Abstract
Mycoplasma pneumoniae infection has been linked to poor asthma outcomes. M. pneumoniae produces an ADP-ribosylating and vacuolating toxin called community-acquired respiratory distress syndrome (CARDS) toxin that has a major role in inflammation and airway dysfunction. The objective was to evaluate the immunopathological effects in primates exposed to M. pneumoniae or CARDS toxin. A total of 13 baboons were exposed to M. pneumoniae or CARDS toxin. At Days 7 and 14, BAL fluid was collected and analyzed for cell count, percent of each type of cell, CARDS toxin by PCR, CARDS toxin by antigen capture, eosinophilic cationic protein, and cytokine profiles. Serum IgM, IgG, and IgE responses to CARDS toxin were measured. All animals had a necropsy for analysis of the histopathological changes on lungs. No animal developed signs of infection. The serological responses to CARDS toxin were variable. At Day 14, four of seven animals exposed to M. pneumoniae and all four animals exposed to CARDS toxin developed histological “asthma-like” changes. T cell intracellular cytokine analysis revealed an increasing ratio of IL-4/IFN-γ over time. Both M. pneumoniae and CARDS toxin exposure resulted in similar histopathological pulmonary changes, suggesting that CARDS toxin plays a major role in the inflammatory response.
Keywords: asthma, mycoplasma, community-acquired respiratory distress syndrome toxin, primate model
Clinical Relevance
This study provides further understanding of the role of community-acquired respiratory distress syndrome toxin and Mycoplasma pneumoniae in the inflammatory response of this infection in the lung.
Asthma is a complex inflammatory disease of the lungs that has reached epidemic proportions in the developed world. It is estimated to affect 300 million worldwide and 10% of the population in the United States and Europe (1, 2). Both viral and bacterial infections are felt to contribute to the etiology and exacerbation rate of asthma. Early reports demonstrated an association between Mycoplasma pneumoniae and asthma symptoms (3, 4). More recently, atypical bacterial infections or colonization with M. pneumoniae have been increasingly associated with the development of asthma and the worsening of asthma control (5–7). Both human and animal studies suggest that M. pneumoniae may play an important role in both the initiation and progression of chronic asthma (8–12). Even in individuals without atopy, M. pneumoniae infection has been linked to the development of asthma in both early and late stages (13). Furthermore, therapies targeting M. pneumoniae have shown potential beneficial effects in asthma control (14).
Until recently, no virulence factors of M. pneumoniae have been directly linked to asthma pathogenesis. We identified an ADP-ribosylating and vacuolating toxin produced by M pneumoniae, community-acquired respiratory distress (CARDS) toxin, which is strongly linked with M. pneumoniae–associated disease in humans and directly linked to asthma-like inflammatory patterns in animal models (15–17). In children, M. pneumoniae colonization is seasonal, and CARDS toxin is readily detected in the respiratory secretions of children in the winter months. In fact, approximately 50% of hospitalized children with acute exacerbation of asthma had detectable CARDS toxin in their respiratory secretions (11). This is in agreement with what we reported in adults with refractory asthma, where 52% of the subjects with refractory asthma had detectable CARDS toxin in the respiratory secretions (18). Importantly, within this patient group, we observed a subset of patients who were persistently positive for CARDS toxin for up to 600 days.
Despite these observations suggesting a link between asthma and CARDS toxin, there is still limited understanding on the inflammatory mechanisms of CARDS toxin and its effect on the development of asthma and its control. The objective of this study was to further characterize the immunological and histopathological response to M. pneumoniae infection and exposure to CARDS toxin using a primate model.
Methods
A total of 13 adult baboons (Papio sp.) was used for the experiment. Animals were individually housed and had free access to food, water, and toys. After anesthesia, all baboons underwent bronchoscopy using an Olympus P20-D bronchoscope (Olympus Medical Systems Corp.). The animals’ vital signs (heart rate, blood pressure, temperature, and oxygen saturation) were monitored closely before and after the experiment. The animals were weighed daily, measured for nutritional and fluid intake, and examined twice daily for normal interactions. The baboons were treated in accordance with guidelines established by the Weatherall report (19). All experiments were performed in accordance with the Institutional Biosafety Committee and Institutional Animal Use and Care Committee protocols of the University of Texas Health Science Center at San Antonio (San Antonio, TX).
All animals had baseline serum drawn, and underwent bronchial lavage with instillation of 20 ml of saline solution, with a return of 10 ml of BAL fluid. After the acquisition of the baseline BAL fluid from the left lower lobe, M. pneumoniae (108 color-changing units/ml; n = 9) or CARDS toxin (5 mg/10 ml; n = 4) was instilled into the bronchus intermedius. This dose was based on the concentration of toxin per milliliter of secretions detected in patients with severe asthma and the estimated volume of distribution. M. pneumoniae strain S1, grown on SP4 liquid media, was used. The process of production and purification of recombinant CARDS toxin has been described previously (15).
