Abstract
Alcohol use disorder (AUD) is a frequent comorbidity among people living with HIV/AIDS (PLWHA). Alcohol consumption is a significant predictor of nonadherence to antiretroviral therapy (ART), as well as worsening immunological and virological indicators among PLWHA. Clinical studies indicate that higher viral loads increase sensitivity to alcohol in PLWHA. The factors that influence alcohol kinetics after HIV infection and initiation of ART are not well understood, limiting the information upon which interventions can be designed to ameliorate the impact of alcohol misuse on this vulnerable patient population. To better understand the relationship between viral load and alcohol kinetics, we measured changes in doses of intragastric ethanol administration to achieve target blood ethanol concentration (BEC) in a rhesus macaque model of chronic binge alcohol (CBA) administration and acute changes following a single acute binge dose of alcohol (ABA) pre- and post-simian immunodeficiency virus (SIV) infection, and following ART initiation. Our results from CBA (14 months)-administered SIV-infected male macaques showed that, following ART initiation, macaques required higher doses of alcohol to achieve a target peak BEC compared with non-ART-treated SIV-infected macaques. In animals given ABA, we found prolonged duration of elevated BEC and decreased elimination rate of alcohol that was not corrected following 7 weeks of ART. These findings suggest that binge drinking associated with AUD could negatively interact with HIV infection and enhance disease progression. These findings further support the need for implementation of behavioral or therapeutic interventions to decrease alcohol consumption to improve the quality of life in PLWHA.
Keywords: : SIV (simian immunodeficiency virus), blood ethanol concentration, acute binge alcohol, chronic binge alcohol
Introduction
There are about 1.2 million people living with HIV/AIDS (PLWHA)1 and approximately 35% of them meet the criteria for alcohol use disorder (AUD).2 With the advent of antiretroviral therapy (ART), HIV infection is a chronic disease, and the number of PLWHA engaging in alcohol misuse surpasses that for the general population.3,4 Alcohol consumption is a significant predictor of nonadherence to ART,5–7 as well as worsening immunological and virological indicators among PLWHA.8 The factors that influence drinking trajectories pre-HIV infection and after initiation of ART are not well understood, limiting the evidence upon which interventions to ameliorate the impact of alcohol misuse on this vulnerable patient population can be constructed. The contribution of altered alcohol elimination rates following HIV infection or initiation of ART could change the subjective response to the effects of alcohol, or in reward pathways that potentially influences drinking patterns in PLWHA.
Investigators from the Veterans Aging Cohort Study (VACS) and from University of California San Francisco, Department of Psychiatry have reported differential effects of HIV infection and ART use on subjective responses to alcohol and on blood ethanol concentrations (BEC) among PLWHA.9–11 PLWHA with detectable viral loads self-reported fewer drinks to achieve a positive subjective effect compared with uninfected males.9 In addition, in PLWHA, BEC achieved after consumption of a known amount of alcohol was greater before than after initiation of ART.10,11 These studies concluded that detectable/higher viral loads increase sensitivity to alcohol in PLWHA. Self-reported number of drinks needed to achieve positive subjective effect, although validated, provides limited information on the mechanisms underlying interactions of alcohol and HIV. Additional information such as BEC achieved following a given dose of alcohol before and following HIV infection and how this responds to ART initiation is challenging to obtain in a clinical setting. History of alcohol consumption, including chronic or occasional acute binge alcohol consumption, can impact BEC achieved. Thus, interpretation of findings should be done in the context of factors that could influence BEC achieved following a given dose of alcohol, including biochemical changes associated with the development of tolerance.
Studies conducted by investigators at the LSUHSC Comprehensive Alcohol-HIV/AIDS Research Center (CARC) in New Orleans aim to elucidate the interactions of alcohol on HIV disease progression using a rhesus macaque model of simian immunodeficiency virus (SIV) infection. This model allows systematic analysis of alcohol-SIV-ART interactions in a controlled environment and allows manipulation of the timing and pattern of alcohol administration and permits us to experimentally validate anecdotal observations reported by PLWHA. Thus, the aim of this study was to determine the acute changes in BEC achieved following a given binge dose of alcohol (ABA) in macaques pre- and post-SIV infection and how these are affected by ART initiation. Moreover, using data collected longitudinally from chronic binge alcohol (CBA)-administered SIV-infected macaques, we examined the temporal patterns of BEC achieved through the course of infection and ART treatment.
