Abstract
Radiation-induced pulmonary fibrosis is a severe complication of patients treated with thoracic irradiation. We have previously shown that syndecan-2 reduces fibrosis by exerting alveolar epithelial cytoprotective effects. Here, we investigate whether syndecan-2 attenuates radiation-induced pulmonary fibrosis by inhibiting fibroblast activation. C57BL/6 wild-type mice and transgenic mice that overexpress human syndecan-2 in alveolar macrophages were exposed to 14 Gy whole-thoracic radiation. At 24 weeks after irradiation, lungs were collected for histological, protein, and mRNA evaluation of pulmonary fibrosis, profibrotic gene expression, and α-smooth muscle actin (α-SMA) expression. Mouse lung fibroblasts were activated with transforming growth factor (TGF)-β1 in the presence or absence of syndecan-2. Cell proliferation, migration, and gel contraction were assessed at different time points. Irradiation resulted in significantly increased mortality and pulmonary fibrosis in wild-type mice that was associated with elevated lung expression of TGF-β1 downstream target genes and cell death compared with irradiated syndecan-2 transgenic mice. In mouse lung fibroblasts, syndecan-2 inhibited α-SMA expression, cell contraction, proliferation, and migration induced by TGF-β1. Syndecan-2 attenuated phosphoinositide 3-kinase/serine/threonine kinase/Rho-associated coiled-coil kinase signaling and serum response factor binding to the α-SMA promoter. Syndecan-2 attenuates pulmonary fibrosis in mice exposed to radiation and inhibits TGF-β1–induced fibroblast–myofibroblast differentiation, migration, and proliferation by down-regulating phosphoinositide 3-kinase/serine/threonine kinase/Rho-associated coiled-coil kinase signaling and blocking serum response factor binding to the α-SMA promoter via CD148. These findings suggest that syndecan-2 has potential as an antifibrotic therapy in radiation-induced lung fibrosis.
Keywords: syndecan-2, radiation, pulmonary fibrosis, fibroblast, myofibroblast
Clinical Relevance
Radiation-induced pulmonary fibrosis is a severe complication of patients treated with thoracic irradiation and is associated with significant morbidity and mortality. Here, we show that syndecan-2 attenuates pulmonary fibrosis in mice exposed to radiation and inhibits fibroblast activation via CD148. These findings suggest that syndecan-2 has potential as an antifibrotic therapy in radiation-induced lung fibrosis.
Radiation therapy is a mainstay of treatment for thoracic malignancies, yet its use is complicated by lung toxicity, particularly fibrosis. Radiation-induced pulmonary fibrosis, which typically develops months after irradiation and can progress for years, has no effective treatment and is associated with significant morbidity and mortality (1). This often limits the depth, dose, and, ultimately, effectiveness of radiation that can be delivered (1, 2).
Despite substantial progress made in understanding the pathogenesis of fibrotic diseases, the mechanisms underlying radiation-induced pulmonary fibrosis remain poorly understood. Irradiation activates a number of signaling pathways that involve recruitment of immune cells, production of inflammatory cytokines, and generation of reactive oxygen species that result in cell death and pathologic tissue remodeling (3, 4). Transforming growth factor (TGF)-β1 is a pleiotropic cytokine that promotes the differentiation of fibroblasts into myofibroblasts and plays a major role in fibrosis (5, 6). Myofibroblasts contribute to the excessive deposition of extracellular matrix and stress fiber formation by producing contractile proteins, including α-smooth muscle actin (α-SMA), a marker of myofibroblast differentiation (6). There is a need to further characterize the factors that interact with TGF-β1 signaling and develop therapies to productively counteract them.
Syndecans are a family of four transmembrane heparan-sulfate proteoglycans that are expressed in multiple cell types, and may play a protective role in pulmonary fibrosis (7, 8). Syndecan-1 interacts with CXCL8 to promote resolution of experimental lung injury, whereas syndecan-4 has been shown to reduce pulmonary fibrosis by inhibiting fibroblast migration (9, 10). Our group has previously demonstrated that syndecan-2 expression is elevated in alveolar macrophages from subjects with idiopathic pulmonary fibrosis (IPF) and that overexpression of human syndecan-2 (hsdc2) in transgenic (TG) mice reduces bleomycin-induced lung fibrosis (11). Syndecan-2 appears to exert its antifibrotic effects in epithelial cells by promoting internalization and degradation of TGF-β receptor 1, thereby inhibiting TGF-β1 downstream signaling and protecting alveolar epithelial cells from TGF-β1–induced apoptosis (11).
In the present study, we used a murine model to determine the role of syndecan-2 in the development of radiation-induced lung fibrosis. We hypothesized that syndecan-2 would reduce TGF-β1–mediated fibroblast proliferation and differentiation into myofibroblasts, and, ultimately, attenuate radiation-induced pulmonary fibrosis.