Serum and BAL fluid were collected again at Days 7 and 14 after inoculation. All samples of BAL fluid were analyzed for cell count, percent of each type of cell, PCR for CARDS toxin, CARDS toxin by antigen capture, and eosinophilic cationic protein, as previously described (16–18). Animals with M. pneumoniae exposure had bronchoscopic microsampling of the epithelial lining fluid (ELF) on Day 7 using the Olympus microsampling probe (model BC-402; Olympus Optical Co., Ltd.) at the bronchus intermedius. At Day 7, four animals underwent necropsy (two of the M. pneumoniae group and two of the CARDS toxin group), with the remaining animals undergoing necropsy on Day 14.
Pathology Methods
After intrabronchial fixation with 10% neutral-buffered formalin, both right and left lower lung lobes were cut transversely into two levels, the surfaces of which yielded four tissue specimens from each lobe for paraffin processing. Sections were cut at 4 µm and stained with hematoxylin and eosin. All slides were scanned with the Aperio Scanscope XT (Aperio) to create digital images. The hematoxylin and eosin digital images were reviewed in a blinded fashion, and lesions of peribronchiolar and bronchial infiltrates, bronchiolar and bronchial luminal exudates, perivascular infiltrate, and parenchymal pneumonia were evaluated. This method assigns values from 0 to 26 (the greater the score, the greater the inflammatory changes in the lung). Inflammatory cell infiltrates of lymphocytes and polymorphonuclear leukocytes were graded at few (grade 1), numerous (grade 2), or abundant (grade 3) in peribronchial/peribronchiolar, perivascular, and intra-alveolar pneumonitic exudate sites.
A representative slide from both the right and left lung of all animals was immunostained using a monoclonal mouse antibody against eosinophil peroxidase (EPX) (provided by J. Lee, Mayo Clinic, Scottsdale, AZ) at a dilution of 1:100 to identify eosinophils. Using the Aperio image analysis system, image analysis algorithms were used to obtain unbiased estimates of EPX-immunostained cells. Anti-rabbit recombinant CARDS toxin column affinity-purified antibody at a 1:1,200 dilution was incubated with representative lung sections, which were then stained with diaminobenzidene chromagen. The findings were correlated with light microscopic histopathological lesions of pneumonia and airway and vascular cellular infiltrates.
Serology
Serum IgM and IgG responses to CARDS toxin were determined by direct ELISA. ELISA plates were coated with recombinant CARDS toxin, 85 ng/well for IgM and 340 ng/well for IgG assessment, and then incubated with baboon serum diluted 1:200. After extensive washing, relative IgM and IgG concentrations were determined using peroxidase-conjugated goat anti-baboon IgM and IgG antibodies and detected using colorimetric substrate. The optical density (OD) at 630 nm was measured on a plate reader and the mean of duplicate measurements reported. An OD at 630 nm above 0.2 was considered above background.
An IgE capture was developed as an ELISA for measuring CARDS toxin–specific IgE antibody in serum. Microplate wells (Thermo Immulon HBX4; Thermo Fisher Scientific) were first coated with a commercial anti-human IgE monoclonal antibody (Kirkegaard and Perry Laboratory), and then incubated with serial dilutions of test sera with 1% BSA as diluent for the capture of only IgE-class antibodies. CARDS toxin plus anti-CARDS toxin–specific rabbit polyclonal antibodies and alkaline phosphatase–conjugated secondary antibody were then added. Finally, p-NPP (Sigma-Aldrich) was used as the enzyme substrate. The OD of the reaction product was measured at 405 nm on a microplate reader.
Flow Cytometry
Flow cytometry was performed in the Core Laboratory of the University of Texas Health Science Center at San Antonio. Peripheral blood mononuclear cells were obtained using Ficoll-Hypaque density gradient centrifugation. Cells were then incubated and then washed with 0.5% Pharmingen (BD Biosciences Pharmingen) stain buffer, and 0.1% NaN3 in PBS. Cells were then blocked with 5% mouse serum and 10% human Fc fragments, incubated with subsequent washing, permeabilized with 50% diethylene glycol and 15% formaldehyde (Permeabilizing Solution 2; BD Biosciences), blocked with 10% normal mouse serum and 20% human Fc fragments, and stained with monoclonal antibodies (CD3 v500 [UCHTL1], CD4-APC-Cy7 [RPA-T4], CD8-PE-Cy7 [RPA-T8], IL4-PE [8D4–8], IL17-APC [eBio64Dec17], IFN-FITC [B27]) against intracellular antigens. Dead cells were discriminated using Violet Dead stain (Thermo Fisher Scientific).
Results
Clinical Response to CARDS Toxin and M. pneumoniae
No animal developed clinical signs of infection, and all animals maintained normal activity throughout the experiment (see Table E1 in the data supplement). The endoscopic exam appeared normal and without secretions after exposure to either CARDS toxin or M. pneumoniae on Days 7 and 14.