Methods
Experimental protocol
All experiments were approved by the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center in New Orleans (LSUHSC-NO) and Tulane National Primate Research Center, LA (TNPRC) and adhered to NIH guidelines for the care and use of experimental animals. For both CBA and ABA studies, 4- to 6-year-old male rhesus macaques (Macaca mulatta) were used. The detailed experimental design and data pertaining to various clinical and pathological parameters for the CBA studies have been previously published.12–14 All animals were individually housed and provided monkey chow ad libitum (Lab Fiber Plus Primate diet DT; PMI Nutrition International, St. Louis, MO) along with supplemental fruits, vitamins, and treats (Research Diets, New Brunswick, NJ). All experiments were conducted in BSL-2 containment rooms at TNPRC (chronic study) or LSUHSC (acute study). All animals were implanted with intragastric catheters as previously described.15,16
CBA and ABA administration
For the CBA studies (N = 28), animals received daily 30-min intragastric alcohol (30% w/v; 13–14 g of ethanol/kg body weight per week) infusions for 3 months preceding SIV infection (n = 14), as previously described.12 In brief, alcohol administration was initiated with an initial alcohol titration period of 2 weeks. During the titration, macaques were administered alcohol at a rate of 8.4 ml/kg/min of 30% w/v over a 30-min time period, 3 days per week with an interposed rest day. The titration protocol was to determine the necessary, individualized infusion rates that would result in BEC between 55–60 mM at 2 h postinitiation of alcohol administration. Subsequently, alcohol was administered daily over a 30-min interval, using an infusion pump that was preset at the predetermined infusion rate for each individual macaque. Blood was collected under ketamine anesthesia, 2 h after initiation of alcohol administration every week until 25 weeks post-SIV infection and the dose of alcohol was adjusted to achieve BEC between 55–60 mM throughout the duration of the study.17 When BECs were outside of the targeted range, then the infusion was adjusted by 5% (increments or decrements) the following week until the target BEC was achieved.
For the ABA study, animals were chair-trained to sit in a macaque restrainer (Primate Products, Inc., Redwood City, CA) so that alcohol kinetics could be studied in a conscious state. On the day of alcohol administration, a baseline blood sample was collected before a 30-min intragastric alcohol (2.5 g/kg, 30% v/v) administration, and serial blood samples were obtained at 15-min intervals during the first hour of infusion, every 30 min for the next 3 h, and at 6 and 24 h post alcohol infusion. This protocol of ABA administration was repeated twice in all 4 animals, 1 week apart, at each study time point–baseline (pre-SIV), early viremia (3, 4 weeks post-SIV), pre-ART (9, 10 weeks post-SIV), and post-ART (16–17 weeks post-SIV). Data presented reflect average of values collected at each time point.
SIV inoculation and ART administration
Three months after initiating CBA or isocaloric sucrose (SUC) administration, animals were inoculated intrarectally with 1,000 times the 50% infective dose (TCID50) of SIVmac251.12,16 At 10 weeks post-SIV infection, animals were randomized to receive ART (provided by Gilead Sciences, Inc.), consisting of daily subcutaneous injections of 20 mg/kg of tenofovir (TFV, 9-[R-2-(phosphonomethoxy) propyl] adenine, PMPA) and 30 mg/kg of emtricitabine (FTC). Alcohol and ART administration were continued throughout the duration of the study (Fig. 1A). Studies from our group have shown that this ART regimen effectively suppresses viral load and does not lead to any overt adverse effects.12 Animals were euthanized 24 h after the last alcohol administration at ∼12 months post SIV infection. For euthanasia, macaques were fasted overnight and anesthetized with ketamine/xylazine, followed by a lethal i.v. injection of sodium pentobarbital. For the ABA study, animals were inoculated intravenously with 100 times TCID50 of SIVmac25112,16 and 2 × 104 TCID50 of SIV-17E Fr (neurotrophic) virus.18 We coinoculated with SIV-17E Fr to understand the impact of alcohol on viral-induced CNS pathology. At 10 weeks post-SIV infection, ART therapy was initiated in all animals similar to the CBA studies and was continued on ART for 7 weeks (Fig. 1B).
