [PMC free article] [PubMed] [Google Scholar]
- 44.Scheiner R, Baumann A, Blenau W. 2006. Aminergic control and modulation of honey bee behaviour. Curr. Neuropharmacol. 4, 259–276. ( 10.2174/157015906778520791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bicker G. 1999. Biogenic amines in the brain of the honey bee: cellular distribution, development, and behavioral functions. Microsc. Res. Tech. 44, 166–178. ( 10.1002/(SICI)1097-0029(19990115/01)44:2/3%3C166::AID-JEMT8%3E3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- 46.Galizia CG. 2014. Olfactory coding in the insect brain: data and conjectures. Eur. J. Neurosci. 39, 1784–1795. ( 10.1111/ejn.12558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roussel E, Carcaud J, Combe M, Giurfa M, Sandoz JC. 2014. Olfactory coding in the honey bee lateral horn. Curr. Biol. 24, 561–567. ( 10.1016/j.cub.2014.01.063) [DOI] [PubMed] [Google Scholar]
- 48.Alaux C, et al. 2009. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405. ( 10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandrasekaran S, Rittschof CC, Djukovic D, Gu H, Raftery D, Price ND, Robinson GE. 2015. Aggression is associated with aerobic glycolysis in the honey bee brain. Genes Brain Behav. 14, 158–166. ( 10.1111/gbb.12201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nouvian M, Mandal S, Jamme C, Claudianos C, d'Ettorre P, Reinhard J, Barron AB, Giurfa M. 2018. Data from: Cooperative defence operates by social modulation of biogenic amine levels in the honey bee brain Dryad Digital Repository. ( 10.5061/dryad.rj10c) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nouvian M, Mandal S, Jamme C, Claudianos C, d'Ettorre P, Reinhard J, Barron AB, Giurfa M. 2018. Data from: Cooperative defence operates by social modulation of biogenic amine levels in the honey bee brain Dryad Digital Repository. ( 10.5061/dryad.rj10c) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The raw data (excel files, including full statistical tables) are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.rj10c [50]. Videos of the behavioural tests, too voluminous for the online repository, are available upon request to M.N.