Summary
Background
Extended-release naltrexone (XR-NTX), an opioid antagonist, and sublingual buprenorphine-naloxone (BUP-NX), a partial opioid agonist, are pharmacologically and conceptually distinct interventions to prevent opioid relapse. We aimed to estimate the difference in opioid relapse-free survival between XR-NTX and BUP-NX.
Methods
We initiated this 24 week, open-label, randomised controlled, comparative effectiveness trial at eight US community-based inpatient services and followed up participants as outpatients. Participants were 18 years or older, had Diagnostic and Statistical Manual of Mental Disorders-5 opioid use disorder, and had used non-prescribed opioids in the past 30 days. We stratified participants by treatment site and opioid use severity and used a web-based permuted block design with random equally weighted block sizes of four and six for randomisation (1:1) to receive XR-NTX or BUP-NX. XR-NTX was monthly intramuscular injections (Vivitrol; Alkermes) and BUP-NX was daily self-administered buprenorphine-naloxone sublingual film (Suboxone; Indivior). The primary outcome was opioid relapse-free survival during 24 weeks of outpatient treatment. Relapse was 4 consecutive weeks of any non-study opioid use by urine toxicology or self-report, or 7 consecutive days of self-reported use. This trial is registered with ClinicalTrials.gov, NCT02032433.
Findings
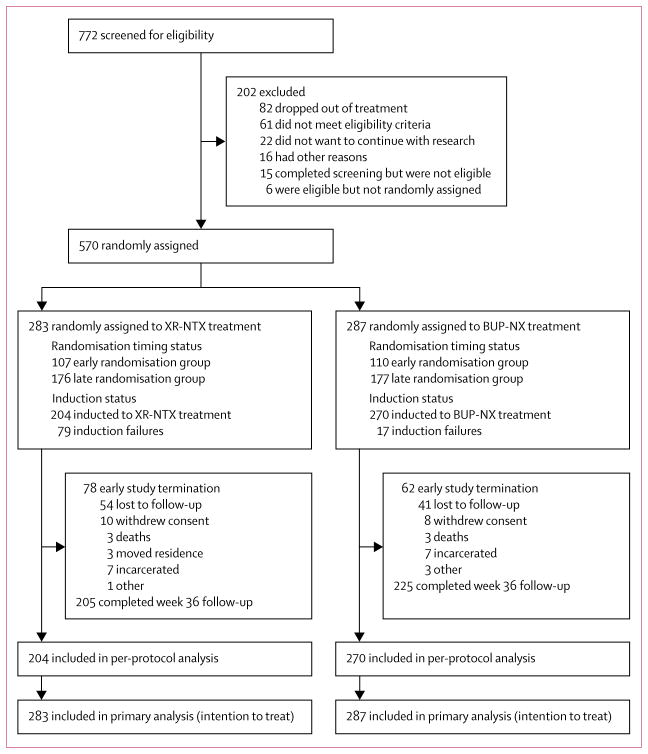
Between Jan 30, 2014, and May 25, 2016, we randomly assigned 570 participants to receive XR-NTX (n=283) or BUP-NX (n=287). The last follow-up visit was Jan 31, 2017. As expected, XR-NTX had a substantial induction hurdle: fewer participants successfully initiated XR-NTX (204 [72%] of 283) than BUP-NX (270 [94%] of 287; p<0·0001). Among all participants who were randomly assigned (intention-to-treat population, n=570) 24 week relapse events were greater for XR-NTX (185 [65%] of 283) than for BUP-NX (163 [57%] of 287; hazard ratio [HR] 1·36, 95% CI 1·10–1·68), most or all of this difference accounted for by early relapse in nearly all (70 [89%] of 79) XR-NTX induction failures. Among participants successfully inducted (per-protocol population, n=474), 24 week relapse events were similar across study groups (p=0·44). Opioid-negative urine samples (p<0·0001) and opioid-abstinent days (p<0·0001) favoured BUP-NX compared with XR-NTX among the intention-to-treat population, but were similar across study groups among the per-protocol population. Self-reported opioid craving was initially less with XR-NTX than with BUP-NX (p=0·0012), then converged by week 24 (p=0·20). With the exception of mild-to-moderate XR-NTX injection site reactions, treatment-emergent adverse events including overdose did not differ between treatment groups. Five fatal overdoses occurred (two in the XR-NTX group and three in the BUP-NX group).