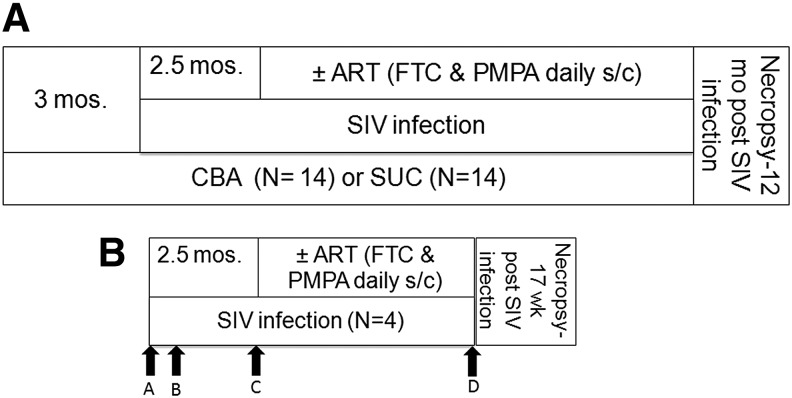
FIG. 1.
Schematic of the study design. (A) CBA (N = 14) or SUC (N = 14) were administered via a gastric catheter daily to achieve a peak BEC of 55–60 mM. All macaques were inoculated intrarectally with SIV virus. Two and half months after SIV infection, half of the CBA and SUC-administered macaques were initiated on ART regimen (N = 7 per group) or vehicle (N = 7 per group) as daily subcutaneous injections. Necropsy was performed 12 mos. post-SIV infection. (B) ABA was administered to all 4 animals at baseline (A), 3 weeks post SIV infection (B), and 10 weeks post SIV infection (C). Two of the animals received an acute binge of alcohol 2 h before necropsy (D). Animals were inoculated intravenously with SIV virus and two and half months later initiated on ART regimen. Necropsy was performed 17 weeks post SIV infection. ABA, acute binge alcohol; CBA, chronic binge alcohol; SIV, simian immunodeficiency virus; SUC, sucrose
Monitoring SIV disease progression and euthanasia
The progression of SIV disease was monitored throughout the study period through clinical, biochemical, and immunological parameters in addition to plasma viral kinetics (SIV gag RNA levels) as described and reported elsewhere.15,16,19 At study end point, macaques were fasted and anesthetized with ketamine/xylazine, whole body perfused with Ringers lactate, followed by a lethal i.v. of sodium pentobarbital. In the ABA study, two macaques were given a binge alcohol bolus, 2 h before euthanizing them.
Alcohol assay & alcohol kinetics
Plasma alcohol concentrations were determined by the alcohol oxidase method (GM7 analyzer, Analox Instruments, London, United Kingdom) according to manufacturer's instructions. Weekly BECs determined at 2 h after initiation of alcohol administration were used for analysis in the CBA study. Serial BECs obtained after an acute binge of alcohol were used for calculations in the ABA study. Area under the 6 h blood alcohol concentration-time curve (AUC 0–6) was calculated. Maximum plasma concentration (Cmax), time of Cmax (Tmax), and alcohol plasma concentration at 6 h post alcohol infusion (C6), were determined from the concentration-time curve. The elimination rate was determined from the slope of the descending arm of the concentration-time curve (4–6 h).