Histopathology and Immunostaining Results
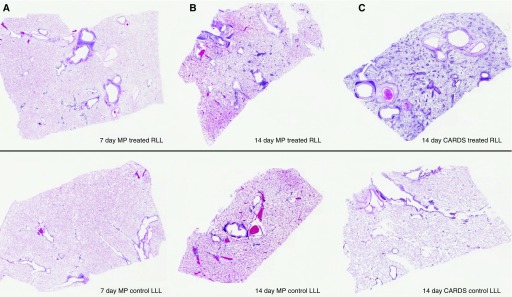
The grades of scanned images and a summary of each animal’s histopathology are presented in Table 1. Overall, the animals with the least visually identified lesions were those exposed to M. pneumoniae at Day 7 (AD12, FA52) and Day 14 (AD67). Conversely, at Day 7, the two CARDS toxin–exposed animals had moderate grades of disease involvement and, at Day 14, both CARDS toxin–exposed baboons had severe lung disease grades. Only two of the Day 14 M. pneumoniae–exposed animals developed severe lesions (FA03, FK73), whereas other M. pneumoniae–treated animals had moderate grades of disease. Figure 1 depicts examples of the mild, moderate, and severe grades of disease in CARDS toxin– or M. pneumoniae–exposed right lung lobes and its left lung control.
Table 1.
Exposure Status, Histopathology, Number of Lung Slides with Disease/Grade, Percent of Eosinophil Peroxidase Cell Staining, Community-acquired Respiratory Distress Syndrome Toxin by Antigen Capture in Mycoplasma pneumoniae–infected Animals, and IgE Community-acquired Respiratory Distress Syndrome before and after Exposure
| Animal | Exposure | Day of Necropsy | Histopathology | Right Lung | Left Lung | Right Lung EPX (%) | Left Lung EPX (%) | CARDS Antigen Capture | IgE CARDS Before (After) |
|---|---|---|---|---|---|---|---|---|---|
| ET92 | CARDS toxin | 7 | Asthma-like disease | 2/4 moderate | 0/4 | 4.52 | 0.18 | 12,790 | 0.047 (0.072)* |
| DA84 | Asthma-like disease | 3/4 moderate | 2/4 mild | 1.03 | 0.34 | 1,991 | 0.036 (0.1) | ||
| EV36 | 14 | Asthma-like disease | 4/4 severe | 4/4 severe | 4.37 | 2.23 | 334 | 0.062 (0.232) | |
| AN53 | Asthma-like disease, focal eosinophilic pneumonia | 4/4 severe | 0/4 | 1.16 | 0.37 | 157 | 0.082 (0.112) | ||
| AD12 | Mp | 7 | Minimal disease with focal sites of lymphocyte aggregates and rare eosinophils in airway and vessel walls | 1/4 mild | 0/4 | 0.36 | 0.30 | 348 | 0.035 (0.083)* |
| FA52 | Minimal disease with focal sites of lymphocyte aggregates and rare eosinophils in airway and vessel walls | 1/4 mild | 0/4 | 0.54 | 0.21 | 229 | 0.058 (0.192) | ||
| DL98 | 14 | Multilayered lymphocytes and rare eosinophils in airway and vessel walls | 4/4 moderate | 2/4 mild | 0.37 | 0.34 | 1,739 [154] | 0.069 (0.125) | |
| BJ14 | Asthma-like disease | 2/4 moderate | 0/4 | 0.81 | 0.43 | 268 [34] | 0.047 (0.096) | ||
| FK73 | Asthma-like disease, focal eosinophilic pneumonia | 3/4 severe | 0/4 | 1.24 | 0.36 | 1,471 [48] | 0.042 (0.116) | ||
| AD93 | Multilayered lymphocytes and rare eosinophils in walls of vessels and airways | 3/4 moderate | 0/4 | 0.43 | 0.21 | 670 [73] | 0.084 (0.123) | ||
| AC86 | Asthma-like disease, eosinophilic pneumonia, and focal foreign body giant cell lesion | 3/4 moderate | 1/4 mild | 2.13 | 0.36 | 247 [469] | 0.032 (0.139) | ||
| AD67 | Focal sites of lymphocytes and rare eosinophils in walls of vessels and airways | 1/4 mild | 1/4 mild | 0.30 | 0.24 | 298 [64] | 0.059 (0.125) | ||
| FA03 | Asthma-like disease mixed cell pneumonia | 2/4 severe | 0/4 | 13.75 | 0.70 | 1,252 [141] | 0.036 (0.078)* |
Definition of abbreviations: CARDS = community-acquired respiratory distress syndrome; EPX = eosinophil peroxidase; Mp = Mycoplasma pneumoniae.
Asthma-like disease: significant lymphocytic and eosinophilic airway disease with more than five eosinophils per 20× field and mucus plugging. Mycoplasma animals have antigen capture (pg/ml) shown at both Day 7 and Day 14 (in brackets). IgE CARDS toxin by antigen capture was measured before exposure and after killing, and considered positive if greater than 0.09 optical density at 450 nm.
Negative postmortem IgE CARDS.
Figure 1.