Interpretation
In this population it is more difficult to initiate patients to XR-NTX than BUP-NX, and this negatively affected overall relapse. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Introduction
Opioid μ-receptor full agonist (methadone), partial agonist (buprenorphine), and antagonist (extended-release naltrexone; XR-NTX) pharmacotherapies are superior to placebo treatment and counselling-only treatment for opioid use disorders.1–4 Buprenorphine (provided a buprenorphine prescribing waiver is obtained) and XR-NTX can be prescribed in any US medical setting, and are key components of a public health response to the current epidemic of opioid use disorders and overdose deaths. Comparative effectiveness data are needed to inform treatment decisions among patients and providers of these two distinct treatment approaches.
Buprenorphine products (sublingual tablets, films, buccal patches, and implants) are now the most commonly prescribed, most accessible form of evidence-based opioid treatment in the USA.5–7 Extended-release injectable naltrexone was developed to provide sustained opioid receptor blockade, improve long-term adherence compared with daily oral naltrexone tablets, and improve overall effectiveness, and was approved by the US Food and Drug Administration, in 2010, for the prevention of opioid relapse following detoxification. Results of clinical trials3,4,8,9 have shown XR-NTX to be superior to placebo treatment3,8 and drug-free treatment-as-usual among participants not interested in opioid-agonist maintenance.4,9 XR-NTX differs from buprenorphine both in terms of induction and ongoing care. XR-NTX cannot be initiated until patients are fully detoxified without risking precipitated withdrawal. Once initiated, XR-NTX produces no opioid-like effects and no physiological dependence, while physiological and subjective effects of exogenous opioids are blocked.10 By contrast, buprenorphine can be initiated as soon as patients are in mild-to-moderate withdrawal. It maintains physiological opioid dependence, and withdrawal is likely to occur on discontinuation; usual effects of other opioids are also blocked.11
Previous opioid antagonist or agonist comparisons have evaluated oral naltrexone and long-term naltrexone implants, but not monthly XR-NTX treatment.12–15 Important clinical issues remain unanswered, beyond the established efficacy of either XR-NTX or BUP-NX. How feasible is XR-NTX induction compared with buprenorphine-naloxone (BUP-NX) among active opioid users admitted voluntarily to real-world, community detoxification centres? Do comparable proportions of individuals remain on medication after induction, and how do they compare in terms of avoiding illicit opioid use? Is the typical community use of XR-NTX as safe as BUP-NX, particularly with regard to overdose events?
This study (X:BOT [CTN-0051]), sponsored by the National Institute on Drug Abuse (NIDA), was a randomised, comparative effectiveness trial of 24 weeks of treatment with XR-NTX versus BUP-NX following an acute inpatient detoxification admission, done at typical community-based treatment programmes across the USA. The primary aim was to estimate the difference, if any, between XR-NTX and BUP-NX treatment for relapse to regular opioid use (time to relapse). Secondary outcomes included failure to initiate medication, opioid use during treatment, and adverse events including overdoses. We hypothesised that XR-NTX would be, relative to BUP-NX, no different in enabling relapse-free-survival, more difficult to initiate, associated with increased opioid abstinence, and no different in adverse events, including overdoses.
Methods
Study design and participants
We did this 24 week, open-label, randomised trial to compare the effectiveness and safety of XR-NTX versus BUP-NX. Eight study sites were National Drug Abuse Treatment Clinical Trials Network (CTN)-affiliated community treatment programmes with high volumes of opioid detoxification admissions and outpatient medical management capabilities. We recruited participants, who gave consent and were screened, at any point during voluntary, usual care, inpatient detoxification admissions. Although community advertising and outreach efforts varied by study site, we primarily recruited participants in person after admission, and they were typically not aware of the study before admission. Methods and design rationale have been published before (appendix).16,17
Participants were 18 years or older, spoke English, had Diagnostic and Statistical Manual of Mental Disorders-5 opioid use disorder, and had used non-prescribed opioids in the past 30 days. We excluded participants if they had other serious medical, psychiatric, or substance use disorders; transaminase concentrations were more than 5 times the upper limit of normal; were suicidal or homicidal; had allergy or sensitivity to XR-NTX or BUP-NX; had methadone maintenance treatment (≥30 mg/day); had chronic pain requiring opioids; had a legal status precluding study completion; and were not able to have safe intramuscular XR-NTX treatment. We excluded women if they were pregnant, breastfeeding, planning conception, or unwilling to use birth control.
