Abstract
Reproductive success relies on the coordination of social behaviours, such as territory defence, courtship and mating. Species with extreme variation in reproductive tactics are useful models for identifying the neural mechanisms underlying social behaviour plasticity. The plainfin midshipman (Porichthys notatus) is a teleost fish with two male reproductive morphs that follow widely divergent developmental trajectories and display alternative reproductive tactics (ARTs). Type I males defend territories, court females and provide paternal care, but will resort to cuckoldry if they cannot maintain a territory. Type II males reproduce only through cuckoldry. We sought to disentangle gene expression patterns underlying behavioural tactic, in this case ARTs, from those solely reflective of developmental morph. Using RNA-sequencing, we investigated differential transcript expression in the preoptic area-anterior hypothalamus (POA-AH) of courting type I males, cuckolding type I males and cuckolding type II males. Unexpectedly, POA-AH differential expression was more strongly coupled to behavioural tactic than morph. This included a suite of transcripts implicated in hormonal regulation of vertebrate social behaviour. Our results reveal that divergent expression patterns in a conserved neuroendocrine centre known to regulate social-reproductive behaviours across vertebrate lineages may be uncoupled from developmental history to enable plasticity in the performance of reproductive tactics.
Keywords: transcriptome, courtship, cuckoldry, social plasticity, alternative reproductive tactics
1. Introduction
Plasticity in social behaviour is crucial for survival and reproduction. To maximize reproductive success, individuals must make decisions such as investing in defending a territory, courting mates, waiting until more resources are accessible [1,2], or by taking any fitness opportunity available if unable to defend a territory or attract mates [3–6]. Species that engage in alternative reproductive tactics (ARTs) are excellent models for studying behavioural plasticity because they provide examples of extreme intrasexual divergence in reproductive behaviour. ARTs are discontinuous variations in reproductive behaviour within a sex, such as alternate morphs [4,6–8]. Furthermore, reproductive tactics or morphs may be fixed during development or remain plastic throughout an individual's life [9]. Though ARTs are relatively common among teleost fishes [7,10], they are also observed among other vertebrate taxa [11–15].
The plainfin midshipman (Porichthys notatus) is a teleost with two developmentally fixed male reproductive morphs that dramatically diverge in a large suite of behavioural and morpho-physiological traits, often with type II males appearing feminized in relation to type I's (electronic supplementary material, table S1). Type I males excavate and defend nests, acoustically court females with long duration advertisement calls known as hums, and provide paternal care [16]. Type II males reach sexual maturity earlier [17] and do not engage in these behaviours, but instead reproduce through cuckoldry by stealing fertilizations from courting type I males [16]. Reflecting differences in reproductive tactic, type I males invest in large vocal muscle mass, while type II males invest significantly more in gonad size [16]. Though neither type II males nor females hum, they both make brief agonistic grunts [16]. Type I and II males also have distinctly different steroid hormone profiles; 11-ketotestosterone (11-KT) and testosterone are the predominant androgens in type I and II males, respectively [18]. Type II males, along with females, also diverge from type I males in the dimensions of vocal neuron (somata, axons, dendrites, neuromuscular junctions) and muscle (total mass, myofibril ultrastructure) traits, and vocal neurophysiology [19]. The two morphs also differ in neuropeptide and steroid modulation of vocal circuit excitability [20–22]: arginine-vasotocin (arginine-vasopressin homologue) and 11-KT are effective in type I males, while isotocin (oxytocin homologue) and testosterone are effective in type II males and females. Unlike type II males that are fixed in their cuckoldry tactic [6,16,23,24], adult type I males that are unable to maintain a nest of their own may resort to cuckoldry [6,24]. Here, we take advantage of this plasticity to investigate patterns in brain gene expression linked to ARTs within a single sex that are influenced by developmental history (morph) or behavioural context (courtship versus cuckoldry). Identifying differentially expressed (DE) transcripts across alternative male morphs and behavioural tactics may also explain how developmental and behavioural decisions are regulated.
Previous brain transcriptomic studies of fishes with ARTs have yielded varied results, potentially owing to variation in reproductive tactics across species, although some trends have emerged [25–32]. In particular, transcripts related to hormone signalling commonly exhibit differential expression patterns. However, these studies are often limited by their use of whole-brain RNA, which prevents drawing conclusions about gene expression in specific brain regions that are important for social behaviour [33]. Studies of species with other forms of reproductive plasticity that investigate expression changes in specific regions focus on candidate genes rather than global gene expression, and may have missed genes of functional importance [34–36].
We took a targeted approach, using RNA-sequencing (RNAseq) to examine transcript expression in one brain region, the preoptic area-anterior hypothalamus (POA-AH), a key node for neuro-hormonal integration and regulation of multiple social behaviours in fishes and other vertebrates [37,38], including reproduction [39–41], aggression [42] and parental care [43,44]. The teleost POA-AH contains homologues of the anterior hypothalamus in tetrapods (hence the designation POA-AH) [45–47], including neurons that produce neuropeptides implicated in the regulation of social behaviour, such as arginine-vasotocin and isotocin [48–51]. The majority of neuroendocrine studies of fishes with ARTs focus on nonapeptides, particularly arginine-vasotocin and gonadal steroids [9,50]. We sought to identify differential expression of transcripts related to these, as well as other hormones or neuromodulators that have not been as well studied in the context of ARTs.
To separate expression patterns linked to behavioural tactic from those reflective of developmentally fixed morph, we compared expression differences in the POA-AH of courting type I, cuckolding type I and cuckolding type II male midshipman. Given the large suite of male morph-specific traits (electronic supplementary material, table S1), including differences in the POA-AH [20,52], we expected POA-AH gene expression to be more strongly linked to morph phenotype; however, our results demonstrate that behavioural tactic is a stronger driver of differential expression. Furthermore, we identified a suite of DE transcripts that are emerging as key regulators of vertebrate social behaviour.
2. Material and methods
(a). Animal subjects and behavioural design
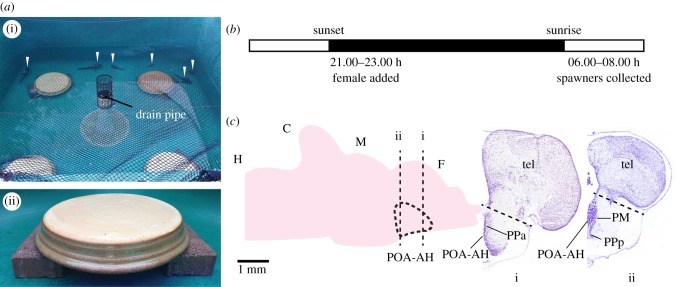
We followed previously established methods for promoting cuckoldry in type I male midshipman [6,24]. Briefly, adult midshipman were collected from nests in Washington state and transferred to outdoor tanks at the University of Washington Big Beef Creek Field Station (Seabeck, WA) in May and June 2014. Males were held in tanks with limited artificial nests made of ceramic plates resting on a rim of bricks (figure 1a). At the start of the experiment, each tank held eight type I males and three to four type II males, with four nests. As with previous studies [6,24], larger type I males were expected to socially dominate smaller males and control nests, while smaller type I males were expected to be forced into cuckolding behaviours for any reproductive success. Females were kept in separate holding tanks. All tanks were exposed to ambient temperature and light. Average daily high temperature during the experiment was 19.6 ± 2.9°C (mean ± s.d.) and average daily low temperature was 7.5 ± 2.2°C. Sunrise time ranged from 05.13 to 05.22 h and sunset time ranged from 20.54 to 21.12 h.