Methods
For additional details regarding materials and methods, please see the data supplement
Animals and Radiation Treatment
C57BL/6 wild-type (WT) mice were purchased from NCI. Syndecan-2 TG mice, described previously (11), were generated using a transgene containing the hsdc2 coding sequence under the control of the scavenger receptor A enhancer/promoter (SREP), a kind gift from Dr. Jeanine D’Armiento (Columbia University, New York, NY). Only female mice were used in this study, as it has previously been demonstrated that they are more susceptible to radiation fibrosis than males (12). Mice were irradiated with 14 Gy of whole-thoracic radiation at 8–11 weeks of age (n = 10 in each respective group). Mice were anesthetized before radiation exposure with 2, 2, 2 tribromoethanol (Avertin, 300 mg/kg; Sigma) via intraperitoneal injection and positioned in a multianimal Plexiglas holder. The mice were shielded in lead with the exception of a 16 × 16-mm window above each animal’s entire thorax. A Philips RT 250 X-ray orthovoltage therapy unit (Philips) was used to deliver 14 Gy to the thorax at a rate of 101.5 cGy/min. Dose rate was calibrated daily by use of an ionization chamber and Condenser R-Meter (Victoreen/Fluke). Dosimetry was confirmed by TLD or nanoDot dosimeters (Landauer). Nonirradiated mice were anesthetized, but did not receive any radiation. All animals were housed in shoebox-type plastic cages with hardwood chip bedding; food and water were provided ad libitum. The Animal Care and Use Committee of Lovelace Respiratory Research Institute approved all animal protocols.
Hydroxyproline Assay
To quantify collagen deposition, the left lung from each mouse was hydrolyzed in HCl 6 N for 24 hours at 110°C, and hydroxyproline levels were quantified as previously described (11). Each sample was tested in triplicate. Data are expressed as micrograms of hydroxyproline per left lung.
Fibroblast Collagen Gel Contraction Assay
Cell pellets of mouse fibroblasts were mixed with eight volumes of rat tail type I collagen suspension, one volume of ×1-concentrated PBS, and one volume of reconstitution buffer (2% sodium bicarbonate and 4.77% Hepes dissolved in 0.05 N NaOH) at a concentration of 2 × 106 cells/ml. Cell-populated collagen solution was immediately poured into a 24-well plate (0.5 ml/well) and incubated at 37°C for 1 hour to permit complete gelation. After 2 days, gels were gently released from the plate with a spatula and overlaid with culture media. Gel images were taken at 0, 12, 24, and 36 hours.
Fibroblast Wound Healing Assay
Fibroblast migration was assessed using a “scratch wound” assay, as described previously (13). Fibroblasts were seeded on a six-well plate to a final concentration of 5 × 105 cells/ml. Once confluent, a cell-free area was created with a pipette in each well. Cells were then treated with either TGF-β1 (10 ng/ml) or TGF-β1 along with recombinant hsdc2 (0.5 μg/ml) for 24 hours. Wound closure and fibroblast migration was evaluated at 0, 4, 8, 12, 16, 20, and 24 hours after treatment.
Statistical Analyses
Data are expressed as mean (±SEM). Comparisons of mortality were made by analyzing Kaplan–Meier survival curves, and then log-rank tests to assess for differences in survival. For comparisons between two groups, we used Student’s unpaired t test, and statistical significance was defined as P less than 0.05. One-way ANOVA, followed by Newman–Keuls or Tukey’s post-test analysis, was used for analysis of more than two groups. The numbers of samples per group (n), or the numbers of experiments, are specified in the figure legends.
Results
Overexpression of Syndecan-2 Improves Survival in Mice Exposed to Thoracic Radiation
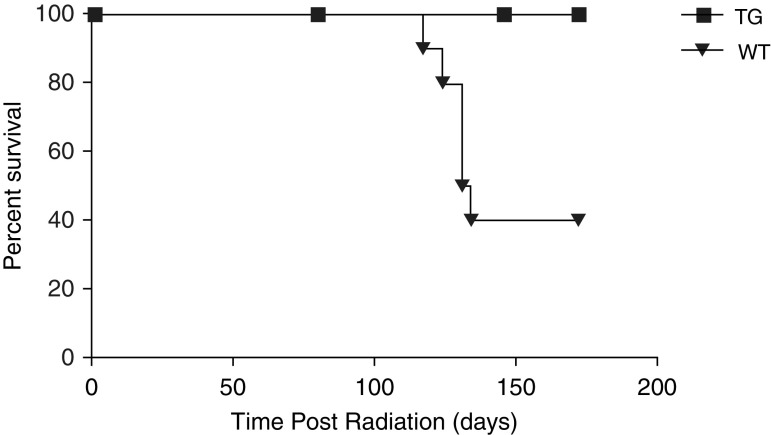
To examine the effects of syndecan-2 in a murine model of radiation-induced pulmonary fibrosis, female C57BL/6 WT and TG mice that overexpress hsdc2 were exposed to a single dose of 14 Gy radiation at 8–11 weeks of age. These TG mice have macrophage-specific overexpression of hsdc2 (scavenger receptor A enhancer/promoter–hsdc2) and have been previously described in detail (11). The two exposure groups were then followed for a total of 24 weeks (Figure 1). Irradiated WT mice began dying at 112 days (16 wk) after radiation exposure. Among irradiated groups, WT mice experienced 60% mortality at the end of 24 weeks, in contrast to TG mice, which had no mortality. Nonirradiated WT and TG mice had no mortality during 24 weeks as well (data not shown).