All sites obtained local Institutional Review Board approval and all participants provided written informed consent. The CTN Greater New York Node had primary responsibility for leading the study; the Emmes Corporation (CTN’s Data and Statistics Center and Clinical Coordinating Center) provided data management and analysis, and monitored safety and quality. The NIDA Center for CTN (CCTN) coordinated the Data Safety Monitoring Board.
Randomisation and masking
Randomisation to XR-NTX or BUP-NX (1:1) followed eligibility determination and was stratified by treatment site and opioid use severity (high severity was ≥6 bags or equivalent intravenous heroin per day in the 7 days before admission). We chose these stratification variables because we expected site differences in the magnitude of the detoxification hurdle, and we expected that high-severity participants would have worse outcomes than low-severity participants. We used a web-based permuted block design with random equally weighted block sizes of four and six for randomisation. This open-label trial involved no masking of treatment or outcomes.
Procedures
Detoxification protocols and length of stay were not protocol-derived and varied by site. Detoxification approaches included no opioids (clonidine or so-called comfort medications only at two sites), 3–5 day methadone tapers (four sites), and 3–14 day buprenorphine tapers (two sites). Timing of randomisation was flexible. Participants were randomised early, during methadone or buprenorphine tapers, or later, after completion of detoxification. We expected participants in the early randomisation group to have more difficulty completing detoxification and initiating XR-NTX than those participants in the late randomisation group. We designated participants a priori to the early randomisation group (randomised within 72 h of last opioid use—including opioids used for detoxification) or the late randomisation group (>72 h following last opioid use). A prespecified interim analysis plan required a minimum of 350 participants to be randomised later. Following randomisation, participants were inducted as quickly as possible.
XR-NTX (4 mL, about 380 mg naltrexone base) was Vivitrol (Alkermes, Dublin, Ireland). Before XR-NTX induction, participants had to complete detoxification (≥3 days from last opioid use), have opioid-negative urine, and a negative naloxone challenge (no or minimal opioid withdrawal symptoms following intramuscular, subcutaneous, or intravenous administration of ≥0·4 mg dose of naloxone, a short-acting opioid antagonist). Subsequent XR-NTX injections were scheduled every 28 days. If injections were missed and physical re-dependence was likely to have occurred, a repeat naloxone challenge or another detoxification programme was required to reinitiate XR-NTX treatment.
BUP-NX was Suboxone (Indivior, Slough, UK) sublingual film, 4 mg/1 mg and 8 mg/2 mg strengths. Typical induction included observed dosing on the detoxification unit once substantial withdrawal symptoms emerged. Subsequently, the study team dispensed BUP-NX to participants at weeks 0, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 20 for self-administration at daily doses of 8–24 mg (higher or lower as clinically indicated). Both study medications were provided free of charge.
Study medications were discontinued following the primary outcome (a relapse event), at the end of 24 weeks, or per safety concerns or participant preference. Thereafter, participants were managed by the same treatment programmes or referred elsewhere in the community as clinically indicated. We universally encouraged extended treatment with buprenorphine, methadone, or naltrexone after the study.
Standard physician or nurse-led office-based medical management was done at each outpatient visit and guided medication treatment. Medical management focused on provider–patient rapport, medication adherence and side-effects, non-study opioid abstinence, and promoted other psychosocial treatment. Additional voluntary ancillary psychosocial counselling was recommended and available at all sites.
Research visits occurred weekly and post-treatment at weeks 28 and 36. We assessed demographic, medical, psychiatric, drug use, and treatment history, quality of life and current health status, and blood and urine testing at baseline. Treatment phase assessments included weekly monitoring of self-reported opioid and other substance use, analysis of urine toxicology samples, ratings of opioid cravings, and adverse events.