Figure 1.
Experiment design (a) Overhead view of behaviour tank with four nests (i) with non-nesting males visible (arrows), and close up of individual nest (ii). (b) Timeline for spawning trials. (c) Left: sagittal view of midshipman brain. Preoptic area-anterior hypothalamus (POA-AH) highlighted in blue. Right: coronal sections through midshipman forebrain at the level of the dashed lines labelled i and ii through POA-AH in left panel. Dashed lines indicate level of cuts separating POA-AH and telencephalon during dissection. Stained with cresyl violet. H, hindbrain; C, cerebellum; M, midbrain; F, forebrain; PM, magnocellular preoptic area; PPa, anterior parvocellular preoptic area; PPp, posterior parvocellular preoptic area; Tel, telencephalon.
Spawning trials (figure 1b) took place 10–27 days after collection. Females were added to male tanks between 21.00 and 23.00 h after audible courtship advertisement humming was observed in a tank. The following morning, between 06.00 and 08.00 h, nests were checked for the presence of a female. Nests where spawning occurred were observed by the experimenters for cuckolding males for up to 30 min. Fish that were observed spawning, or found in the nest were collected and sacrificed. Type I males found inside the nest were identified as courters or cuckolders on the basis of size, as larger type I males dominate nest sites [6,24]. After spawning, nests were removed to ensure that the number of available nests remained limited.
Fish were deeply anesthetized in 0.025% benzocaine (Sigma-Aldrich, St Louis, MO) and exsanguinated from the heart. The brain was removed, transected at the midbrain-hindbrain boundary and stored in RNAlater (Life Technologies, Carlsbad, CA) overnight at 4°C, then at −20°C. Brains were shipped overnight to Cornell University (Ithaca, NY) on dry ice, then stored at −20°C until use. Morph type was confirmed at sacrifice on the basis of body, gonad, and swim bladder muscle size and body coloration [16,24,53].
(b). RNA extraction and sequencing
Brains in RNAlater (n = 5 per group) of courting type I males (75–245 g, 17–26 cm standard length (s.l.)), cuckolding type I males (35–140 g, 12–21 cm s.l.) and cuckolding type II males (20–65 g, 11–16 cm s.l.) were thawed on ice, then the POA-AH was isolated by cutting away the telencephalic lobes and then cutting the POA-AH from the remaining brain (figure 1c). Dissection was done in RNAlater. Additional dissected brains were sectioned frozen in the transverse plane at 30 µm to confirm that the dissected region contained the POA-AH (see [54–56] for description of POA-AH in midshipman fish). RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), treated with DNase I (Invitrogen) and reverse transcribed using Superscript III (Invitrogen) following manufacturer's protocols. cDNA (2.228–4.56 µg) was sent to Polar Genomics (Ithaca, NY) for strand-specific RNAseq library preparation. Each barcoded library contained cDNA from one individual. Sequencing was performed by the Cornell University Biotechnology Resource Center Genomics Facility on the Illumina NextSeq 500.
(c). Transcriptome assembly and annotation
Illumina quality filtering removed read pairs in which either read was poor quality. Trimmomatic (v. 0.32) [57] removed adapter sequences and trimmed low quality nucleotides from reads (PHRED = 5, SLIDINGWINDOW = 4:15). Reads of fewer than 25 base pairs (bp) after trimming were discarded. Only read pairs that both survived trimming were used for transcriptome assembly. Libraries from the courting type I male, cuckolding type I male and cuckolding type II male with the most reads after trimming were pooled to assemble a reference transcriptome using Trinity (v. 2.1.1) [58] on the Pittsburgh Super Computing Center's Greenfield system. Length distribution statistics were obtained using Prinseq (v.0.20.4) [59]. Transrate [60] was used to assess assembly quality and remove sequences with low read support. Reference transcriptome completeness was evaluated using BUSCO [61], and Kallisto was used to estimate abundance [62]. Transcripts with estimated expression below 0.5 transcripts per million (TPM) reads across any library were removed using a Trinity accessory script. The Transrate- and TPM-filtered reference transcriptome was annotated using Transdecoder [63] and Blast2Go [64]. Transdecoder identified open reading frames (ORFs) and translated nucleotide sequences to peptide sequences, which were annotated using Blast2Go with the NCBI non-redundant database. Sequences were submitted to blastp with an e-value cut-off of 1 × 10−10. If transcripts produced multiple peptide sequences with different BLAST hits, the hit with the lowest e-value was retained. Sequences without a vertebrate BLAST hit were removed prior to differential expression analyses. Gene ontology (GO) enrichment analysis was performed in Blast2Go using single tailed Fisher's exact test with false discovery rate (FDR) < 0.05 as a cut-off.
(d). Differential expression
Differential expression analyses were conducted using edgeR (Bioconductor, v. 3.16.5) [65,66]. Transcript-level abundance estimates were imported using tximport (Bioconductor, v. 1.2.0) [67]. Abundance data were fit to a glm model, and comparisons made between all type I and type II males, all cuckolding and courting males, as well as paired comparisons between each morph-behaviour group. FDR < 0.05 was used as a cut-off for significance. Differences between groups were reported as log2-fold change (logFC) in transcript expression. Hierarchical clustering of DE transcripts was performed using limma (Bioconductor, v. 3.30.13) [68].
3. Results
(a). Reference transcriptome
Illumina sequencing resulted in 41 814 685 ± 3 208 582.186 (mean ± s.d.) read pairs per library. Of the total, 92.79% ± 2.88% of reads survived quality trimming. Trinity assembly produced 887 303 sequences with a mean length of 498.79 ± 635.76 bp, Transrate score of 0.0429, 79.50% complete BUSCOs and 13.9% missing BUSCOs. Transrate and TPM filtering of the original assembly resulted in a transcriptome with 196 626 sequences with a mean length of 926.25 ± 1063.60 bp, Transrate score of 0.2291, 72.97% complete BUSCOs and 19.88% missing BUSCOs. Transdecoder identified 78 370 ORFs and generated peptide sequences for each, of which 85.87% had significant BLAST hits and 47.64% received full functional annotation from Blast2Go. Each of the top 20 BLAST hit species were ray-finned fish (electronic supplementary material, figure S1); however, five of the top 30 species were plants of the genera Solanum or Nicotiana. To remove contamination from non-vertebrate transcripts, sequences were removed if they did not receive a vertebrate BLAST hit, resulting in a transcriptome of 58 878 sequences used for differential expression analyses (available through Dryad, DOI: https://doi.org/10.5061/dryad.d08g0) [69]. The majority of filtered sequences at this step (11 083) were removed owing to lack of significant BLAST hit. The remainder had significant hits for ‘Plants and Fungi’ (4243), or other lineages, but lacked a vertebrate hit or were duplicate ORFs from the same transcript. Just one GO term, ‘hormone activity’, was found to be overexpressed among DE transcripts (FDR = 0.00653).
