The article by Bryant and colleagues in this issue of the Journal (pp. 170–180) focuses on the pathogenesis of pulmonary hypertension (PH) associated with idiopathic pulmonary fibrosis (IPF), a disease associated with early mortality and a diminished quality of life for which there is no therapy (1). The authors examined the role of granulocytic myeloid-derived suppressor cells (G-MDSCs) in the pathogenesis of PH and pulmonary fibrosis by studying human blood samples and using murine experimental models. They focused on analyzing C-X-C motif chemokine receptor 2 (CXCR2) inhibition, which blocks the trafficking of G-MDSCs in bleomycin-induced pulmonary fibrosis (2) and in development of PH (3).
Previous studies suggested a role for arginase-expressing MDSCs in the pathogenesis of PH (4). Furthermore, increased numbers of circulating MDSCs were found in patients with IPF (5). A chemokine receptor, CXCR2 (also known as IL-8 receptor-b) has been shown to be relevant to the pathogenesis of both PH (3) and pulmonary fibrosis (2). Bryant and colleagues (1) induced pulmonary fibrosis and PH in association with an increase in circulating bone marrow–derived cells by giving clodronate liposomes to bleomycin-treated wild-type mice. Clodronate liposomes chronically deplete macrophages and destabilize the immune system. A maladjusted, dysregulated immune response is believed to be of significant importance in the pathogenesis of IPF and IPF-associated PH (6). They performed fibrosis scoring, hydroxyproline microplate assays, invasive hemodynamic measurements, histologic analysis of the lungs, flow cytometry, and T lymphocyte proliferation assays. The data were also supported by clinical patient samples.
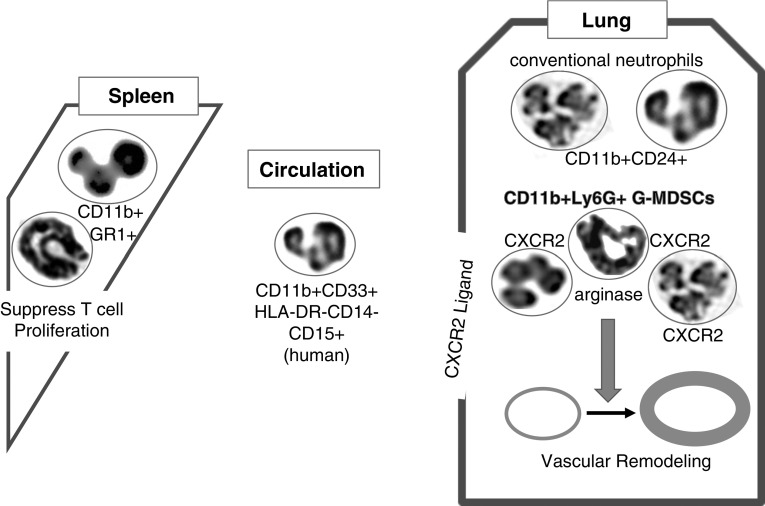
As summarized in Figure 1, they found that a population of G-MDSCs was significantly increased in experimental PH associated with fibrosis, together with highly significantly increased right ventricular systolic pressure measurements. Right ventricular systolic pressure increases, pulmonary vascular remodeling, and arginase expression were significantly inhibited by CXCR2 inhibition. However, recruitment of monocytic MDSCs (M-MDSCs) to the lungs was not decreased, and right ventricular hypertrophy was also not affected by CXCR2 inhibition. The authors confirmed the proposed role for elevated G-MDSCs in interstitial pulmonary fibrosis and interstitial pulmonary fibrosis with PH by performing molecular and cellular analyses of clinical patient samples. They concluded that G-MDSCs play a key role in the pathogenesis of PH associated with lung fibrosis via trafficking to the lung through CXCR2 signaling and the production of arginase-1. Their study suggests a potential therapeutic role for CXCR2 inhibitors.
Figure 1.