(A) Mild (<50%) grade of multilayered cellular infiltrates reflected visually in scanned lung section; lesions with fewer cell infiltrates are not evident in the scans. (B) Moderate (50–75%) grade of multilayered cellular infiltrates reflected visually in scanned lung section. (C) Severe (75–100%) grade of multilayered cellular infiltrates reflected visually in scanned lung section. Treated right lower lobe (upper panel) and “untreated control” left lower lobe (lower panel) of the same animal are illustrated in each case. CARDS = community-acquired respiratory distress syndrome; LLL = left lower lobe; MP = Mycoplasma pneumoniae; RLL = right lower lobe.
All animals exposed to CARDS toxin at Days 7 and 14 developed “asthma-like” histopathologic changes characterized by significant lymphocytic and eosinophilic airway disease with more than five eosinophils per 20× field and mucus plugging (Table 1). On Day 14, one animal (AN53) had severe multilayering of lymphocytes and eosinophils around the vessels and airways. The EPX value on this animal was 1.16%. The left lung was histologically normal. After instilling CARDS toxin endobronchially to EV36, the animal was noted to vomit, and was turned on his left side and suctioned. The animal subsequently developed significant disease in both lower lobes (Table 1). EV36 developed greater than five eosinophils per 20× field in both lungs, with a slight increase in polymorphonuclear leukocytes. The EPX value on this animal was 4.37% on the right and 2.23% on the left.
At Days 14, four of seven animals exposed to M. pneumoniae (BJ14, FK73, AC86, and FA03) developed “asthma-like” histopathologic changes (Figure 1, Figures E1 and E2). Only one animal (AC86) had eosinophils on baseline BAL (1%), and this value increased to 30% at the time of necropsy. As noted in the summary of lesions in Table 1, this animal had histologic signs of aspiration and was also noted to have 20% polymorphonuclear leukocytes in both the right and left lung BAL at necropsy (data not shown). Another animal (FA03) had asthma-like disease with elevated EPX levels determined by the Aperio system (13.75% of cells were eosinophils in the right lung and 0.70% in the left lung). This animal also demonstrated focal eosinophilic pneumonia and an area of mucus plugging in the left lung. Although this animal had only scattered eosinophils in the walls of distal airways, abundant eosinophils in the wall of the large bronchus plus the focal sites of eosinophilic pneumonia were likely contributors to the EPX value. Figures E1A, E1B, and E2A depicts the multilayering of lymphocytes in both M. pneumoniae– and CARDS toxin–treated infected lungs; of note is the striking eosinophilic presence in CARDS toxin–treated lung at 14 days (Figure E2B) and the EPX stain for eosinophils at 7 days after CARDS toxin treatment (Figure E2C).