(b). Differential expression—general trends
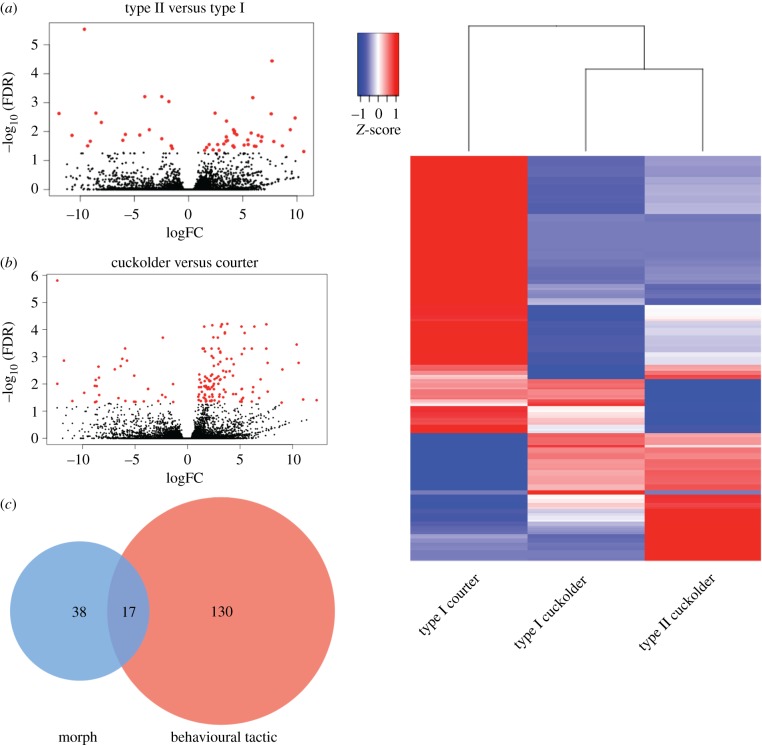
There were a greater number of DE POA-AH transcripts across males engaged in different behavioural tactics than between morphs (figure 2a,b). A total of 147 transcripts were significantly DE when comparing all cuckolding males to courting males, while 55 were significantly DE when comparing all type I males to type II males (figure 2c). Furthermore, hierarchical clustering of DE transcripts showed that cuckolding type I males clustered more closely to cuckolding type II males than to courting type I males (figure 2d).
Figure 2.
Differential expression trends. (a) Volcano plot showing differentially expressed (DE) transcripts across morph. Transcripts on the left are upregulated in type II and those on the right in type I males. (b) Volcano plot of DE transcripts across behavioural tactic. Transcripts on the left are upregulated in cuckolding males, those on the right are upregulated in courting males. Each transcript is represented by a dot; red dots indicate DE transcripts (FDR < 0.05). (c) Venn diagram showing number of transcripts DE in comparisons of morph (blue), behavioural tactic (red) or both (purple). (d) Heatmap of DE transcripts clustered by morph/behaviour group.
Of the 202 transcripts DE in either the morph or behavioural tactic comparison (electronic supplementary material, table S2), 17 were significant in both comparisons (figure 2c). To better understand which expression differences were driving the significance, we also compared each morph/behaviour group in pairs (electronic supplementary material, figure S2). From these comparisons, 59 transcripts were found to be DE between courting type I males and type II males, which was the highest of the three comparisons. Fifty-one transcripts were DE between type I males engaged in courtship versus cuckoldry. Only 17 transcripts were DE between cuckolding type I males and type II males. Thus, hierarchical clustering of DE transcripts and pairwise comparisons support the conclusion that POA-AH transcriptional activity is more strongly associated with behavioural tactic than developmental morph.
(c). Differential expression—genes of interest
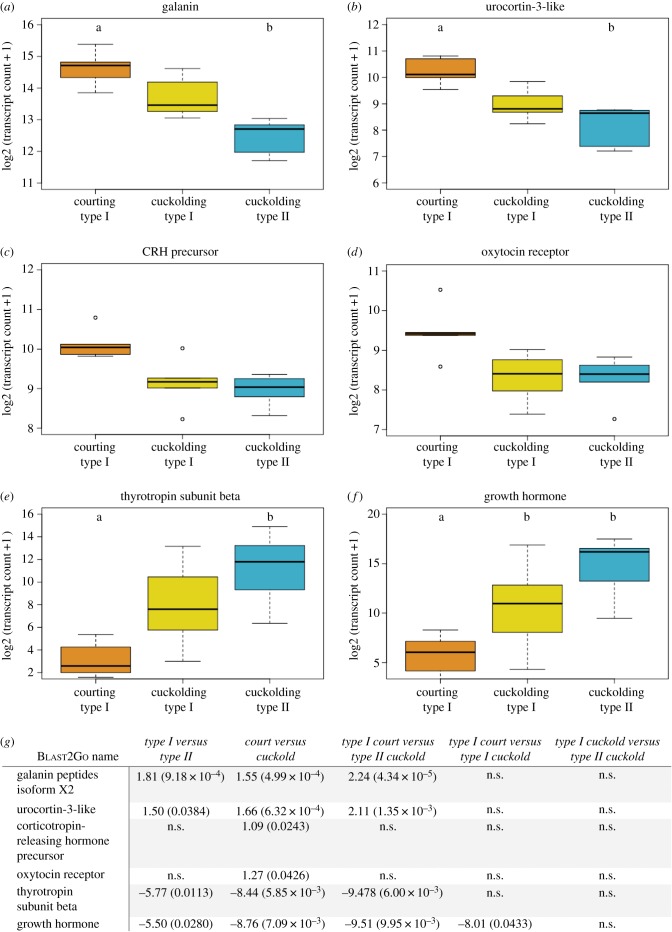
Based on the results of the enrichment analysis, we searched for DE transcripts that mapped to the GO term ‘hormone activity’ to identify transcripts of potential functional interest. Given prior work highlighting the importance of nonapeptides in regulating teleost reproductive behaviour plasticity, including midshipman [20,70–74], we also searched the GO terms ‘oxytocin receptor activity’ and ‘vasopressin receptor activity’. Six transcripts were linked to one or more of these GO terms—galanin, urocortin-3-like (UC-3), corticotropin-releasing hormone (CRH) precursor, oxytocin receptor (OTR), thyrotropin subunit beta and growth hormone (GH). Other GO terms associated with these transcripts of interest were ‘signal transduction’ (galanin, UC-3, CRH, thyrotropin and GH), and ‘response to stress’ (UC-3 and CRH).
Consistent with general expression trends, cuckolding type I males had intermediate expression levels or were more similar to type II males for each transcript of interest. Four genes were most highly expressed in courting type I males. Galanin (figure 3a) and UC-3 (figure 3b) were significantly upregulated in courting type I's compared to all cuckolders and in all type I compared to type II males. Pairwise comparisons showed each were upregulated in courting type I's compared to type II males (see figure 3 for all statistics). CRH and OTR were each upregulated in courting type I males compared to all cuckolders (figure 3c,d).
Figure 3.