Figure 1.
Overexpression of syndecan-2 increases survival in transgenic (TG) mice 24 weeks after radiation exposure. C57BL/6 wild-type (WT; n = 10) and scavenger receptor A enhancer/promoter–human syndecan-2 (hsdc2) TG mice (n = 10) with macrophage-specific overexpression of hsdc2 were exposed to a single dose of 14 Gy radiation delivered to the thorax by a Philips RT 250 X-ray therapy unit at 8–11 weeks of age. Survival was 100% in TG mice at Day 168 (Week 24) compared with 40% in irradiated WT (WT Rad) mice (P < 0.001). Survival for WT versus TG mice (P < 0.001); data are presented as a Kaplan–Meier survival curve.
Overexpression of Syndecan-2 Reduces Radiation-induced Pulmonary Fibrosis
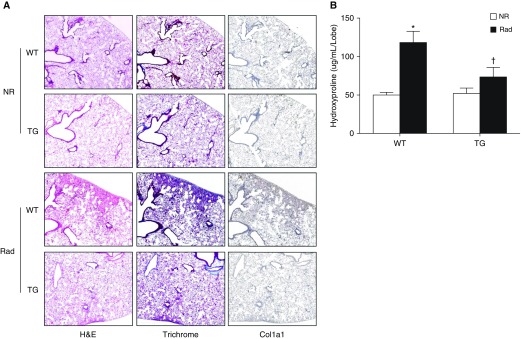
To determine the effect of syndecan-2 overexpression on radiation-induced pulmonary fibrosis, lung sections of subsets of WT and TG mice were stained with hematoxylin and eosin, trichrome, and collagen-1a1 antibody 16 weeks after radiation exposure. Hematoxylin and eosin staining demonstrated less interstitial thickening and inflammation in irradiated TG (TG Rad) mice compared with their WT counterparts (Figure 2A). Staining for trichrome and collagen1a1 demonstrated less collagen deposition in the lung interstitium of TG mice. Collagen content was quantified using a hydroxyproline assay (Figure 2B). Lungs from TG Rad mice had significantly less hydroxyproline compared with irradiated WT mice (73.59 ± 20.56 versus 118.25 ± 41.40 μg/ml/lobe). Apoptosis of alveolar epithelial cells plays a key role in the development of pulmonary fibrosis, and is induced by a variety of injuries, including thoracic irradiation (14, 15). Irradiated mouse lungs had increased terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining compared with nonirradiated lungs at Week 16, but those from TG mice had significantly fewer TUNEL-positive cells (P < 0.01; see Figure E1 in the data supplement). Moreover, syndecan-2 overexpression significantly attenuated apoptosis only in lung epithelial cells. There was a trend toward decreased apoptosis in endothelial cells that did not reach statistical significance. No differences were observed in fibroblasts/myofibroblasts compared with WT mice after irradiation (Figure E2). Immunoblots of whole-lung homogenates 16 weeks after radiation exposure showed decreased expression of caspase-9 in TG mice compared with WT mice (Figure E3). Finally, we measured total cell count in BAL fluid and found that TG mice had fewer cells after radiation injury compared with WT mice (Figure E4). Taken together, these findings demonstrate that overexpression of syndecan-2 attenuates development of radiation-induced injury and pulmonary fibrosis in mice.
Figure 2.
Overexpression of syndecan-2 significantly attenuates radiation-induced pulmonary fibrosis. (A) Lung sections of WT and syndecan-2–overexpressing TG mice 24 weeks after radiation exposure were stained with hematoxylin and eosin (H&E), trichrome, and collagen-1a1 (Col1a1) antibody. Pulmonary fibrosis and inflammatory cell infiltration were decreased in TG radiated (Rad) mice compared with WT Rad mice. (B) Hydroxyproline content (left lung) was measured in the left lung of TG and WT mice (n = 20) 24 weeks after irradiation. TG Rad mice had significantly less hydroxyproline compared with WT mice (n = 8–10; WT nonirradiated [NR] versus WT Rad, *P < 0.05; WT Rad versus TG Rad, †P < 0.05).