The EPX data consistently show higher values in the right lung when compared with those in the left lung (Table 1). By Day 14, five of seven M. pneumoniae–exposed animals demonstrated higher EPX values (range, 2- to 20-fold) when compared with those of left lung. Three had eosinophils within the alveoli that were likely contributors to higher EPX values. All CARDS toxin–treated animal lungs had consistently higher right-lobe EPX values (range, 2- to 26-fold) and numerous to abundant eosinophils in the walls of distal airways and vascular structures. Because animals were noted to cough after extubation, the histological findings on the left lung were not unexpected. Immunostaining with CARDS toxin antibody was present in alveolar macrophages in peribronchiolar/alveolar spaces in CARDS toxin–treated animals at Day 14.
Serological Responses to CARDS Toxin
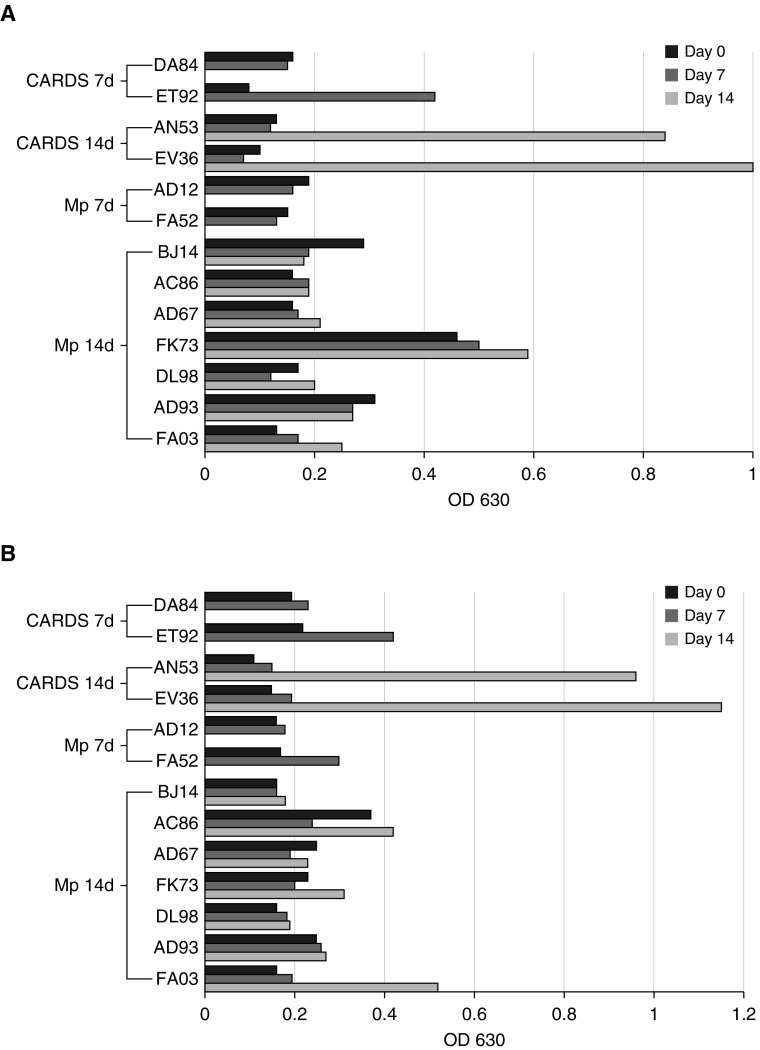
Animals exposed to recombinant CARDS toxin or infected with M. pneumoniae had variable serological responses (Figure 2). Three animals (BJ14, FK73, and AD93) showed CARDS toxin–specific IgM at baseline, and four animals (AC86, AD67, FK73, and FA52) had low, but detectable, levels of CARDS toxin–specific IgG. Of note, 75% of the animals treated with recombinant CARDS toxin showed increases in both toxin-specific IgM and IgG over the course of the experiment, whereas only 22% of the M. pneumoniae–infected animals showed increased IgM, and 44% had increases in IgG. Overall, animals exposed to recombinant CARDS toxin had better IgM and IgG responses than animals infected with M. pneumoniae. One animal (AC86) had increased IgG without a corresponding change in IgM. Four animals (DA84, DL98, AD12, and BJ14) did not increase IgM or IgG titers above baseline throughout the course of the experiment.
Figure 2.
(A) IgM antibody response to CARDS toxin and Mycoplasma pneumoniae organisms as determined by ELISA. (B) IgG antibody response to CARDS toxin and M. pneumoniae organisms as determined by ELISA. OD = optical density.
All animals had negative IgE to CARDS at baseline and, with the exception of three animals (ET92, AD12, and FA03), developed IgE to CARDS by the time they were killed. IgE levels did not correlate with CARDS toxin levels or eosinophil counts (Table 1).
Intracellular Cytokine Staining of Peripheral Blood Lymphocytes
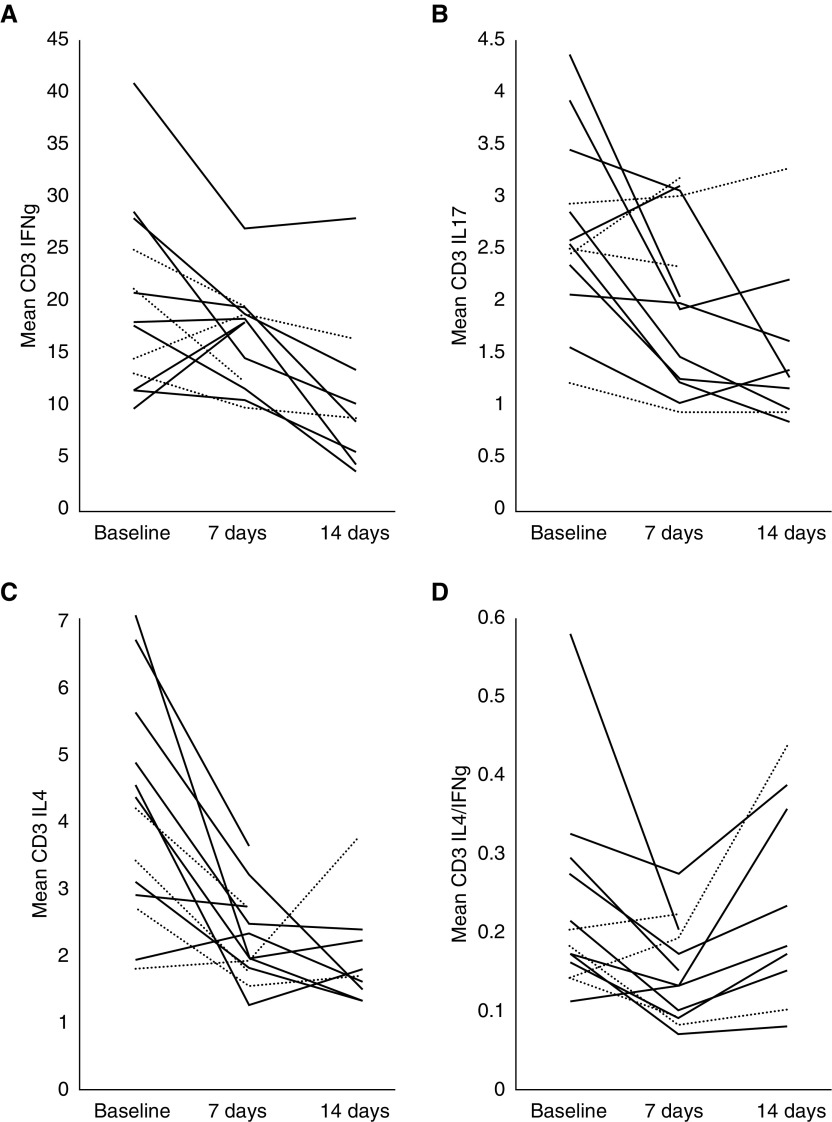
Intracellular cytokines were evaluated on Days 0 and 7 for all animals, and on Day 14 for those animals not killed on Day 7. Intracellular IL-4, IL-17, and IFN-γ were reduced in T cells (CD3+) on Days 7 and 14 after either CARDS toxin or M. pneumoniae inoculation (Figure 3). The mean reduction at Day 7 was 22.4% for IFN-γ, 90.6% for IL-4, and 43.1% for IL-17. However, by Day 14, IL-4 rebounded toward baseline in five of seven animals, whereas IFN-γ continued to decline. The ratio of IL-4:IFN-γ, although initially reduced at Day 7 (mean, 0.145) compared with Day 0 (mean, 0.225), increased at Day 14 (mean, 0.230). However, two CARDS toxin–treated animals with asthma-like lesions (EV36 and ET92) had very little change in their IL-4:IFN-γ ratio on Day 7 (0.06 and −0.1).