Differentially expressed transcripts of functional interest (n = 5 animals per group). Boxplots showing expression levels of (a) galanin, (b) urocortin-3-like, (c) corticotropin-releasing hormone precursor, (d) oxytocin receptor, (e) thyrotropin subunit beta and (f) growth hormone. Expression displayed as log2 of transcript count estimate +1. Median, maximum and minimum values are indicated. Open circles represent outliers. Letters indicate significant differences between groups. Courting males significantly different from all cuckolding males in (c) and (d); see (g) for statistics. (g) Statistics for all comparisons of transcripts annotated by ‘hormone activity’, ‘oxytocin receptor activity’ or ‘vasopressin receptor activity’. Values are log2FC (FDR), ns, not significant. (Online version in colour.)
Two genes exhibited highest expression in type II males. Thyrotropin (figure 3e) was upregulated in all cuckolders compared to courters, in type II males compared to all type I's, and in type IIs compared to courting type I's. Growth hormone (figure 3f) showed the same pattern but was also significantly upregulated in cuckolding type I's compared to courting type I's.
4. Discussion
The findings reported here represent an advance in understanding brain gene expression related to ARTs in two key ways. First, while some previous studies compared behaving animals [27,29–31], this is, to our knowledge, the first investigating differential expression of specific transcripts to include individuals of different morphs engaged in the same tactic. This is critical for identifying which transcripts are DE owing to developmental differences between morphs and which are related to behavioural tactic. This strengthens our ability to identify candidate genes that are most probably regulating reproductive behaviour plasticity in species with ARTs and, more broadly, that underlie plasticity in social behaviour across vertebrate taxa. Second, this is, also to our knowledge, the first study of global patterns of transcript expression in fishes with ARTs to focus on a single brain region, in this case the POA-AH, rather than whole brain. We are able to replicate key findings of prior studies and advance their findings by identifying the brain region where previously observed differential expression is likely to be acting. Additionally, because our region of interest is a major neuroendocrine centre of the brain, our dataset highlights testable candidate genes that may play a role in the control of ARTs, namely peptide hormones and receptors that can be manipulated in future experiments. Thus, we are probably better able to identify DE transcripts relevant to reproductive behaviour plasticity specifically, and decrease the number of DE transcripts in our dataset that do not play a predominant role in the behaviour.
The results reveal a strong influence of behavioural tactic in determining global transcript expression patterns in the POA-AH. This result was somewhat unexpected as prior studies of midshipman and other teleosts have emphasized morph-specific patterns for many traits, including gene expression profiles, neurophysiology, and neuropeptide neuron size and number in the POA-AH (electronic supplementary material, table S1) [16,20–22,75]. GO enrichment analysis of DE transcripts identified a suite of neuro-hormonal transcripts coupled to ARTs that includes genes such as galanin and oxytocin receptor that are strongly implicated in the control of social behaviour across vertebrate lineages. As discussed, this further leads to the proposal that an evolutionarily conserved set of genes plays a predominant role in regulating the choice to follow one social behaviour tactic over another, for example, between a mating tactic, parental behaviour or aggression.
(a). Differential expression—general trends
Prior studies in midshipman that focused on the actions of specific hormones in adulthood have demonstrated a strong influence of developmental history in determining the behavioural and neural phenotypes of the two male morphs [20–22,45,76]. However, these studies, along with most prior transcriptomic studies of teleosts with ARTs [25,26,28–31], did not include animals of different morphs engaged in the same tactic, so they may have missed tactic-specific changes. One study, conducted in sailfin mollies (Poecilia latipinna), found a stronger influence of male morph on whole brain transcript expression, though behavioural tactic, influenced by social environment, also had a significant effect [27]. There are two key differences between that and the present study that may explain the divergence with our results. First, the molly study used whole brain RNA, while we used RNA from one brain region, the POA-AH. This suggests that regulation of expression may differ between one specific neuroendocrine centre and the brain as a whole. Second, sailfin molly morphs are determined by genetic alleles, while morph determination in midshipman appears to be strongly influenced by environment (rearing studies suggest morph determination is dependent on the density of animals raised together [77]). This may explain the stronger link between morph and gene expression in mollies.
(b). Differential expression—genes of interest
Consistent with the strong influence of behavioural tactic, the expression of neurohormonal transcripts of interest showed two general patterns: significantly different between cuckolding and courtship tactics alone (OTR and CRH) or with cuckolding type I males having intermediate levels between the morphs (galanin, UC-3, thyrotropin and GH). As discussed below, integrating the results of comparative studies of reproductive plasticity, inclusive of ARTs, among teleosts together with the present results for midshipman lead to identification of one group of transcripts (galanin, OTR, UC-3 and CRH) most strongly associated with male territorial defence, courtship and parental care, and a second (thyrotropin and GH) with male cuckoldry.
(i). Transcripts associated with territorial-courting-parental phenotype
Two of the DE transcripts with highest expression levels in type I male courters, galanin and OTR, are linked to a territorial-courting-parental (TCP) phenotype. Similar to our results, galanin prepropeptide transcripts are also upregulated in whole brain of TCP males compared to cuckolder-sneaker bluegill sunfish (Lepomis macrochirus) [30]. The bluegill result is notable, as its mating system is the most similar to midshipman among brain transcriptomic studies of teleosts with ARTs. Male bluegills develop into either type I-like TCP or type II-like earlier maturing cuckolder morphs [78,79]. In Astatotilapia burtoni cichlids, galanin is also upregulated in whole brain of reproductive (R) males that defend a territory and court females (but do not provide parental care) compared to non-reproductive (NR) males [2,80]. Among mammals, galanin is also a potent regulator of reproductive and parental behaviour; microinjection in the medial preoptic nucleus (MPON) of female rats primed with steroids elicits sexual behaviour [41], and mouse MPON-galanin neuron activity is critical for parental behaviour [44].
OTR (isotocin in teleosts) and CRH (below) were the most strongly associated with behavioural tactic, with significantly higher expression in courting than cuckolding males of both midshipman morphs. The OTR result parallels the bluegill transcriptome; cuckolder/sneaker males have the lowest expression levels of oxytocin peptide [30]. Together with studies in other teleosts [43,81–83], the results point to a specific role for isotocin in promoting territorial defence and paternal care, two hallmark features of the type I midshipman phenotype. Additionally, a large body of work demonstrates the importance of oxytocin in regulating social behaviours in tetrapods, including parental [84] and reproductive behaviour [85,86] and territorial aggression [87,88].
Two closely related transcripts, UC-3 and CRH that are members of the corticotropin releasing factor protein family, were also DE in the POA-AH. UC-3 expression paralleled that of galanin, while CRH was significant across behavioural tactics alone. Both genes were most highly expressed in courting type I males. Upregulation of UC-3 and CRH in courting type I males may be linked to energetic demands and behavioural trade-offs [89] of courtship humming during consecutive nights [90], and defending a nest and caring for developing eggs for weeks throughout the breeding season [16,91,92]. Among mammals, UC-3 increases blood glucose levels [93], and injection into the ventromedial hypothalamus of rats suppresses feeding, but does not activate the hypothalamic-pituitary-adrenal axis [94]. Similarly, CRH acts as a feeding suppressant in goldfish [95]. Thus, upregulation of UC-3 in courting type I male POA-AH could reduce appetite in the breeding season, during which type I males show a significant reduction in body weight [96]. Although CRH receptor (CRHr) was upregulated in sneaker male bluegills [30], a pattern reversed from that observed for CRH in midshipman, the bluegill study was of whole brain samples, and the relationship between expression of CRH and CRHr is not necessarily positively related, as CRH injection can decrease CRHr in rats [97].