Overexpression of Syndecan-2 Reduces Expression of TGF-β1 Target Genes in Radiation-induced Pulmonary Fibrosis
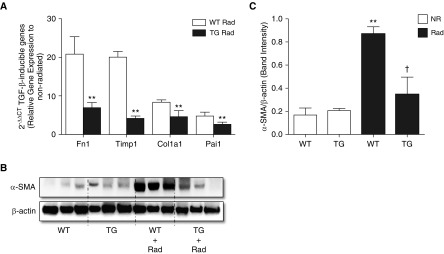
TGF-β1 plays a central role in the induction of fibrogenesis caused by radiation (16, 17). We therefore investigated the effect of syndecan-2 on TGF-β1 activity by evaluating the expression of known TGF-β1 target genes with real-time PCR. Gene expression of fibronectin-1, collagen 1a1, tissue inhibitor of metalloproteinase-1, and plasminogen activator inhibitor 1 (Pai1) in whole-lung homogenates 16 weeks after radiation exposure was significantly decreased in TG Rad mice compared with WT mice, consistent with decreased TGF-β1 activity (Figure 3A).
Figure 3.
Overexpression of syndecan-2 inhibits transforming growth factor (TGF)-β1 target gene and α-smooth muscle actin (α-SMA) expression in the lungs of irradiated mice. (A) Gene expression, as measured by quantitative PCR, of fibronectin-1 (Fn1), Col1a1, tissue inhibitor of metalloproteinase-1 (Timp1), and plasminogen activator inhibitor 1 (Pai1) was significantly downregulated in lungs of TG mice compared with WT mice 16 weeks after radiation lung injury (**P < 0.01 for all four genes). (B) α-SMA protein levels were decreased in TG Rad mice compared with WT Rad mice. (C) Relative levels of α-SMA were determined by densitometry (n = 8–10; WT NR versus WT Rad, **P < 0.001; and WT Rad versus TG Rad, †P < 0.05).
Overexpression of Syndecan-2 Reduces Radiation-induced Fibroblast-to-Myofibroblast Differentiation
Fibroblast-to-myofibroblast differentiation is a hallmark of fibrogenesis, the latter cell type functioning to produce contractile proteins, such as α-SMA. Immunoblotting and immunohistochemistry demonstrated that lung tissue from TG mice had significantly less α-SMA compared with WT mice in response to radiation (Figure 3B), suggesting that in vivo expression of syndecan-2 inhibits fibroblast-to-myofibroblast differentiation.
Syndecan-2 Decreases TGF-β1–induced Fibroblast Contraction, Migration, and Proliferation
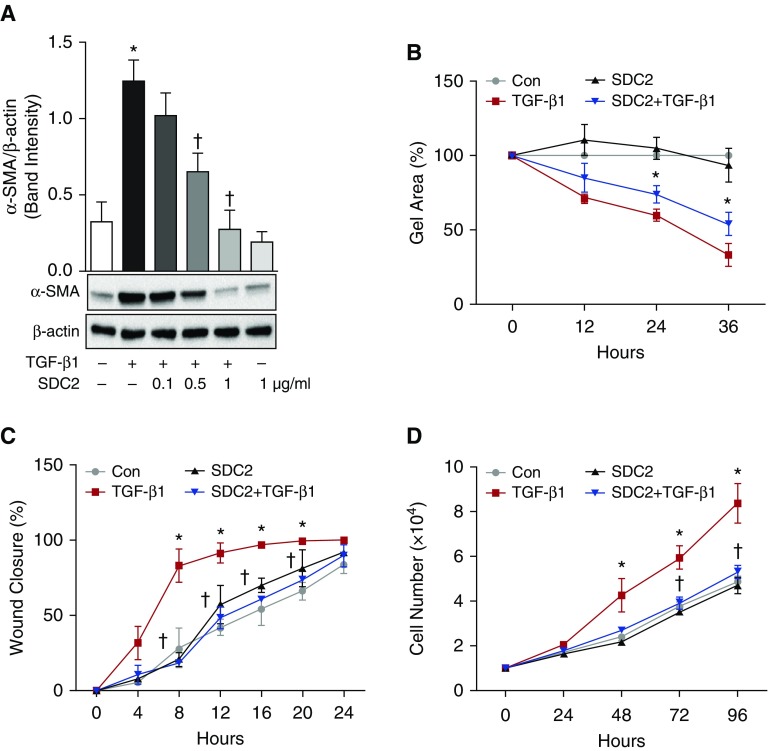
Given the importance of fibroblast-to-myofibroblast differentiation in the development of lung fibrosis (6, 18–20), we next sought to determine the extent to which syndecan-2 regulates fibroblast-to-myofibroblast differentiation in vitro. To investigate the direct effects of syndecan-2 on these processes, we treated a mouse lung fibroblast cell line (MLg2908) with TGF-β1 in the presence or absence of recombinant hsdc2. Myofibroblast differentiation was determined by measuring α-SMA expression in fibroblasts. As expected, TGF-β1–induced expression of α-SMA in fibroblasts was abrogated by concurrent administration of recombinant hsdc2 in a dose-dependent fashion (Figure 4A). We also evaluated the effect of syndecan-2 on cell contractility by culturing fibroblasts on a three-dimensional collagen gel. Cell contractility was significantly decreased in fibroblasts exposed to both TGF-β1 and syndecan-2 compared with those exposed to TGF-β1 alone (P < 0.05; Figure 4B). Next, we observed that syndecan-2 inhibits migration without affecting cell proliferation at 24 hours after TGF-β1 stimulation, whereas significant differences in cell proliferation were observed at 3 and 4 days after induction. (Figures 4C and 4D). Consistent with our results in MLg2908 cells, syndecan-2 treatment also inhibited α-SMA expression in primary mouse lung fibroblasts after TGF-β1 stimulation (Figure E5). These data suggest that syndecan-2 potently inhibits TGF-β1–induced lung fibroblast migration and proliferation, and myofibroblast differentiation.