Assessment of alcohol metabolic capacity
Liver alcohol metabolism enzyme expression
Liver samples collected at necropsy were homogenized in T-PER (Pierce Thermo Scientific, Rockford, IL) buffer with Halt protease and phosphatase inhibitor cocktail (Thermo Scientific) and protein concentration determined using Pierce BCA Protein Assay Kit (Thermo Scientific). For cytochrome P450 2E1 expression, protein (30 μg) was separated using Tris-glycine 4%–15% Criterion-protein gels (Bio-Rad). Western blots were probed using rabbit polyclonal cytochrome P450 2E1 (Abcam; #ab19140) and bands visualized using ECL kit (Millipore, Billerica, MA). Beta-actin (CST, #3972) was used as loading control. Alcohol dehydrogenase (ADH) expression was determined in liver and plasma using Human ADH ELISA Kit (#LS-F13054; LS Biosciences, Seattle, WA).
Liver alcohol metabolism enzyme gene expression
Total RNA was isolated from liver collected at necropsy from the ABA- and CBA- administered macaques using the RNeasy Mini Kit (Qiagen). cDNA was synthesized from 1 μg of the resulting total RNA using the Quantitect Reverse Transcriptase Kit (Qiagen), in accordance with the manufacturer's instructions. Primers for ADH (Forward GACATGAGGATCGCCAAAGA; Reverse CCTCTCAATCACCTCCTCAATC), ALDH (Forward GCTCTACCTGTGCTGTGTTT; Reverse CCTGGATGGGTTTCTTGTAGTC) were designed to span exon–exon junctions (IDT, Coralville, IA) and used at a concentration of 500 nmol. The final reactions were made to a total volume of 20 μl with Quantitect SYBR Green PCR Kit (Qiagen). All reactions were carried out in duplicate on a CFX96 system (Bio-Rad Laboratories, Hercules, CA) for qPCR detection. qPCR data were analyzed using the comparative Ct (delta-delta-Ct, ΔΔCT) method. Target genes were compared with the endogenous control (ribosomal protein S13 (RPS13).
Liver and plasma alcohol metabolism enzyme activity
ADH activity and aldehyde dehydrogenase (ALDH) activity were determined in liver homogenates and plasma samples using colorimetric assays (#K731 and #K787 respectively, BioVision, California) according to manufacturer's instructions.
Statistical analysis
For ABA studies, one-way analysis of variance was used to test the significance of the differences in pharmacokinetic parameters (within-subject analyses) using Prism Pad (La Jolla, CA). The same software was used to calculate the area under curve. The elimination rate was determined from the slope of the linear portion of the concentration-time curve, which was generally between 4 and 6 h. A difference was considered statistically significant if the p-value was ≤.05. For CBA studies, the analysis of dose of alcohol administered over time was completed using a piecewise linear mixed effect spline model with a knot at 10 weeks.20 A linear trend model examined the measurements of BEC over the course of the study period. All models generated used PROC MIXED with SAS software, (Version 9.4 of the SAS System for Windows, SAS Institute, Inc., Cary, NC). Two-way analysis of variance was used to test changes in gene and protein expression using Prism Pad.
Results
SIV infection and ART differentially alter the dose of alcohol required to achieve target BEC in CBA-administered macaques
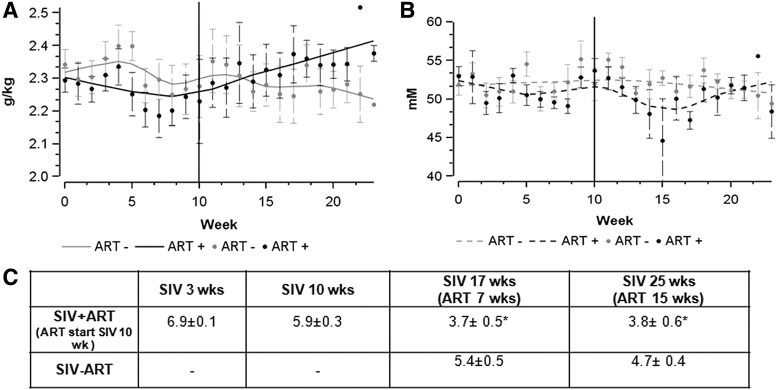
In the CBA study, macaques were administered alcohol daily to achieve a target BEC of ∼55–60 mM at 2 h post infusion and the dose of alcohol was adjusted (by changing the dose of alcohol infusion) to maintain the target BEC. Post SIV infection, but before initiation of ART, the dose required to achieve the target BEC decreased in both groups of animals. After the initiation of ART, macaques required a higher dose of alcohol to achieve a target BEC (p = .0010; Fig. 2A) compared with those that did not receive ART. Due to the titration of dose for this group, there were no statistically significant differences in BEC between macaques that were ART naive or those receiving ART over the course of the study period (p = .7635; Fig. 2B).