(ii). Transcripts associated with cuckolder male phenotype
Two transcripts, thyrotropin beta subunit and GH, were upregulated in cuckolding compared to courting male POA-AH. Relatively little work has been done investigating the social behavioural role of these hormones. Thyrotropin is a glycoprotein hormone related to gonadotropins [98]. It has also been proposed that CRH and thyrotropin work synergistically to regulate vertebrate life stage transitions [99], although it is unclear how these two hormones may be interacting in adult midshipman or other species with ARTs. Our results for GH reflect those from whole-brain microarray experiments in Atlantic salmon (Salmo salar), which found that GH-related genes were upregulated in mature parr (sneaker males) compared to immature males [25], and that GH itself was upregulated in parr compared to early migrating, mature anadromous males [26].
(c). Summary: divergent behavioural phenotypes
While each of the transcripts discussed here are candidate regulators of reproductive-related social plasticity, it is important to acknowledge that differences in transcript expression relate to a suite of behaviours and developmental history extending beyond the minutes to hours involved in the act of spawning itself that we used as a timepoint for tissue collection. Over weeks to months in the breeding season, nesting type I males engage in courtship, parental care and driving off cuckolders, while cuckolders must choose between attempts to sneak or satellite spawn, escape aggression from nesting males or attempt a nest take-over in the case of type I cuckolders [6,16,24,92]. Additionally, morph-related traits (electronic supplementary material, table S1) may explain, in part, the cases where type I male cuckolders have intermediate levels of expression compared to courting type I and type II males. Furthermore, although we identified relationships between behavioural tactic and transcript expression, influence of internal state cannot be discounted, for example for UC-3 [94], oxytocin [100], CRH [95] and galanin [101], which are regulators of feeding [89]. Thus, DE transcript patterns probably reflect both external and internal stimuli related to broad differences in state associated with courtship and cuckoldry tactics, such as the case of type I male courters that show weight loss [96] during consecutive days and weeks of nest fidelity and more limited foraging opportunities [90–92].
Surprisingly, DE patterns did not emerge for steroid signalling pathways despite numerous studies, including those of midshipman, documenting their abundant expression in the POA-AH [9,42,102]. Differential steroid action may have occurred earlier in morph development, and morph-specific steroid action in adulthood may be carried out by differences in circulating steroid levels [18], rather than changes in expression of their receptor in the POA-AH.
5. Conclusion
This study demonstrates that behavioural tactic is more strongly coupled to POA-AH transcript expression than developmental history in a species exhibiting extreme intrasexual variation, and identifies key candidate transcripts for regulating ARTs that represent extremes in social behaviour plasticity in vertebrates. We found that males of different morphs engaged in the same behavioural tactic had more similar patterns of differential transcript expression than males of the same morph engaged in different behavioural tactics. In addition, we identified DE transcripts related to hormone activity and nonapeptide signalling that may be key regulators of behavioural plasticity. These include galanin, UC-3, CRH and OTR that were most strongly associated with a TCP phenotype, as well as GH and thyrotropin that were strongly associated with cuckoldry. This suite or module (see [80]) of genes may function as a behavioural switch mechanism between cuckoldry and courtship in adult type I males. Such a switch may be suppressed or permanently turned on to cuckoldry in type II males during development or in adulthood. By focusing in on a specific brain region of interest, we conclude that the expression of a conserved suite of genes in a conserved neuroendocrine centre, the POA-AH, plays a central role in the flexible selection of social tactics across multiple vertebrate lineages, exemplified here by a species with extreme intrasexual variation known as ARTs.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Margaret Marchaterre, David Rose and Ian Pengra for field support, Joe Sisneros for assistance in obtaining collection permits, the Cornell University Statistical Consulting Unit and Computational Biology Service Unit, particularly Qi Sun, for guidance in analysing the data, and Aubrey Kelly and two anonymous reviewers for constructive feedback on drafts of this manuscript.
Ethics
Animals were collected with a permit from the Washington Department of Fish and Wildlife (no. 14–147). All procedures were approved by the Institutional Animal Care and Use Committees of Cornell University and University of Washington.
Data accessibility
The assembled transcriptome and reads from each sample supporting the results of this article are available in the NCBI Transcriptome Shotgun Assembly and Sequence Read Archive databases under BioProject accession number PRJNA414410. Annotation information for assembled transcripts is available through Dryad (https://doi.org/10.5061/dryad.d08g0) [69].
Authors' contributions
J.A.T. and A.H.B. conceived the study and wrote the manuscript. J.A.T. and N.Y.F. performed the experiments. J.A.T., N.Y.F. and A.H.B. analysed and interpreted the data. All authors edited and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation (NSF) ACI-1548562. Other support from NSF IOS-1120925 and NSF IOS-1457108 (A.H.B.); Cornell University Center for Vertebrate Genomics and Department of Neurobiology and Behaviour (J.A.T.).