Outcomes
The primary outcome measure was the time to a relapse event. Relapse was defined as the use of non-study opioids any time after day 20 post-randomisation: at the start of 4 consecutive opioid use weeks or at the start of 7 consecutive days of self-reported opioid use days. A so-called use week was defined as any week during which the participant reported at least 1 day of non-study opioid use with the Timeline Followback method,18 provided a urine toxicology sample that was positive for non-study opioids (buprenorphine, methadone, morphine [heroin, codeine, morphine], or oxycodone), or did not provide a urine sample (missed visits or refusals). Day 21 was the start of the relapse-event observation period and chosen primarily because participants recently detoxified were likely to have positive urine samples for long-acting opioids prescribed as part of the detoxification regimen (non-study buprenorphine or methadone) for 2–3 weeks after being randomly assigned a treatment (not indicating relapse to illicit or non-study opioid use).
Secondary outcomes were the proportion of participants successfully inducted onto an initial dose of study medication, safety (adverse events), frequency of non-study opioid use per Timeline Followback and assessment of weekly urine toxicology samples, and opioid craving. Adverse events, including overdose events, were queried with the standard Medical Dictionary for Regulatory Activities terminology and reviewed by the NIDA CTN Medical Monitor. Opioid cravings were self-rated using a 0–100 visual analogue scale.
Statistical analysis
The target sample size was based on the width of the 95% CI for the hazard ratio (HR) of the difference between treatments (XR-NTX vs BUP-NX), projecting relapse-free survival of about 50% for each medication after induction2 (described previously16). On the basis of simulation results, the 95% CI width for HR decreases as the sample size increases by 50 per group to 250 per group (from a base of 50 per group) by 31%, 19%, 14%, and 11%, respectively. A preplanned interim analysis increased the overall target sample size from an initial 400 participants to about 600 participants to achieve a minimum sample of 350 participants in the late randomisation group. Sample size calculations indicated that 350 participants would yield a similar (only slightly wider) 95% CI to the original sample size target of 400 participants, and preserved the aim to achieve a precise estimate of the difference in relapses between groups.
We analysed endpoints according to the intention-to-treat principle as part of the primary analysis and additionally among a per-protocol population. The per-protocol population consisted of only those participants who were successfully inducted onto an initial dose of study medication.
The primary outcome analysis was the construction of the asymptotic 95% CI for the HR of the difference between the treatment groups among the intention-to-treat population in the time-to-event (relapse) distribution with the earliest relapse day assessed at day 21. We administratively censored participants at week 24. The binary baseline covariate of early versus late randomisation was examined for an interaction with treatment; this covariate was not significant (p>0·10), and thus dropped from the final model. Unadjusted Kaplan-Meier survival curves and the extended Cox model HRs compared relapse by group. We examined the proportional hazard assumption via the interaction of treatment and time.
Logistic regression yielding odds ratios contrasted induction success and overall 24 week opioid relapse by group. We used Pearson’s χ2 or Fisher’s exact tests, and logistic regression for analyses of dichotomous secondary outcomes. We used Cox models for time-to-event secondary outcomes and Wilcoxon rank-sum tests and mixed effects models for continuous outcomes.
We considered missing urine samples to be opioid positive and contributed to the definition of a relapse event. Thus, treatment dropouts (who stopped contributing data) were scored as having relapsed, an assumption which is likely in this population.19–22 We did statistical analyses with SAS software (version 9.3 or higher). This study is registered with ClinicalTrials.gov, NCT02032433.
Role of the funding source
The authors and the study sponsor designed and implemented the study, collected and analysed the data, wrote the initial manuscript draft, and are responsible for data integrity. Indivior donated Suboxone (BUP-NX) and had access to periodic safety data only, with no input or review of this manuscript. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Between Jan 30, 2014, and May 25, 2016, we randomly assigned 570 participants to receive XR-NTX (n=283) or BUP-NX (n=287; figure 1). The final study visit occurred on Jan 31, 2017. 369 participants (65%; n=175 XR-NTX, n=194 BUP-NX) completed week 28 follow-up. 430 participants (75%) completed an end-of-study visit at week 36 (figure 1). Most participants were white men, aged 25–45 years, had a primary heroin use disorder, were using by injection, were stratified as low-severity opioid use, and were single, unemployed, and Medicaid-insured (table 1).
Figure 1. Trial profile.
XR-NTX=extended-release naltrexone. BUP-NX=buprenorphine-naloxone.
Table 1.