FIG. 2.
Alcohol dose and BEC achieved in CBA-administered macaques. Smoothing (LOESS) plots of (A) average dose of alcohol infusion rate (g/kg). Solid gray line represents CBA/SIV/ART- and gray dots are the individual data points, solid black line represents CBA/SIV/ART+ and gray dots are the individual data points. (B) BEC (mM) over the 25 weeks of SIV infection. Dotted gray line represents CBA/SIV/ART- and gray dots are the individual data points, dotted black line represents CBA/SIV/ART+ and gray dots are the individual data points. Vertical line (10 weeks) reflects start of ART (n = 7 for each group). (C) Plasma viral loads (log copies/ml) at the time points corresponding to the acute binge alcohol study *p < .05. BEC, blood ethanol concentration; CBA, chronic binge alcohol.
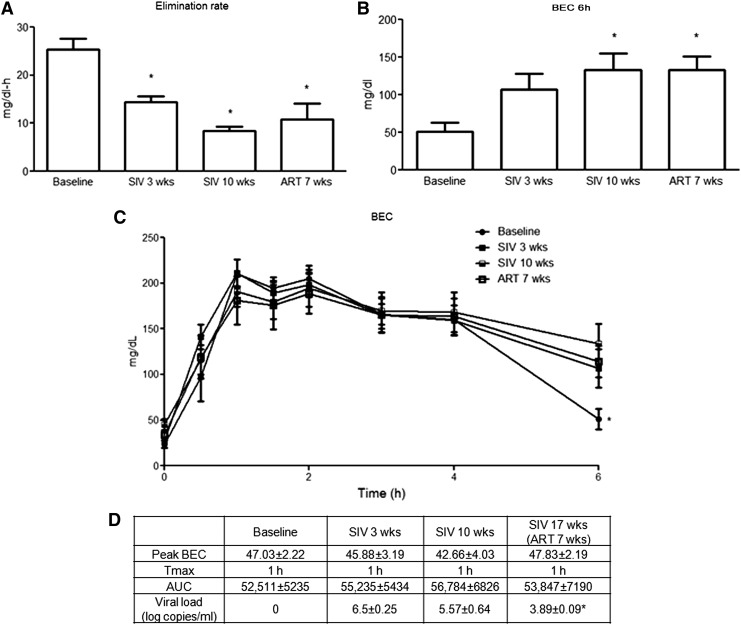
SIV infection decreases alcohol elimination rate following ABA administration
In the ABA study, plasma alcohol kinetics was monitored by measures of BEC over a 6-h period following ethanol infusion at discreet time points before and following SIV-infection and ART. Animals showed significantly lower elimination rate from baseline at SIV 3 weeks, SIV 10 weeks, and ART 7 weeks (Fig. 3A). There were no statistically significant differences in the elimination rates between post-SIV and post-ART time points. The BEC at 6 h was significantly higher at SIV 10 weeks and ART 7 weeks compared with baseline (Fig. 3B). However, no statistically significant differences were observed in the target BEC, Tmax, and AUC0-6 at any of the other time points examined (Fig. 3C, D).
FIG. 3.