Figure 3.
Proportion of peripheral blood intracellular IFN-γ (A) IL-17, (B) IL-4, and (C) in total T cells in baboons exposed to CARDS toxin (dotted lines) or M. pneumoniae at baseline, Day 7, and Day 14. (D) Shows the intercellular IL-4/IFN-γ ratio of peripheral blood cells at baseline, Day 7, and Day 14.
Production of CARDS Toxin in Animals Exposed to M. pneumoniae
All M. pneumoniae–infected animals had measurable CARDS toxin on Days 7 and 14 by antigen capture (Table 1). With the exception of one animal, all levels of CARDS toxin were lower at Day 14 (mean, 849.1 ± 629.6 versus 140 ± 151.9 pg/ml; P = 0.013). Five of seven animals had measureable CARDS toxin in the ELF on Day 7. The mean level of CARDS toxin was 353.5 pg/ml (range, 11.7–912.7 pg/ml) in ELF, which is comparable to levels we detected in patients with severe/refractory asthma. The degree of histologic severity was correlated to level of CARDS toxin by antigen capture on Day 7 (r = 0.676, P = 0.046), but not on Day 14 (r = 0.306, P = 0.504).
Discussion
The results of this study demonstrate that both M. pneumoniae and CARDS toxin exposure are capable of producing asthma-like histopathologic changes in primates and IgE to CARDS toxin. All CARDS toxin–treated animals had asthma-like changes on histologic exam. On Day 7, M. pneumoniae–exposed animals had mild lymphocytic reactions around vessels and airways, but only rare eosinophils, but by Day 14, progression of the lymphocytic and eosinophilic responses was more robust and “asthma like” in four of seven animals. In general, the number of eosinophils reflected in the EPX values and documented histopathological findings correlated with the severity and extent of disease in the digital images.
Based on clinical observations, we developed animal models of CARDS toxin–induced inflammation. Our previous studies demonstrated that recombinant CARDS toxin induces peribronchiolar inflammation in both mice and baboons (16). Subsequent studies in mice demonstrated that CARDS toxin induces asthma-like inflammation (17). This inflammation consists of a robust eosinophilia, induction of IL-4 and IL-13 expression, mucus metaplasia, and airway hyperreactivity. It was demonstrated that this response is dependent on CD4+ T cells, and shows a trend of polarization toward a type-2 inflammation pattern. Altogether, these data suggest that CARDS toxin can induce allergic inflammation in rodents.
In our mouse models, treatment with recombinant CARDS toxin or exposure with M. pneumoniae resulted in an early (by Day 7) and robust polarization of the T cell response to a type-2 phenotype (16, 17, 20). In contrast, the response in baboons was delayed, and demonstrated a mixed immunologic response characterized by a decline in IL-4, IL-17, and IFN-γ by Day 7, with a trend for IL-4 to return toward baseline, whereas IL-17 and IFN-γ continued to decline in the majority of animals. This resulted in an increase in the IL-4:IFN-γ ratio, suggesting a shift toward a type-2 polarization of the T cell compartment. However, this is purely speculative, as we could not determine from our cytokine data the mechanism of the asthma-like response that we observed on histology. The mechanism of CARDS toxin–mediated cytokine suppression remains unknown, but it might be more obvious in the baboon, as the kinetics of the T cell response to CARDS toxin or M. pneumoniae is slower than what is observed in the mouse.