Polymorphonuclear leukocytes are not always what they appear to be. Bryant and colleagues (1) show that granulocytic myeloid-derived suppressor cells (G-MDSCs) migrate into the lungs via C-X-C motif chemokine receptor 2 (CXCR2) signaling. The cells are attracted by ligands for CXCR2 that are expressed in the lungs of animals challenged with bleomycin and subjected to macrophage depletion. The G-MDSCs produce arginase-1 and induce pulmonary arterial remodeling and increases in right ventricular systolic pressure. MDSCs isolated from the spleens of the same animals inhibit T cell proliferation. The numbers of G-MDSCs are increased in the circulation of patients with interstitial pulmonary fibrosis. The authors used state-of-the-art flow-cytometric markers (8) to identify the G-MDSCs. These findings are interesting given that human (18) and mouse (19) G-MDSCs have a microscopic morphology that resembles polymorphonuclear neutrophils. Further, the mouse GR1 marker (20) has been extensively used to characterize the activity of neutrophils (17,000 results in Google Scholar) and recognizes Ly-6G and Ly-6C, which are markers for mouse G-MDSCs and monocytic MDSCs, respectively (8). HLA-DR = human leukocyte antigen-antigen D related; Ly6G = lymphocyte antigen 6 complex locus G6D.
The two subsets of MDSCs (M-MDSCs and G-MDSCs) have been studied for their key permissive function in cancer (reviewed in References 7 and 8). MDSCs accumulate in cancerous tissue, strongly inhibit the antitumor immune response, and can provide factors that promote cancer cell proliferation and facilitate metastasis by inducing tissue remodeling (7). Confirming the immune-suppressive role of the G-MDSCs, Bryant and colleagues isolated these cells from the spleens of experimental mice and showed that splenic G-MDSCs significantly suppressed T-lymphocyte proliferation. In the case of PH associated with fibrosis, the G-MDSCs could enhance PH by providing growth signals (e.g., arginase [9–11]) to smooth muscle cells, thereby enhancing the remodeling of the pulmonary vasculature.
The identification of G-MDSCs can be problematic; for example, under light microscopy, G-MDSCs are similar to neutrophils in that both are polymorphonuclear leukocytes (PMNs) (7). Furthermore, alternative names for these cells are PMN-MDSCs, or Gr-MDSCs based on flow cytometry (see below). Flow cytometry is commonly used to definitely identify G-MDSCs, and Bryant and colleagues used the most recently recommended methods for identifying these cells in mice (CD11b+ and Ly6G+) and humans (CD11b+CD33+CD15+CD14−) (8). In mice, the markers for MDSCs include CD11b, Ly6C, and Ly6G (G-MDSCs are CD11b+ and Ly6G+, and M-MDSCs are CD11b+ and Ly6C+) (8). A commonly used mouse neutrophil marker is GR1, which recognizes both Ly6C and Ly6G (8). In many studies, investigators have used CD11b and GR1 for flow cytometry–based identification of neutrophils. Therefore, microscopic and flow-cytometric analyses of granulocytes in experimental models of inflammatory lung diseases may have already included G-MDSCs, as G-MDSCs and conventional neutrophils coexist in lung tissue. In the model used by Bryant and colleagues, both G-MDSCs and neutrophils were present in the lung tissue, and, importantly, CXCR2 inhibition only blocked G-MDSC trafficking into the tissue. G-MDSCs differ from conventional neutrophils in that they have fewer granules and express more arginase-1. Bryant and colleagues distinguished G-MDSCs and conventional neutrophils using the CD24 marker, another surface molecule that is highly expressed by conventional neutrophils and precursors of conventional neutrophils (12). Their studies strongly suggest that future experimental work in chronic lung diseases should distinguish G-MDSCs from neutrophils.