Baseline characteristics
| Intention-to-treat population
|
Per-protocol population
|
|||
|---|---|---|---|---|
| XR-NTX group (n=283) | BUP-NX group (n=287) | XR-NTX group (n=204) | BUP-NX group (n=270) | |
| Demographics | ||||
|
| ||||
| Sex | ||||
| Female | 88 (31%) | 81 (28%) | 66 (32%) | 77 (29%) |
| Male | 195 (69%) | 206 (72%) | 138 (68%) | 193 (71%) |
| Age (years) | 34·0 (9·5) | 33·7 (9·8) | 33·7 (9·3) | 33·7 (9·8) |
| Ethnic origin | ||||
| Hispanic or Latino | 45 (16%) | 54 (19%) | 27 (13%) | 53 (20%) |
| Black or African American | 29 (10%) | 28 (10%) | 20 (10%) | 27 (10%) |
| White | 206 (73%) | 215 (75%) | 157 (77%) | 201 (74%) |
| Marital status | ||||
| Never married | 187 (66%) | 189 (66%) | 134 (66%) | 180 (67%) |
| Have been married | 96 (34%) | 98 (34%) | 70 (34%) | 90 (33%) |
| Employment | ||||
| Working now | 48 (17%) | 57 (20%) | 34 (17%) | 50 (19%) |
| Unemployed | 179 (63%) | 181 (63%) | 125 (61%) | 172 (64%) |
|
| ||||
| Clinical characteristics | ||||
|
| ||||
| Intravenous drug use | 177 (63%) | 183 (64%) | 131 (64%) | 171 (63%) |
| Primary opioid used in the 7 days before detox admission | ||||
| Buprenorphine | 6 (2%) | 2 (1%) | 4 (2%) | 2 (1%) |
| Opioid analgesics | 43 (15%) | 47 (16%) | 36 (18%) | 45 (17%) |
| Methadone | 3 (1%) | 4 (1%) | 3 (1%) | 4 (1%) |
| Heroin | 230 (81%) | 233 (81%) | 160 (78%) | 218 (81%) |
| Cost per day for primary opioid (US$) | $90·7 (76) | $96·3 (74) | $91·0 (84) | $94·1 (73) |
| Age at onset of any opioid use | 21·2 (6·5) | 21·4 (7·6) | 20·8 (6·5) | 21·4 (7·6) |
| Duration of opioid use (years) | 12·8 (9.0) | 12·2 (9·0) | 12·9 (9·1) | 12·3 (9·1) |
| Index admission is first opioid treatment episode | 100 (35%) | 109 (38%) | 75 (37%) | 105 (39%) |
| Stimulant use (past 30 days) | 133 (47%) | 164 (57%) | 99 (49%) | 155 (57%) |
| Sedative use (past 30 days) | 72 (25%) | 93 (32%) | 53 (26%) | 86 (32%) |
| Heavy alcohol use (past 30 days) | 71 (25%) | 77 (27%) | 56 (27%) | 74 (27%) |
| Cannabis use (past 30 days) | 122 (43%) | 133 (46%) | 86 (42%) | 130 (48%) |
| Hamilton Depression Scale (0–50) | 8·6 (6·5) | 9·3 (6·6) | 8·5 (6·4) | 9·5 (6·7) |
| History of psychiatric disorders, self-report | 190 (67%) | 191 (67%) | 141 (69%) | 183 (68%) |
| Subjective opioid withdrawal scale (0–64) | 15·6 (13·4) | 15·6 (13·2) | 15·3 (13·5) | 15·9 (13·2) |
Data are n (%) or mean (SD). XR-NTX=extended-release naltrexone. BUP-NX=buprenorphine-naloxone.
More participants in the BUP-NX group were successfully inducted than in the XR-NTX group (p<0·0001; table 2). XR-NTX induction was successful in fewer participants in the early randomisation group (53%) than the late randomisation group (84%), and varied by site, ranging from 52% at a short-stay, methadone-taper unit, to 95% at an extended-stay, opioid-free programme. 204 participants inducted to XR-NTX treatment completed an average of 3·9 monthly injections (about 16 weeks treatment); 96 (47%) did not end medication early and completed the planned 24 week treatment phase. 270 participants inducted to BUP-NX treatment completed a median of 14 weeks of treatment (IQR 4·6–24·0) at a median maintenance dose of 16 mg/day (12–18); 115 (43%) did not end medication early and completed the planned 24 week treatment phase.
Table 2.