Figure 4.
Syndecan-2 reduces mouse fibroblast contractility, migration, and proliferation induced by TGF-β1. (A) Lung fibroblasts were treated with TGF-β1 (10 ng/ml) in the presence or absence of syndecan-2 (SDC2; 0.1, 0.5, 1 μg/ml) for 24 hours and were then harvested for Western blot analysis. Fibroblasts treated with syndecan-2 had significantly less α-SMA expression. P < 0.05; significant comparisons: * versus untreated control cells; † versus cells treated with TGF-β1 alone. (B) Fibroblast contractility assays were performed. Syndecan-2 significantly attenuated gel contraction in fibroblasts exposed to TGF-β1 at 24 and 36 hours (*P < 0.05). Con = control. (C) Syndecan-2 significantly reduced fibroblast migration in an in vitro model of wound healing. (D) Fibroblasts were plated and stimulated with TGF-β1 (10 ng/ml) in the presence or absence of syndecan-2 (0.5 μg/ml). At different time points, cell proliferation was measured by counting of live cells using trypan blue exclusion assay. The data are presented as mean (±SEM), n = 3/group, with testing by one-way ANOVA (P < 0.05; significant comparisons: * versus untreated control cells (con) and † versus cells treated with TGF-β1 alone at the corresponding time point).
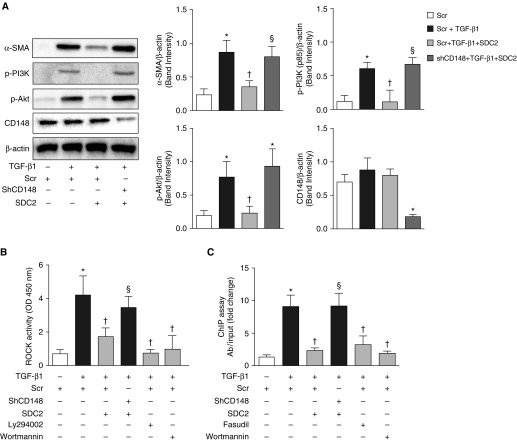
Syndecan-2 Attenuates TGF-β1–induced Fibroblast-to-Myofibroblast Differentiation by Regulating Phosphoinositide 3-Kinase/Serine/Threonine K/Rho-associated Coiled-Coil Kinase Signaling via CD148
It has been shown that TGF-β1 can activate phosphoinositide 3-kinase (PI3K)/serine/threonine kinase (Akt) and Rho-associated coiled-coil kinase (ROCK) signaling proteins to induce the expression of α-SMA and myofibroblast transformation (21, 22). Interestingly, it has also been shown that syndecan-2 can bind to and activate phosphatase activity of membrane-bound protein tyrosine phosphatase eta (PTPRj/CD148) (23, 24). We thus examined whether syndecan-2 inhibits lung fibroblast activation via CD148. TGF-β1–induced α-SMA expression was significantly reduced by syndecan-2 in scramble-transfected cells, but not in short hairpin (sh)CD148 cells, suggesting that CD148 plays an important role in syndecan-2–mediated antifibrotic effects (Figure 5A). Syndecan-2 also significantly attenuated the activation of PI3K/Akt and ROCK proteins in CD148-expressing fibroblasts, but not in CD148 knockdown cells (Figures 5A and 5B). In addition, PI3K/Akt inhibitors, wortmannin and Ly294002, significantly downregulated ROCK activity in TGF-β1–induced lung fibroblasts (Figure 5B), suggesting that PI3K/Akt signaling is upstream of ROCK activation, as previously described (25).
Figure 5.