They also identified arginase-1 as a key product of G-MDSCs that could contribute to pulmonary vascular remodeling. It is intriguing that arginase-1 is strongly induced by IL-13 and IL-4 (13), cytokines that have also been implicated in experimental pulmonary vascular remodeling and PH (14–16). The T helper type 2 (Th2) immune response, characterized by the production of IL-13, is inhibited by immune responses that support neutrophilic inflammation, such as the Th17 response associated with the key cytokine IL-17A, and vice versa. However, studies of patients with chronic asthma exacerbated by air pollution (17), as well as an experimental animal model of PH (15), have shown that IL-13 and IL-17A responses can occur simultaneously. Further, in experimental lung inflammation, the bone marrow shows significantly increased activity, and inflammatory cells are rapidly recruited from bone marrow–derived progenitors. It is tempting to speculate that under these conditions, the IL-17A arm of the response recruits granulocytic bone marrow cells to the lungs, and once in the lungs, the IL-13 arm of the immune response initiates arginase-1 expression in the granulocytic bone marrow cells, giving these cells a G-MDSC phenotype.
In conclusion, the study by Bryant and colleagues (1) highlights the significance of G-MDSCs, arginase-1, and CXCR2 in PH associated with fibrosis. Their intriguing findings are supported by previous studies that found increased numbers of circulating MDSCs in PH (4) and IPF (5). The data strongly suggest that investigators need to be more discriminating when studying PMNs in inflammatory lung diseases, because G-MDSCs and conventional neutrophils have dramatically distinct functions.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bryant AJ, Shenoy V, Fu C, Marek G, Lorentsen KJ, Herzog EL, et al. Myeloid-derived suppressor cells are necessary for development of pulmonary hypertension Am J Respir Cell Mol Biol 201858170–180.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffê E, Souza AL, et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2009;40:410–421. doi: 10.1165/rcmb.2007-0364OC. [DOI] [PubMed] [Google Scholar]
- 3.Burton VJ, Holmes AM, Ciuclan LI, Robinson A, Roger JS, Jarai G, et al. Attenuation of leukocyte recruitment via CXCR1/2 inhibition stops the progression of PAH in mice with genetic ablation of endothelial BMPR-II. Blood. 2011;118:4750–4758. doi: 10.1182/blood-2011-05-347393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, et al. Circulating myeloid-derived suppressor cells are increased and activated in pulmonary hypertension. Chest. 2012;141:944–952. doi: 10.1378/chest.11-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez IE, Greiffo FR, Frankenberger M, Bandres J, Heinzelmann K, Neurohr C, et al. Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur Respir J. 2016;48:1171–1183. doi: 10.1183/13993003.01826-2015. [DOI] [PubMed] [Google Scholar]
- 6.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 7.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1151–L1159. doi: 10.1152/ajplung.00183.2009. [DOI] [PubMed] [Google Scholar]
- 11.Cowburn AS, Crosby A, Macias D, Branco C, Colaço RD, Southwood M, et al. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci USA. 2016;113:8801–8806. doi: 10.1073/pnas.1602978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MH, Yang D, Kim M, Kim SY, Kim D, Kang SJ. A late-lineage murine neutrophil precursor population exhibits dynamic changes during demand-adapted granulopoiesis. Sci Rep. 2017;7:39804. doi: 10.1038/srep39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 14.Cho W, Chen N, Tang C, Elias JA, Lee C. IL-13 induces vascular remodeling and pulmonary arterial hyptertension via arginase 2-dependent pathway. Am J Respir Crit Care Med. 2010;181:A6323. [Google Scholar]
- 15.Park SH, Chen WC, Esmaeil N, Lucas B, Marsh LM, Reibman J, et al. Interleukin 13- and interleukin 17A-induced pulmonary hypertension phenotype due to inhalation of antigen and fine particles from air pollution. Pulm Circ. 2014;4:654–668. doi: 10.1086/678511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Mickael C, Chabon J, Gebreab L, Rutebemberwa A, Garcia AR, et al. The causal role of IL-4 and IL-13 in Schistosoma mansoni pulmonary hypertension. Am J Respir Crit Care Med. 2015;192:998–1008. doi: 10.1164/rccm.201410-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204.e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:pii: aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 20.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]