The serologic response in this study was also variable. Although 75% of animals exposed to CARDS toxin showed increases in both toxin-specific IgM and IgG, animals exposed to M. pneumoniae showed more variability, with only 65% of animals developing significant levels of either IgM or IgG. However, all animals were initially negative for IgE directed to CARDS toxin, and 11/13 developed IgE to CARDS toxin by Day 14. Clinical studies have also shown a variable immunologic response to M. pneumoniae. One study in children with asthma with M. pneumoniae infection revealed higher levels of IgE and IL-9, and lower levels of IFN-γ (21). In contrast to these findings, another study showed no difference in IL-2, IFN-γ, or IL-4 levels in patients with M. pneumoniae infection (22). Nevertheless, in those patients with asthma symptoms and M. pneumoniae infection, IL-5 levels were found to be significantly higher compared with those with asymptomatic infection (21, 22). In line with these findings, an analysis of serum samples of subjects with asthma and concomitant M. pneumoniae infection revealed higher levels of IL-5 and IgE compared with patients with only M. pneumoniae infection (12). Therefore, even though the inflammatory patterns seen in patients exposed to M. pneumoniae are not uniform, there is strong evidence of a Th2 type response in a subset of patients.
The human pulmonary anatomy and physiology, compared with rodents and other small animals, differs significantly (23), and, therefore, nonhuman primate models are favored. Particularly in asthma, where there is an emphasis on the inflammatory mechanisms of disease, nonhuman primate models offer significant advantages to murine and other animal models. For example, leukotrienes and other important cytokines in asthma may have minimal or no effect on airway inflammation in mice (24). In fact, compared with other phylogenetically similar nonhuman primates, the baboon exhibited more robust responses to leukotriene D4 (24). Research baboons often live in corrals that provide environmental exposures to dust, nonfiltered air, and other particulates similar to humans (25). In addition to these advantages, baboons are large enough to allow the use of medical equipment, such as bronchoscopes.
Similar to our baboon data, we reported that both adult and pediatric patients with asthma who test positive for CARDS toxin by PCR or antigen capture assays had variable serologic responses, with few patients exhibiting a robust response to CARDS toxin, and the majority displaying a minimal to low antibody response (11, 18). Similar patterns of serological responses are reflected in the baboon. Some animals exposed to recombinant CARDS toxin exhibited a robust antibody response over the 14-day course of the experiment, whereas overall weaker responses were observed in animals infected with M. pneumoniae, similar to what we have observed in patients with asthma (11, 18). The exposure to M. pneumoniae, inciting a subclinical infection, may partially explain M. pneumoniae–associated exacerbation of asthma in humans and asthma-like pulmonary histopathologic changes in baboons. In our study, we demonstrate that, in baboons, exposure to M. pneumoniae did not produce a clinical infection, but resulted in an asthma-like inflammatory response. In addition, we were able to demonstrate the production of significant levels of CARDS toxin at Days 7 and 14 after instillation of M. pneumoniae. Although the bronchus intermedius appeared normal at the time of sampling the ELF, levels of toxin measured by antigen capture were similar to those reported to induce airway hyperresponsiveness in our murine models of CARDS toxin–induced asthma (20). The degree of histological severity correlated with levels of CARDS toxin by antigen capture on Day 7, but not on Day 14. This suggests that the initial degree of exposure may determine the degree of resulting inflammation. In CARDS toxin–exposed animals, the levels of toxin detected by antigen capture were mostly higher on Day 7 compared with Day 14. Despite this, the histopathologic severity was higher on Day 14. It is plausible that, by Day 14, toxin levels in the ELF were lower due to clearance mechanisms of airway secretions and local or systemic absorption. This suggests that CARDS toxin–induced inflammation may persist even after initial exposure. Because CARDS toxin binds to and is internalized by target cells, its persistence in the airways and continued ADP-ribosylating and vacuolating activities promote intracellular events that lead to increased histopathology and hyperinflammation (26). However, these observations are limited by the design of the experiment, given that only two bronchoscopic measurements of toxin levels were possible.
Evidence continues to emerge regarding the important association between M. pneumoniae infection and asthma. This study demonstrates that both M. pneumoniae and CARDS toxin are capable of producing an allergic response in healthy, nonhuman primates with all of the histological features of asthma. Pulmonary instillation of M. pneumoniae resulted in the production of high levels of CARDS toxin by Day 7, despite the normal bronchoscopic appearance of the airway. The pathological features induced by M. pneumoniae and CARDS toxin were very similar. Although there are likely multiple underlying pathologic mechanisms, the results of this study suggest that CARDS toxin plays a major role in the induction of an “asthma-like” histological response after exposure to M. pneumoniae. M. pneumoniae is the leading cause of community-acquired pneumonia among children worldwide. Furthermore, the alarming increase in macrolide resistance and the absence of an effective vaccine further reinforce the need to better understand M. pneumoniae and CARDS toxin–mediated airway pathologies and their potential role in mediating or exacerbating chronic airway disease.
Footnotes
This work was supported by National Institutes of Health grants U19AI070412, RO1AI067716, and RO1AG030119, and Divisional Funds from the Division of Pulmonary and Critical Care Medicine, University of Texas Health Science Center, San Antonio, Texas.
Author Contributions: D.J.M.—performed bronchoscopies, collected and analyzed data, and wrote the manuscript; J.L.M., V.T.W., M.P., and M.P.C.—collected and analyzed data; E.G.B., J.J.C., J.B.B., and P.H.D.—designed the study, collected and analyzed data, and wrote the manuscript; T.R.K.—collected and analyzed data and wrote the manuscript; J.I.P.—designed the study, performed bronchoscopies, collected and analyzed data, and wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0006OC on September 15, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sembajwe G, Cifuentes M, Tak SW, Kriebel D, Gore R, Punnett L. National income, self-reported wheezing and asthma diagnosis from the World Health Survey. Eur Respir J. 2010;35:279–286. doi: 10.1183/09031936.00027509. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2016 [accessed 2016 Dec 1]. Available from: http://ginasthma.org/2016-gina.