Syndecan-2 inhibits phosphoinositide 3-kinase (PI3K)/serine/threonine kinase (Akt)/Rho-associated coiled-coil kinase (ROCK)/serum response factor (SRF) signaling and α-SMA expression in TGF-β1–stimulated mouse lung fibroblasts via CD148. MLg2908 fibroblasts were transfected with scramble (scr) or short hairpin (sh)CD148 using lentiviral particles. After puromycin selection, cells were treated with TGF-β1 (10 ng/ml) for 24 hours. Knockdown of CD148 reversed the inhibitory effects of syndecan-2 on (A) expression of α-SMA, phosphorylated (p)-PI3K (p-p85), and phosphorylated (p)-Akt, (B) ROCK activity, and (C) binding of SRF to the α-SMA promoter in TGF-β1–stimulated fibroblasts. TGF-β1–stimulated scr cells were treated with PI3K/Akt inhibitors Ly294002 (5 μM) and wortmannin (10 nM), and ROCK inhibitor fasudil (10 μM) as positive controls. The data are presented as mean (±SEM), n = 3/group, with testing by one-way ANOVA (P < 0.05; significant comparisons: * versus cells treated with scr alone; † versus cells treated with scr and TGF-β1; § versus cells treated with scr, TGF-β1 and SDC2). ChIP = chromatin immunoprecipitation; OD = optical density.
It has been demonstrated that increased ROCK activity may lead to serum response factor (SRF) activation (26, 27), which, in turn, binds to serum response elements in the promoter regions of the human and rodent α-SMA gene (28). We observed that Rho/ROCK pathway inhibition downregulates α-SMA expression in lung fibroblasts after TGF-β1 stimulation (Figure E6) (21). Thus, we asked whether inhibition of PI3K/Akt/ROCK signaling by syndecan-2 via CD148 regulates SRF binding to the α-SMA promoter. Using a chromatin immunoprecipitation assay, we found that TGF-β1 stimulated binding of SRF to the α-SMA promoter in mouse lung fibroblasts. Syndecan-2 significantly attenuated this binding in scramble, but not in short hairpin (sh)CD148-transfected cells (Figure 5C).
Taken together, these findings suggest that syndecan-2 modulates PI3K/Akt/ROCK signaling via CD148, thereby reducing SRF binding to the α-SMA promoter and inhibiting lung fibroblast-to-myofibroblast differentiation.
Discussion
More than half of cancer patients receive radiation therapy during their treatment course. Pulmonary fibrosis is a frequent complication of radiation therapy for thoracic malignancies, most commonly non–small-cell lung cancer and breast cancer. Incidental irradiation of normal lung tissue is unavoidable, but can severely compromise lung function, often preventing delivery of the dose and field of radiation necessary to effectively treat tumors. Ionizing radiation stimulates a number of molecular pathways that lead to generation of reactive oxygen species, release of proinflammatory and profibrotic cytokines, and, ultimately, replacement of normal lung parenchyma with fibrotic tissue (29). Radiation fibrosis is largely refractory to medical therapy (30), and there is an unmet need to develop therapies that can prevent or minimize its development.
In this study, we investigated the potential therapeutic role of syndecan-2 in radiation-induced pulmonary fibrosis. Previous work done by our group demonstrated that syndecan-2 expression is elevated in alveolar macrophages of IPF lungs, and that syndecan-2 overexpression may be protective against bleomycin-induced pulmonary fibrosis in mice. Furthermore, exogenous administration of recombinant hsdc2 abrogated lung fibrosis in WT mice by inhibiting alveolar epithelial apoptosis, highlighting its cytoprotective effects and therapeutic potential (11). Here, we demonstrate that TG Rad mice with macrophage-specific overexpression of syndecan-2 have improved survival and less pulmonary fibrosis compared with their WT irradiated counterparts. Lungs of syndecan-2–overexpressing mice exhibited reduced expression of profibrotic TGF-β1 target genes, less cell death, and decreased α-SMA in response to radiation.
In contrast to radiation-induced fibrosis, which is usually preceded by an acute phase of intense inflammation initiated by a definitive insult (1), no single etiologic agent has been identified in IPF, and its histopathologic pattern, usual interstitial pneumonia, is characterized by the absence of significant inflammation (26). Nonetheless, both diseases share fibrosis as a common endpoint, with up-regulation of TGF-β1 signaling pathways leading to aberrant fibroblast proliferation, differentiation into myofibroblasts, and excessive production of extracellular matrix (16–18). In vitro, syndecan-2 was previously shown to promote caveolin-1–dependent internalization of TGF-β receptor 1, thereby inhibiting TGF-β1 signaling and alveolar epithelial apoptosis (11). However, the effect of syndecan-2 on specific downstream events in lung fibrogenesis, including fibroblast proliferation and migration, were previously unknown.