Alcohol pharmacokinetics in ABA-administered macaques. (A) Elimination rate of blood alcohol at baseline (pre-SIV), SIV 3, and 10 weeks and at 7 weeks post-ART initiation. (B) BEC at 6 h post-alcohol infusion (BEC 6 h) was significantly higher at SIV 10 weeks and after 7 weeks of ART administration. (C) BEC over the 6 h period after ABA administration at baseline, SIV 3, and 10 weeks and ART 7 week. (D) Peak BEC, Tmax, AUC1-6 and viral load in ABA administered SIV-infected macaques. N = 4 animals, ABA administration was repeated twice at each time point (within animal design). *p < .05. BEC, blood ethanol concentration; ABA, acute binge alcohol.
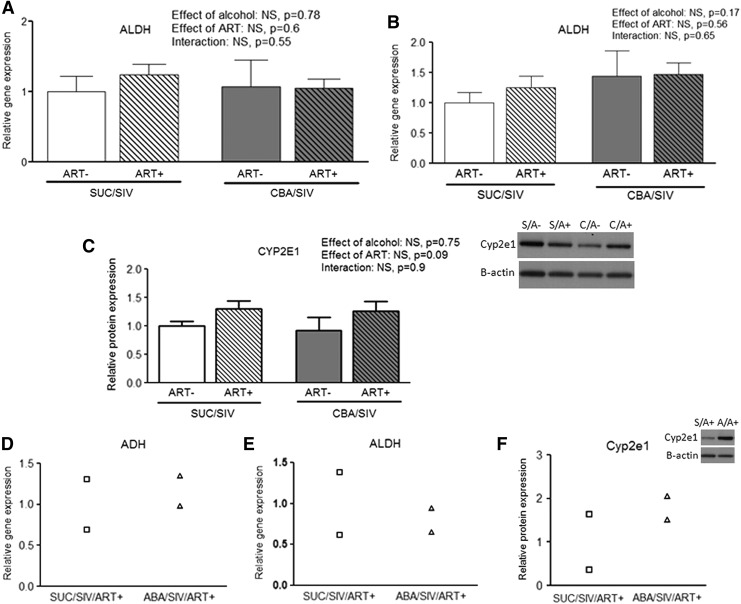
SIV or ART do not alter expression or activity of liver alcohol metabolizing enzymes
ADH and ALDH gene expression and Cyp2e1 protein expression were not significantly different in the livers of CBA or ABA macaques (Fig. 4). We were unable to detect activity of ADH and ALDH in the liver or expression in the plasma (data not shown).
FIG. 4.
Hepatic alcohol metabolizing capacity. (A) Relative hepatic gene expression of ADH, (B) ALDH, and (C) cytochrome P450 2E1 (Cyp2e1) expression in livers of SUC/SIV/ART- (white bars, n = 6), SUC/SIV/ART+ (white hatched bars, n = 7), CBA/SIV/ART- (gray bars, n = 6), and CBA/SIV/ART+ (gray hatched bars, n = 7) obtained at necropsy. Relative hepatic gene expression of (D) ADH and (E) ALDH, and (F) Cyp2e1protein expression in SUC/SIV/ART+ (□) and ABA/SIV/ART+ (Δ) obtained at necropsy. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase.
Discussion
Our results from the CBA study using a cohort of SIV-infected male rhesus macaques administered alcohol daily for approximately 14 months showed that post-SIV infection, the dose required to achieve target BEC was lower than pre-SIV infection. In contrast, post initiation of ART, SIV-infected animals required higher doses of alcohol to achieve a target peak BEC compared to non-ART treated SIV-infected macaques. The results from the ABA study showed that SIV decreased the elimination rate of alcohol without changing target BEC achieved. Taken together, these findings suggest that SIV infection decreases alcohol clearance and prolongs the time of increased BEC following a binge alcohol dose. This is the first study in rhesus macaques that demonstrates that SIV infection prolongs the time of elevated BECs compared with those achieved in the uninfected state and suggests that ART partially corrects this alteration. These findings are in agreement with studies on interactions of HIV and alcohol pharmacokinetics, which demonstrated that PLWHA needed lesser amounts of alcohol to feel positive subjective effects, and that peak BEC was higher in untreated PLWHA compared with those treated with ART.9,10
The ABA study demonstrates that peak and mean BEC during the absorption phase (up to Tmax) were not different at any of the time points tested. This is in contrast to the findings reported by McCance-Katz et al. that showed that the peak and mean BEC during the absorption phase were significantly higher in untreated PLWHA compared with after ART therapy. The authors of that study proposed a contribution of HIV-induced intestinal damage as a possible mechanism.10 SIV infection results in a significant loss of CD4+ T cells, disruption of gut-associated lymphoid tissue, and increased intestinal permeability.21,22 We have also demonstrated that there is higher viral replication in the intestine with significant depletion of CD4+ T cells in CBA-administered SIV macaques.23 Therefore, it is possible that gut leak could contribute to increased peak BEC later in the infection or due to chronic alcohol administration. Since BEC was measured only at a single time point postadministration, it precludes us from reporting changes in peak BEC. However, our results indicate that a lower amount of alcohol was required to achieve the target BEC, indirectly suggesting that higher peak BEC concentrations could have been achieved in the macaque reflecting the findings from the human study.