- 3.Berkovich S, Millian SJ, Snyder RD. The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child. Ann Allergy. 1970;28:43–49. [PubMed] [Google Scholar]
- 4.Seggev JS, Lis I, Siman-Tov R, Gutman R, Abu-Samara H, Schey G, et al. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy. 1986;57:263–265. [PubMed] [Google Scholar]
- 5.Lieberman D, Lieberman D, Printz S, Ben-Yaakov M, Lazarovich Z, Ohana B, et al. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med. 2003;167:406–410. doi: 10.1164/rccm.200209-996OC. [DOI] [PubMed] [Google Scholar]
- 6.Cosentini R, Tarsia P, Canetta C, Graziadei G, Brambilla AM, Aliberti S, et al. Severe asthma exacerbation: role of acute Chlamydophila pneumoniae and Mycoplasma pneumoniae infection. Respir Res. 2008;9:48. doi: 10.1186/1465-9921-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz G, Kraft M. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am. 2010;30:575–585 (vii–viii.). doi: 10.1016/j.iac.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–25. [PubMed] [Google Scholar]
- 9.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–1353. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 10.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 11.Wood PR, Hill VL, Burks ML, Peters JI, Singh H, Kannan TR, et al. Mycoplasma pneumoniae in children with acute and refractory asthma. Ann Allergy Asthma Immunol. 2013;110:328–334.e1. doi: 10.1016/j.anai.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Chen Q, Shi C, Lv H, Xu X, Yu L. Changes of serum TNF-α, IL-5 and IgE levels in the patients of mycoplasma pneumonia infection with or without bronchial asthma. Int J Clin Exp Med. 2015;8:3901–3906. [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh JJ, Wang YC, Hsu WH, Kao CH. Incident asthma and Mycoplasma pneumoniae: a nationwide cohort study. J Allergy Clin Immunol. 2016;137:1017–23.e1, 6. doi: 10.1016/j.jaci.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015;9:CD002997. doi: 10.1002/14651858.CD002997.pub4. [DOI] [PubMed] [Google Scholar]
- 15.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci USA. 2006;103:6724–6729. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4:e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina JL, Coalson JJ, Brooks EG, Winter VT, Chaparro A, Principe MF, et al. Mycoplasma pneumoniae CARDS toxin induces pulmonary eosinophilic and lymphocytic inflammation. Am J Respir Cell Mol Biol. 2012;46:815–822. doi: 10.1165/rcmb.2011-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters J, Singh H, Brooks EG, Diaz J, Kannan TR, Coalson JJ, et al. Persistence of community-acquired respiratory distress syndrome toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest. 2011;140:401–407. doi: 10.1378/chest.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weatherall D. The use of non-human primates in research. 2006 [accessed 2006 Dec 5]. Available from: http://www.royalsoc.ac.uk/weatherall.
- 20.Medina JL, Coalson JJ, Brooks EG, Le Saux CJ, Winter VT, Chaparro A, et al. Mycoplasma pneumoniae CARDS toxin exacerbates ovalbumin-induced asthma-like inflammation in BALB/c mice. PLoS One. 2014;9:e102613. doi: 10.1371/journal.pone.0102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao L, Cong Z, Li X, Zou H, Cao L, Guo Y. Changes in levels of IL-9, IL-17, IFN-γ, dendritic cell numbers and TLR expression in peripheral blood in asthmatic children with Mycoplasma pneumoniae infection. Int J Clin Exp Pathol. 2015;8:5263–5272. [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito S, Droghetti R, Bosis S, Claut L, Marchisio P, Principi N. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr Pulmonol. 2002;34:122–127. doi: 10.1002/ppul.10139. [DOI] [PubMed] [Google Scholar]
- 23.Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Shenoy AT, Gilley RP, et al. A non-human primate model of severe pneumococcal pneumonia. PLoS One. 2016;11:e0166092. doi: 10.1371/journal.pone.0166092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seehase S, Schlepütz M, Switalla S, Mätz-Rensing K, Kaup FJ, Zöller M, et al. Bronchoconstriction in nonhuman primates: a species comparison. J Appl Physiol (1985) 2011;111:791–798. doi: 10.1152/japplphysiol.00162.2011. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin WJ, Coelho AM., Jr Development of a large scale baboon breeding program. Lab Anim Sci. 1982;32:672–676. [PubMed] [Google Scholar]
- 26.Krishnan M, Kannan TR, Baseman JB. Mycoplasma pneumoniae CARDS toxin is internalized via clathrin-mediated endocytosis. PLoS One. 2013;8:e62706. doi: 10.1371/journal.pone.0062706. [DOI] [PMC free article] [PubMed] [Google Scholar]