A recent report has shown that shed syndecan-2 can bind to the receptor-type protein tyrosine phosphatase eta (PTPRj/CD148) and trigger its phosphatase activity (23, 24). De Rossi and colleagues (24) demonstrated that shed syndecan-2 inhibits blood vessel formation through CD148 binding in vascular endothelial cells. CD148 dephosphorylates tyrosine residues in β1-integrin and inhibits pro-angiogenic signaling (24). It is thought that CD148 exerts tumor suppressor activity due to its ability to dephosphorylate and inactivate cell surface growth factor receptors and inhibit critical downstream signaling events associated with cell survival (31, 32). In addition, it has been shown that CD148 can directly interact with and dephosphorylate the regulatory subunit of PI3K (p85), which, in turn, results in inhibition of PI3K/Akt signaling (33).
Hyperactivation of PI3K/Akt plays an important role in the profibrotic phenotype of IPF-derived lung fibroblasts by promoting cell proliferation and migration and myofibroblast differentiation (34, 35). PI3K/Akt signaling is critical for ROCK activation (25, 36), which has been implicated in hepatic, cardiac, and lung fibrosis. Pharmacological ROCK inhibition has been shown to significantly reduce experimental lung fibrosis induced by bleomycin or radiation (21, 37–40).
These observations led us to investigate whether syndecan-2 exerts its antifibrotic effects via CD148 and by modulating PI3K/Akt/ROCK signaling. We found that syndecan-2 significantly attenuated phosphorylation of PI3K (p85 subunit) and activation of the Akt and ROCK pathway in TGF-β1–activated lung fibroblasts, but this effect was lost in CD148-deficient cells. We then demonstrated that syndecan-2 inhibits TGF-β1–mediated effects of SRF binding to the α-SMA promoter in lung fibroblasts.
SRF is a multifaceted transcription factor, encoded by a single gene that is abundantly expressed in most cell types (41). SRF binds with high affinity to the palindromic CC(A/T)6GG DNA sequence (called the CArG-box), and more than 200 SRF target genes have been identified (42). Such versatile transcriptional activity can be dictated by SRF’s ability to bind different cofactors, such as the ternary complex factor subclass of Ets-type cofactors and the myocardin family of coactivators (myocardin and myocardin related transcription factors) (43–45). In the current study, syndecan-2 inhibits SRF binding to α-SMA promoter region by down-regulation of PI3K/Akt/ROCK signaling. However, future studies could focus on elucidating the effect of syndecan-2 on SRF cofactors, such as ternary complex factor and MRTF.
Importantly, activated ROCK can directly affect actin filament assembly and cell contraction through phosphorylation and inactivation of myosin light-chain phosphatase, which, in turn, leads to phosphorylation and activation of myosin light chain (46). Further investigation is necessary to identify how syndecan-2 regulates other key features of fibrogenesis, such as actin filament assembly, stress fiber formation, and cell contraction.
In conclusion, we have demonstrated that syndecan-2 significantly attenuates radiation-induced pulmonary fibrosis in mice and reduces lung fibroblast-to-myofibroblast differentiation, proliferation, and migration induced by TGF-β1 through CD148-mediated signaling. These findings build upon our prior work in which syndecan-2 reduces bleomycin-induced fibrosis, and highlight its therapeutic potential in the prevention and treatment of pulmonary fibrosis.
Acknowledgments
Acknowledgments
The authors acknowledge and thank Dr. Jeanine D’Armiento (Columbia University, New York, NY) for kindly providing the scavenger receptor A enhancer/promoter used for generating the syndecan-2 transgenic mice that were central to the experiments described in this article.
Footnotes
This work was supported by National Institutes of Health grants HL114501 and HL15024.
Author Contributions: K.T., N.G.P.-J., and I.O.R. designed the study; K.T., S.G.C., N.G.P.-J., J.W., J.V., and I.O.R. performed the experiments, contributed materials, and analyzed data; M.D.-E., J.M., X.L., S.E.-C., and M.A.P. contributed intellectual input; K.T., S.G.C., and I.O.R. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0088OC on September 8, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 2.Omarini C, Thanopoulou E, Johnston SR. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res Treat. 2014;146:245–258. doi: 10.1007/s10549-014-3016-5. [DOI] [PubMed] [Google Scholar]
- 3.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Yue X, Shan B, Lasky JA. TGF-β: titan of lung fibrogenesis. Curr Enzym Inhib. 2010;6(6) doi: 10.2174/10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stempien-Otero A, Kim DH, Davis J. Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol. 2016;97:153–161. doi: 10.1016/j.yjmcc.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Gochuico BR, Yu G, Tang X, Osorio JC, Fernandez IE, et al. Syndecan-2 exerts antifibrotic effects by promoting caveolin-1–mediated transforming growth factor-β receptor I internalization and inhibiting transforming growth factor-β1 signaling. Am J Respir Crit Care Med. 2013;188:831–841. doi: 10.1164/rccm.201303-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haston CK, Travis EL. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 1997;57:5286–5291. [PubMed] [Google Scholar]