References
- 1.Warner RR, Swearer S. 1991. Social control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae). Biol. Bull. 181, 199–204. ( 10.2307/1542090) [DOI] [PubMed] [Google Scholar]
- 2.Fernald RD, Hirata NR. 1977. Field study of Haplochromis burtoni: quantitative behavioral observations. Anim. Behav. 25, 964–975. ( 10.1016/0003-3472(77)90048-3) [DOI] [Google Scholar]
- 3.Eberhard WG. 1982. Beetle horn dimorphism: making the best of a bad lot. Am. Nat. 119, 420–426. ( 10.1086/283920) [DOI] [Google Scholar]
- 4.Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 92–98. ( 10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 5.Lee JSF. 2005. Alternative reproductive tactics and status-dependent selection. Behav. Ecol. 16, 566–570. ( 10.1093/beheco/ari030) [DOI] [Google Scholar]
- 6.Lee JSF, Bass AH. 2004. Does exaggerated morphology preclude plasticity to cuckoldry in the midshipman fish (Porichthys notatus). Naturwissenschaften 91, 338–341. ( 10.1007/s00114-004-0531-y) [DOI] [PubMed] [Google Scholar]
- 7.Taborsky M. 1994. Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 23, 1–100. ( 10.1016/S0065-3454(08)60351-4) [DOI] [Google Scholar]
- 8.Neff BD, Svensson EI. 2013. Polyandry and alternative mating tactics. Phil. Trans. R. Soc. B 368, 20120045 ( 10.1098/rstb.2012.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godwin J. 2010. Neuroendocrinology of sexual plasticity in teleost fishes. Front. Neuroendocrinol. 31, 203–216. ( 10.1016/j.yfrne.2010.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mank JE, Avise JC. 2006. The evolution of reproductive and genomic diversity in ray-finned fishes: insights from phylogeny and comparative analysis. J. Fish Biol. 69, 1–27. ( 10.1111/j.1095-8649.2006.01132.x) [DOI] [Google Scholar]
- 11.Lucas JR, Howard RD. 2017. On alternative reproductive tactics in anurans: dynamic games with density and frequency dependence. Am. Nat. 146, 365–397. ( 10.1086/285805) [DOI] [Google Scholar]
- 12.Sinervo B, Lively CM. 1996. The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243. ( 10.1038/380240a0) [DOI] [Google Scholar]
- 13.Lank DB, Smith CM, Hanotte O, Burke T, Cooke F. 1995. Genetic polymorphism for alternative mating-behavior in lekking male ruff Philomachus pugnax. Nature 378, 59–62. ( 10.1038/378059a0) [DOI] [Google Scholar]
- 14.Maggioncalda AN, Czekala NM, Sapolsky RM. 2002. Male orangutan subadulthood: a new twist on the relationship between chronic stress and developmental arrest. Am. J. Phys. Anthropol. 118, 25–32. ( 10.1002/ajpa.10074) [DOI] [PubMed] [Google Scholar]
- 15.Schradin C, Yuen CH. 2011. Hormone levels of male African striped mice change as they switch between alternative reproductive tactics. Horm. Behav. 60, 676–680. ( 10.1016/j.yhbeh.2011.09.002) [DOI] [PubMed] [Google Scholar]
- 16.Brantley RK, Bass AH. 1994. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae). Ethology 96, 213–232. ( 10.1111/j.1439-0310.1994.tb01011.x) [DOI] [Google Scholar]
- 17.Bass AH, Horvath BJ, Brothers EB. 1996. Nonsequential developmental trajectories lead to dimorphic vocal circuitry for males with alternative reproductive tactics. J. Neurobiol. 30, 493–504. ( 10.1002/(SICI)1097-4695(199608)30:4%3C493::AID-NEU5%3E3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 18.Brantley RK, Wingfield JC, Bass AH. 1993. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm. Behav. 27, 332–347. ( 10.1006/hbeh.1993.1025) [DOI] [PubMed] [Google Scholar]
- 19.Bass A. 1992. Dimorphic male brains and alternative reproductive tactics in a vocalizing fish. Trends Neurosci. 15, 139–145. ( 10.1016/0166-2236(92)90356-D) [DOI] [PubMed] [Google Scholar]
- 20.Goodson JL, Bass AH. 2000. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature 403, 769–772. ( 10.1038/35001581) [DOI] [PubMed] [Google Scholar]
- 21.Remage-Healey L, Bass AH. 2004. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 24, 5892–5900. ( 10.1523/JNEUROSCI.1220-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remage-Healey L, Bass AH. 2007. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 27, 1114–1122. ( 10.1523/JNEUROSCI.4282-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JSF, Bass AH. 2005. Differential effects of 11-ketotestosterone on dimorphic traits in a teleost with alternative male reproductive morphs. Horm. Behav. 47, 523–531. ( 10.1016/j.yhbeh.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 24.Lee JSF, Bass AH. 2006. Dimorphic male midshipman fish: reduced sexual selection or sexual selection for reduced characters? Behav. Ecol. 17, 670–675. ( 10.1093/beheco/ark015) [DOI] [Google Scholar]
- 25.Aubin-Horth N, Landry CR, Letcher BH, Hofmann HA. 2005. Alternative life histories shape brain gene expression profiles in males of the same population. Proc. R. Soc. Lond. B 272, 1655–1662. ( 10.1098/rspb.2005.3125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aubin-Horth N, Letcher BH, Hofmann HA. 2009. Gene-expression signatures of Atlantic salmons plastic life cycle. Gen. Comp. Endocrinol. 163, 278–284. ( 10.1016/j.ygcen.2009.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser BA, Janowitz I, Thairu M, Travis J, Hughes KA. 2014. Phenotypic and genomic plasticity of alternative male reproductive tactics in sailfin mollies. Proc. R. Soc. B 281, 20132310 ( 10.1098/rspb.2013.2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schunter C, Vollmer SV, Macpherson E, Pascual M. 2014. Transcriptome analyses and differential gene expression in a non-model fish species with alternative mating tactics. BMC Genomics 15, 167 ( 10.1186/1471-2164-15-167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiver KA, Harris RM, Townsend JP, Hofmann HA, Alonzo SH. 2015. Neural gene expression profiles and androgen levels underlie alternative reproductive tactics in the ocellated wrasse, Symphodus ocellatus. Ethology 121, 152–167. ( 10.1111/eth.12324) [DOI] [Google Scholar]
- 30.Partridge CG, MacManes MD, Knapp R, Neff BD. 2016. Brain transcriptional profiles of male alternative reproductive tactics and females in bluegill sunfish. PLoS ONE 11, 1–21. ( 10.1371/journal.pone.0167509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugent BM, Stiver KA, Alonzo SH, Hofmann HA. 2016. Neuroendocrine profiles associated with discrete behavioral variation in Symphodus ocellatus, a species with male alternative reproductive tactics. Mol. Ecol. 25, 5212–5227. ( 10.1111/mec.13828) [DOI] [PubMed] [Google Scholar]
- 32.Cardoso SD, Goncalves D, Goesmann A, Canario AVM, Oliveira RF. In press Temporal vjariation in brain transcriptome is associated with the expression of female mimicry as a sequential male alternative reproductive tactic in fish. Mol. Ecol. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira RF. 2012. Social plasticity in fish: integrating mechanisms and function. J. Fish Biol. 81, 2127–2150. ( 10.1111/j.1095-8649.2012.03477.x) [DOI] [PubMed] [Google Scholar]
- 34.Maruska KP, Zhang A, Neboori A, Fernald RD. 2013. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145–157. ( 10.1111/j.1365-2826.2012.02382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weitekamp CA, Nguyen J, Hofmann HA. 2017. Social context affects behavior, preoptic area gene expression, and response to D2 receptor manipulation during territorial defense in a cichlid fish. Genes. Brain. Behav. 16, 601–611. ( 10.1111/gbb.12389) [DOI] [PubMed] [Google Scholar]