The SIV studies were conducted in a strict controlled environment where animals were SIV-inoculated at a fixed time, ART therapy initiated at set point (10 weeks post SIV infection), and animals were on a controlled diet. Conversely, in clinical studies numerous confounds could contribute to increased intestinal damage and the feeling of intoxication with fewer drinks, including malnutrition, opportunistic infections, psychological status, and the like. While the ART regimen used in our studies does not allow for direct comparison to regimens used clinically, it allows for the study of SIV-infected macaques with moderate viral suppression. The ART regimen administered to the macaques is the backbone for combination ART therapy, which is generally paired with a protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI) in the clinical setting. McCance-Katz et al. saw an improvement in peak BEC after an ART regimen of NNRTIs and PIs.10 Whether different results would have been obtained with additional ART (PIs, NNRTIs, and integrase inhibitors) or more complete virological suppression in our non-human primate model remains to be determined.
The limitations of our study include a small number of animals in the ABA study, the use of an ART regimen that does not exactly match that used in the clinical setting, and the relative short time of study for what is now considered a chronic disease. However, the data obtained underscore the need to assess for AUD among PLWHA. From our study, it is evident that alcohol pharmacokinetics are impaired due to SIV infection. Longer times of elevated BEC could contribute to adverse biomedical consequences, adverse neurocognitive effects, and could enhance HIV disease progression. The present study also indicated that ABA is capable of increasing the duration of BEC by decreasing the rate of elimination specifically, a pharmacokinetic effect potentially different from the one that increases BEC duration during CBA. One can speculate that AUD, particularly binge drinking, could increase risk for alcohol-mediated tissue injury in PLWHA. Our results show that continuous uninterrupted ART partially corrected the impaired alcohol kinetics in the CBA study, highlighting the importance of adherence to treatment in overall health in PLWHA. However, it is reported that adherence to ART in PLWH who misuse alcohol is challenging in a clinical setting.24,25 Since chronic alcohol misuse is prevalent in PLWHA, consideration for the impact of infection on alcohol kinetics and the potential for greater risk for alcohol-associated adverse effects should be considered. Taken together, our results in SIV-infected macaques exposed to CBA and ABA and reports from clinical studies support implementation of behavioral or therapeutic interventions to ameliorate the adverse effects of alcohol on end-organ damage and improve the quality of life in PLWHA and AUD.