- 13.Liang CC, Park AY, Guan JL. In vitro scratch assay: convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 14.Kuwano K, Hagimoto N, Nakanishi Y. The role of apoptosis in pulmonary fibrosis. Histol Histopathol. 2004;19:867–881. doi: 10.14670/HH-19.867. [DOI] [PubMed] [Google Scholar]
- 15.Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–8486. doi: 10.1007/s13277-016-5035-9. [DOI] [PubMed] [Google Scholar]
- 16.Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor β gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28:621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Lefaix J, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 19.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-β1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–43009. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 20.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tada S, Iwamoto H, Nakamuta M, Sugimoto R, Enjoji M, Nakashima Y, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. J Hepatol. 2001;34:529–536. doi: 10.1016/s0168-8278(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Tang H, Lin H, Mao J, Gao L, Liu J, et al. Rho/Rock cross-talks with transforming growth factor-β/Smad pathway participates in lung fibroblast-myofibroblast differentiation. Biomed Rep. 2014;2:787–792. doi: 10.3892/br.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteford JR, Xian X, Chaussade C, Vanhaesebroeck B, Nourshargh S, Couchman JR. Syndecan-2 is a novel ligand for the protein tyrosine phosphatase receptor CD148. Mol Biol Cell. 2011;22:3609–3624. doi: 10.1091/mbc.E11-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rossi G, Evans AR, Kay E, Woodfin A, McKay TR, Nourshargh S, et al. Shed syndecan-2 inhibits angiogenesis. J Cell Sci. 2014;127:4788–4799. doi: 10.1242/jcs.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Signorello MG, Leoncini G. Effect of 2-arachidonoylglycerol on myosin light chain phosphorylation and platelet activation: the role of phosphatidylinositol 3 kinase/AKT pathway. Biochimie. 2014;105:182–191. doi: 10.1016/j.biochi.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 27.Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits α-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem. 2014;289:17151–17162. doi: 10.1074/jbc.M114.558130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury. Assessment, management, and prevention. Oncology. 2008;22:37–47. [Discussion, pp. 52–33.] [PubMed] [Google Scholar]
- 30.Abratt RP, Morgan GW, Silvestri G, Willcox P. Pulmonary complications of radiation therapy. Clin Chest Med. 2004;25:167–177. doi: 10.1016/S0272-5231(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 31.Omerovic J, Clague MJ, Prior IA. Phosphatome profiling reveals PTPN2, PTPRJ and PTEN as potent negative regulators of PKB/Akt activation in Ras-mutated cancer cells. Biochem J. 2010;426:65–72. doi: 10.1042/BJ20091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortuso F, Paduano F, Carotenuto A, Gomez-Monterrey I, Bilotta A, Gaudio E, et al. Discovery of PTPRJ agonist peptides that effectively inhibit in vitro cancer cell proliferation and tube formation. ACS Chem Biol. 2013;8:1497–1506. doi: 10.1021/cb3007192. [DOI] [PubMed] [Google Scholar]
- 33.Tsuboi N, Utsunomiya T, Roberts RL, Ito H, Takahashi K, Noda M, et al. The tyrosine phosphatase CD148 interacts with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 2008;413:193–200. doi: 10.1042/BJ20071317. [DOI] [PubMed] [Google Scholar]
- 34.Kral JB, Kuttke M, Schrottmaier WC, Birnecker B, Warszawska J, Wernig C, et al. Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci Rep. 2016;6:23034. doi: 10.1038/srep23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One. 2014;9:e94616. doi: 10.1371/journal.pone.0094616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin MN, Shang DS, Sun W, Li B, Xu X, Fang WG, et al. Involvement of PI3K and ROCK signaling pathways in migration of bone marrow-derived mesenchymal stem cells through human brain microvascular endothelial cell monolayers. Brain Res. 2013;1513:1–8. doi: 10.1016/j.brainres.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–518. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu Y, Dobashi K, Iizuka K, Horie T, Suzuki K, Tukagoshi H, et al. Contribution of small GTPase Rho and its target protein rock in a murine model of lung fibrosis. Am J Respir Crit Care Med. 2001;163:210–217. doi: 10.1164/ajrccm.163.1.2001089. [DOI] [PubMed] [Google Scholar]
- 40.Monceau V, Pasinetti N, Schupp C, Pouzoulet F, Opolon P, Vozenin MC. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets. 2010;11:1395–1404. doi: 10.2174/1389450111009011395. [DOI] [PubMed] [Google Scholar]
- 41.Miano JM. Role of serum response factor in the pathogenesis of disease. Lab Invest. 2010;90:1274–1284. doi: 10.1038/labinvest.2010.104. [DOI] [PubMed] [Google Scholar]
- 42.Cooper SJ, Trinklein ND, Nguyen L, Myers RM. Serum response factor binding sites differ in three human cell types. Genome Res. 2007;17:136–144. doi: 10.1101/gr.5875007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 44.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- 46.Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev. 2015;67:103–117. doi: 10.1124/pr.114.009381. [DOI] [PMC free article] [PubMed] [Google Scholar]