- 36.Nyman C, Fischer S, Aubin-Horth N, Taborsky B. 2017. Effect of the early social environment on behavioural and genomic responses to a social challenge in a cooperatively breeding vertebrate. Mol. Ecol. 26, 3186–3203. ( 10.1111/mec.14113) [DOI] [PubMed] [Google Scholar]
- 37.Newman SW. 1999. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 877, 242–257. ( 10.1111/j.1749-6632.1999.tb09271.x) [DOI] [PubMed] [Google Scholar]
- 38.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. ( 10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demski LS, Bauer DH, Gerald JW. 1975. Sperm release evoked by electrical stimulation of the fish brain: a functional-anatomical study. J. Exp. Zool. 191, 215–232. ( 10.1002/jez.1401910209) [DOI] [PubMed] [Google Scholar]
- 40.Demski LS, Sloan HE. 1985. A direct magnocellular-preopticospinal pathway in goldfish: implications for control of sex behavior. Neurosci. Lett. 55, 283–288. ( 10.1016/0304-3940(85)90449-5) [DOI] [PubMed] [Google Scholar]
- 41.Bloch GJ, Butler PC, Kohlert JG. 1996. Galanin microinjected into the medial preoptic nucleus facilitates female- and male-typical sexual behaviors in the female rat. Physiol. Behav. 59, 1147–1154. ( 10.1016/0031-9384(95)02087-X) [DOI] [PubMed] [Google Scholar]
- 42.Forlano PM, Bass AH. 2011. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm. Behav. 59, 616–629. ( 10.1016/j.yhbeh.2010.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connell LA, Matthews BJ, Hofmann HA. 2012. Isotocin regulates paternal care in a monogamous cichlid fish. Horm. Behav. 61, 725–733. ( 10.1016/j.yhbeh.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. ( 10.1038/nature13307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bass AH, Forlano PM. 2008. Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of reproductive and social plasticity. In Alternative reproductive tactics - an integrative approach (eds RF Oliveira, M Taborsky, J Brockman), pp. 109–131. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 46.Herget U, Wolf A, Wullimann MF, Ryu S. 2014. Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic-hypothalamic area in zebrafish larvae. J. Comp. Neurol. 522, 1542–1564. ( 10.1002/cne.23480) [DOI] [PubMed] [Google Scholar]
- 47.Affaticati P, Yamamoto K, Rizzi B, Bureau C, Peyriéras N, Pasqualini C, Demarque M, Vernier P. 2015. Identification of the optic recess region as a morphogenetic entity in the zebrafish forebrain. Sci. Rep. 5, 8738 ( 10.1038/srep08738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornbrooks EB, Parsons RL. 1991. Sexually dimorphic distribution of a galanin-like peptide in the central-nervous-system of the teleost fish Poecilia latipinna. J. Comp. Neurol. 304, 639–657. ( 10.1002/cne.903040410) [DOI] [PubMed] [Google Scholar]
- 49.Hofmann HA, Fernald RD. 2000. Social status controls somatostatin neuron size and growth. J. Neurosci. 20, 4740–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godwin J, Thompson R. 2012. Nonapeptides and social behavior in fishes. Horm. Behav. 61, 230–238. ( 10.1016/j.yhbeh.2011.12.016) [DOI] [PubMed] [Google Scholar]
- 51.Goodson JL, Evans AK, Bass AH. 2003. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J. Comp. Neurol. 462, 1–14. ( 10.1002/cne.10679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grober MS, Fox SH, Laughlin C, Bass AH. 1994. GnRH cell size and number in a teleost fish with two male reproductive morphs: sexual maturation, final sexual statues and body size allometry. Brain Behav. Evol. 43, 61–78. ( 10.1159/000113625) [DOI] [PubMed] [Google Scholar]
- 53.Bass AH, Marchaterre MA. 1989. Sound-generating (sonic) motor system in a teleost fish (Porichthys notatus): sexual polymorphism in the ultrastructure of myofibrils. J. Comp. Neurol. 286, 141–153. ( 10.1002/cne.902860202) [DOI] [PubMed] [Google Scholar]
- 54.Foran CM, Myers DA, Bass AH. 1997. Modification of gonadotropin releasing hormone (GnRH) mRNA expression in the retinal-recipient thalamus. Gen. Comp. Endocrinol. 106, 251–264. ( 10.1006/gcen.1997.6875) [DOI] [PubMed] [Google Scholar]
- 55.Foran CM, Bass AH. 1998. Preoptic AVT immunoreactive neurons of a teleost fish with alternative reproductive tactics. Gen. Comp. Endocrinol. 111, 271–282. ( 10.1006/gcen.1998.7113) [DOI] [PubMed] [Google Scholar]
- 56.Goodson JL, Bass AH. 2000. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J. Comp. Neurol. 422, 363–379. ( 10.1002/1096-9861(20000703)422:3%3C363::AID-CNE4%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 57.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grabherr MG, et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. ( 10.1038/nbt.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864. ( 10.1093/bioinformatics/btr026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith-Unna R, Boursnell C, Patro R, Hibberd JM, Kelly S. 2016. TransRate: reference-free quality assessment of de novo transcriptome assemblies. Genome Res. 26, 1134–1144. ( 10.1101/gr.196469.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212. ( 10.1093/bioinformatics/btv351) [DOI] [PubMed] [Google Scholar]
- 62.Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527. ( 10.1038/nbt.3519) [DOI] [PubMed] [Google Scholar]
- 63.Haas BJ, et al. 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512. ( 10.1038/nprot.2013.084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. 2005. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676. ( 10.1093/bioinformatics/bti610) [DOI] [PubMed] [Google Scholar]
- 65.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. ( 10.1093/bioinformatics/btp616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarthy DJ, Chen Y, Smyth GK. 2012. Differential expression analysis of multifactor RNA-seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. ( 10.1093/nar/gks042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soneson C, Love MI, Robinson MD. 2015. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research 4, 1521 ( 10.12688/f1000research.7563.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 ( 10.1093/nar/gkv007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tripp JA, Feng NY, Bass AH. 2017. Data from: Behavioral tactic predicts preoptic-hypothalamic gene expression more strongly than developmental morph in fish with alternative reproductive tactics Dryad Digital Repository. ( 10.5061/dryad.d08g0) [DOI] [PMC free article] [PubMed]
- 70.Semsar K, Kandel FL, Godwin J. 2001. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm. Behav. 40, 21–31. ( 10.1006/hbeh.2001.1663) [DOI] [PubMed] [Google Scholar]
- 71.Grober MS, George AA, Watkins KK, Carneiro LA, Oliveira RF. 2002. Forebrain AVT and courtship in a fish with male alternative reproductive tactics. Brain Res. Bull. 57, 423–425. ( 10.1016/S0361-9230(01)00704-3) [DOI] [PubMed] [Google Scholar]