Acknowledgments
The authors acknowledge Gilead Sciences, Inc. for providing tenofovir and emtricitabine for these studies. The authors acknowledge from TNPRC, Dr. Jason Dufour, DVM, and DACLAM for veterinary and animal study expertise and Larissa Devlin, Wayne A. Cyprian, and Nancy Dillman for excellent care of study animals. We acknowledge the TNPRC Pathology Laboratory for assistance with blood and tissue analysis and the TNPRC Virology Core for the virus stocks used in the study. From LSUHSC-NO, we are thankful for the veterinary and animal study expertise of Drs. Leslie Birke and Jeffrey Schumacher. We are greatly indebted to the personnel of the LSUHSC-NO Comprehensive Alcohol-HIV/AIDS Research Center (CARC, P60 AA00980) Experimental Core, including Larry Coleman and Amy Weinberg for excellent animal care and technical expertise. We acknowledge the personnel of the CARC Analytical Core, including Constance Porretta, Jean Carnal, Jane Schexnayder, and Rhonda R. Martinez for performing flow cytometry and viral load analysis. Research reported in this publication was supported by the National Institutes of Health under award numbers P60 AA009803, 1 K01AA024494-01A1, and P51 RR000164.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Prevention, CDC: HIV Surveillance Report 2013;25
- 2.Justice AC, et al. : Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care 2006;44:S52–S60 [DOI] [PubMed] [Google Scholar]
- 3.Galvan FH, et al. : The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. J Stud Alcohol 2002;63:179–186 [DOI] [PubMed] [Google Scholar]
- 4.Lefevre F, et al. : Alcohol consumption among HIV-infected patients. J Gener Intern Med 1995;10:458–460 [DOI] [PubMed] [Google Scholar]
- 5.Parsons JT, Rosof E, Mustanski B: The temporal relationship between alcohol consumption and HIV-medication adherence: A multilevel model of direct and moderating effects. Health Psychol 2008;27:628–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samet JH, Walley AY: Interventions targeting HIV-infected risky drinkers: Drops in the bottle. Alcohol Res Health 2010;33:267–279 [PMC free article] [PubMed] [Google Scholar]
- 7.Braithwaite RS, et al. : Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care 2007;19:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima VD, et al. : The effect of history of injection drug use and alcoholism on HIV disease progression. AIDS Care 2014;26:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinnis KA, et al. : Number of drinks to “Feel a Buzz” by HIV status and viral load in men. AIDS Behav 2016;20:504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCance-Katz EF, et al. : Untreated HIV infection is associated with higher blood alcohol levels. J Acquir Immune Defic Syndr 2012;60:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM: Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med 2013;7:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina PE, et al. : Chronic binge alcohol consumption does not diminish effectiveness of continuous antiretroviral suppression of viral load in simian immunodeficiency virus-infected macaques. Alcohol Clin Exp Res 2014;38:2335–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford SM Jr., et al. : Chronic binge alcohol administration impairs glucose-insulin dynamics and decreases adiponectin in asymptomatic simian immunodeficiency virus-infected macaques. Am J Physiol Regul Integr Comp Physiol 2016;311:R888–R897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon L, et al. : Decreased myoblast differentiation in chronic binge alcohol administered simian immunodeficiency virus-infected male macaques: Role of decreased miR-206. Am J Physiol Regul Integr Comp Physiol 2017;ajpregu 00146:02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S: Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 2008;32:138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagby GJ, et al. : The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res 2003;27:495–502 [DOI] [PubMed] [Google Scholar]
- 17.Nelson S, Bagby GJ: Alcohol and HIV infection. Trans Am Clin Climatol Assoc 2011;122:244–253 [PMC free article] [PubMed] [Google Scholar]
- 18.Zink MC, et al. : Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol 1997;151:793–803 [PMC free article] [PubMed] [Google Scholar]
- 19.Molina PE, et al. : Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res 2006;30:2065–2078 [DOI] [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, Larid N, Ware JH: Applied Longitudinal Analysis. Wiley, New Jersey, 2004 [Google Scholar]
- 21.Estes JD, et al. : Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010;6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Wang X, Lackner AA, Veazey RS: Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus-infected macaques. FASEB J 2015;29:5072–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poonia B, et al. : Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses 2006;22:589–594 [DOI] [PubMed] [Google Scholar]
- 24.Grierson J, Koelmeyer RL, Smith A, Pitts M: Adherence to antiretroviral therapy: Factors independently associated with reported difficulty taking antiretroviral therapy in a national sample of HIV-positive Australians. HIV Med 2011;12:562–569 [DOI] [PubMed] [Google Scholar]
- 25.Golin CE, et al. : A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med 2002;17:756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]