- 72.Greenwood AK, Wark AR, Fernald RD, Hofmann HA. 2008. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. R. Soc. B 275, 2393–2402. ( 10.1098/rspb.2008.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huffman LS, Hinz FI, Wojcik S, Aubin-Horth N, Hofmann HA. 2014. Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni. Gen. Comp. Endocrinol. 212, 106–113. ( 10.1016/j.ygcen.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 74.Almeida O, Oliveira RF. 2015. Social status and arginine vasotocin neuronal phenotypes in a cichlid fish. Brain Behav. Evol. 85, 203–213. ( 10.1159/000381251) [DOI] [PubMed] [Google Scholar]
- 75.Bass AH, Grober MS. 2009. Reproductive plasticity in fish: evolutionary lability in the patterning of neuroendocrine and behavioral traits underlying divergent sexual phenotypes. In Hormones, brain, and behavior (eds Pfaff D, Arnold A, Etgen A, Farhbach R, Moss R, Rubin R), pp. 579–609. New York, NY: Academic Press. [Google Scholar]
- 76.Forlano PM, Bass AH. 2005. Steroid regulation of brain aromatase expression in glia: female preoptic and vocal motor nuclei. J. Neurobiol. 65, 50–58. ( 10.1002/neu.20178) [DOI] [PubMed] [Google Scholar]
- 77.Foran CM. 1998. Phenotypic plasticity in the neuroendocrine axis of a teleost fish. PhD thesis, Cornell University, Ithaca, New York, USA. [Google Scholar]
- 78.Gross MR, Charnov EL. 1980. Alternative male life histories in bluegill sunfish. Proc. Natl Acad. Sci. USA 77, 6937–6940. ( 10.1073/pnas.77.11.6937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominey WJ. 1980. Female mimicry in male bluegill sunfish—a genetic polymorphism? Nature 284, 546–548. ( 10.1038/284546a0) [DOI] [Google Scholar]
- 80.Renn SCP, Aubin-Horth N, Hofmann HA. 2008. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J. Exp. Biol. 211, 3041–3056. ( 10.1242/jeb.018242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kulczykowska E, Kleszczyńska A. 2014. Brain arginine vasotocin and isotocin in breeding female three-spined sticklebacks (Gasterosteus aculeatus): the presence of male and egg deposition. Gen. Comp. Endocrinol. 204, 8–12. ( 10.1016/j.ygcen.2014.04.039) [DOI] [PubMed] [Google Scholar]
- 82.DeAngelis R, Gogola J, Dodd L, Rhodes JS. 2017. Opposite effects of nonapeptide antagonists on paternal behavior in the teleost fish Amphiprion ocellaris. Horm. Behav. 90, 113–119. ( 10.1016/j.yhbeh.2017.02.013) [DOI] [PubMed] [Google Scholar]
- 83.Oldfield RG, Hofmann HA. 2011. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol. Behav. 102, 296–303. ( 10.1016/j.physbeh.2010.11.022) [DOI] [PubMed] [Google Scholar]
- 84.Pedersen CA, Prange AJ. 1979. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl Acad. Sci. USA 76, 6661–6665. ( 10.1073/pnas.76.12.6661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian A V., Ziegler TE. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm. Behav. 58, 614–618. ( 10.1016/j.yhbeh.2010.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakajima M, Görlich A, Heintz N. 2014. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159, 295–305. ( 10.1016/j.cell.2014.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harmon AC, Huhman KL, Moore TO, Albers HE. 2002. Oxytocin inhibits aggression in female Syrian hamsters. J. Neuroendocrinol. 14, 963–969. ( 10.1046/j.1365-2826.2002.00863.x) [DOI] [PubMed] [Google Scholar]
- 88.Goodson JL, Schrock SE, Kingsbury MA. 2015. Oxytocin mechanisms of stress response and aggression in a territorial finch. Physiol. Behav. 141, 154–163. ( 10.1016/j.physbeh.2015.01.016) [DOI] [PubMed] [Google Scholar]
- 89.Fischer EK, O'Connell LA. 2017. Modification of feeding circuits in the evolution of social behavior. J. Exp. Biol. 220, 92–102. ( 10.1242/jeb.143859) [DOI] [PubMed] [Google Scholar]
- 90.Feng NY, Bass AH. 2016. ‘Singing’ fish rely on circadian rhythm and melatonin for the timing of nocturnal courtship vocalization. Curr. Biol. 26, 2681–2689. ( 10.1016/j.cub.2016.07.079) [DOI] [PubMed] [Google Scholar]
- 91.DeMartini EE. 1988. Spawning success of the male plainfin midshipman. I. Influences of male body size and area of spawning site. J. Exp. Mar. Biol. Ecol. 121, 177–192. ( 10.1016/0022-0981(88)90254-7) [DOI] [Google Scholar]
- 92.Bose APH, Mcclelland GB, Balshine S. 2016. Cannibalism, competition, and costly care in the plainfin midshipman fish, Porichthys notatus. Behav. Ecol. 27, 628–636. ( 10.1093/beheco/arv203) [DOI] [Google Scholar]
- 93.Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. 2003. Urocortin III is expressed in pancreatic B-cells and stimulates insulin and glucagon secretion. Endocrinology 144, 3216–3224. ( 10.1210/en.2002-0087) [DOI] [PubMed] [Google Scholar]
- 94.Chen P, Vaughan J, Donaldson C, Vale W, Li C. 2010. Injection of urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels, and hypothalamic POMC gene expression but not the HPA axis. Am. J. Physiol. Endocrinol. Metab. 298, E337–E345. ( 10.1152/ajpendo.00402.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pedro N De, Alonso-Gómez AL, Gancedo B, Delgado MJ, Alonso-Bedate M. 1993. Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish. Physiol. Behav. 53, 517–520. ( 10.1016/0031-9384(93)90146-7) [DOI] [PubMed] [Google Scholar]
- 96.Sisneros JA, Alderks PW, Leon K, Sniffen B. 2009. Morphometric changes associated with the reproductive cycle and behaviour of the intertidal-nesting, male plainfin midshipman Porichthys notatus. J. Fish Biol. 74, 18–36. ( 10.1111/j.1095-8649.2008.02104.x) [DOI] [PubMed] [Google Scholar]
- 97.Aguilera G, Rabadan-Diehl C, Nikodemova M. 2001. Regulation of pituitary corticotropin releasing hormone receptors. Peptides 22, 769–774. ( 10.1016/S0196-9781(01)00390-4) [DOI] [PubMed] [Google Scholar]
- 98.Pierce JG, Parsons TF. 1981. Glycoprotein hormones: structrue and function. Annu. Rev. Biochem. 50, 465–495. ( 10.1146/annurev.bi.50.070181.002341) [DOI] [PubMed] [Google Scholar]
- 99.Watanabe Y, Grommen SVH, De Groef B. 2016. Corticotropin-releasing hormone: mediator of vertebrate life stage transitions? Gen. Comp. Endocrinol. 228, 60–68. ( 10.1016/j.ygcen.2016.02.012) [DOI] [PubMed] [Google Scholar]
- 100.Arletti R, Benellis A, Bertolini A. 1970. Influence of oxytocin on feeding behavior in the rat. J. Comp. Physiol. Psychol. 70, 103–112. ( 10.1037/h0028404) [DOI] [PubMed] [Google Scholar]
- 101.Corwin RL, Robinson JK, Crawley JN. 1993. Galanin antagonists block galanin-induced feeding in the hypothalamus and amygdala of the rat. Eur. J. Neurosci. 5, 1528–1533. ( 10.1111/j.1460-9568.1993.tb00221.x) [DOI] [PubMed] [Google Scholar]
- 102.Feng NY, Bass AH. 2017. Neural, hormonal, and genetic mechanisms of alternative reproductive tactics: vocal fish as model systems. In Hormones, brain, and behavior (eds Pfaff DW, Joels M), pp. 47–68. Oxford, UK: Oxford Academic Press. ( 10.1016/B978-0-12-803592-4.00018-3) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tripp JA, Feng NY, Bass AH. 2017. Data from: Behavioral tactic predicts preoptic-hypothalamic gene expression more strongly than developmental morph in fish with alternative reproductive tactics Dryad Digital Repository. ( 10.5061/dryad.d08g0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The assembled transcriptome and reads from each sample supporting the results of this article are available in the NCBI Transcriptome Shotgun Assembly and Sequence Read Archive databases under BioProject accession number PRJNA414410. Annotation information for assembled transcripts is available through Dryad (https://doi.org/10.5061/dryad.d08g0) [69].