Opioid treatment outcomes
| XR-NTX group (n=283) | BUP-NX group (n=287) | Treatment effect | |
|---|---|---|---|
| Inducted to study medication | |||
|
| |||
| Intention-to-treat group | 204 (72%) | 270 (94%) | OR 0·16, 95% CI 0·09–0·28; p<0·0001 |
|
| |||
| Opioid relapse, weeks 3–24 | |||
|
| |||
| Intention-to-treat group | 185 (65%) | 163 (57%) | OR 1·44, 95% CI 1·02–2·01; p=0·036 |
| Per-protocol group | 106/204 (52%) | 150/270 (56%) | OR 0·87, 95% CI 0·60–1·25; p=0·44 |
|
| |||
| Relapse-free-survival (weeks), range 3–24 | |||
|
| |||
| Intention-to-treat group | 8·4 (3·0–23·4) | 14·4 (5·1–23·4) | HR 1·36, 95% CI 1·10–1·68; p=0·0040 |
| Per-protocol group | 20·4 (5·4–23·4) | 15·2 (5·7–23·4) | HR 0·92, 95% CI 0·71–1·18; p=0·49 |
|
| |||
| Total number of weekly opioid-negative urine samples, range 0–24 | |||
|
| |||
| Intention-to-treat group | 4 (0–19) | 10 (3–20) | p<0·0001 |
| Per-protocol group | 13 (3–21) | 11 (3–20) | p=0·81 |
|
| |||
| Total number of self-reported opioid-abstinent days, range 0–144 | |||
|
| |||
| Intention-to-treat group | 39 (1–144) | 81 (16–144) | p<0·0001 |
| Per-protocol group | 123 (18–144) | 87 (20–144) | p=0·67 |
Data are n (%), n/N (%), or median (IQR). XR-NTX=extended-release naltrexone. BUP-NX=buprenorphine-naloxone. OR=odds ratio. HR=hazard ratio.
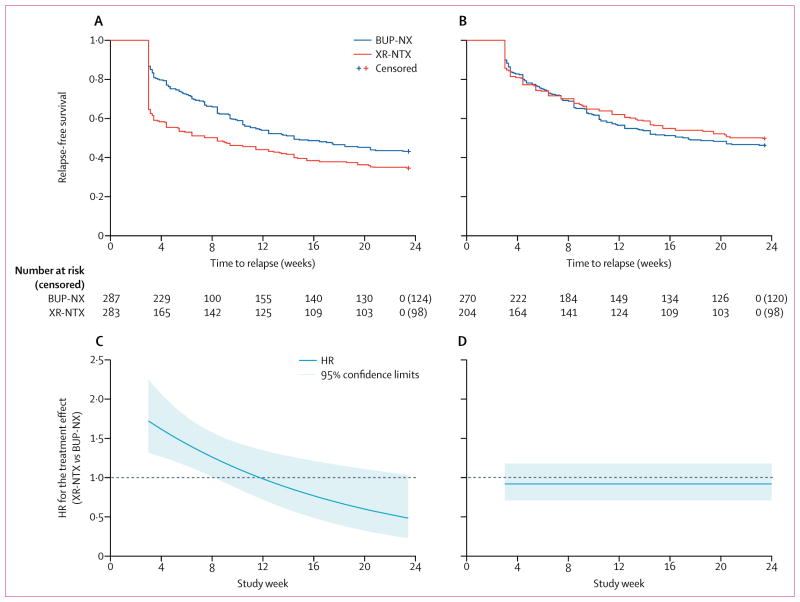
For the primary intention-to-treat sample, the proportion of opioid-relapse events was 185 (65%) of 283 participants for XR-NTX treatment versus 163 (57%) of 287 participants for BUP-NX treatment (table 2). In the survival analysis, BUP-NX treatment was favoured when compared with XR-NTX treatment (HR 1·36, 95% CI 1·10–1·68; table 2, figure 2A, C). The constancy of the relative hazard assumption was violated, as evidenced by a treatment-by-time interaction (p=0·0050). The risk of relapse was lower in the BUP-NX group than the XR-NTX group at the start of the study period, but this risk was not sustained (figure 2C). Participants in the early randomisation group had a higher risk of relapse than participants in the late randomisation group (1·32, 1·06–1·63) for both treatments, with no interaction with treatment (p=0·70). Dropout followed by missing urine data and a relapse event was a common pattern in both treatment groups: 63% (220/348) of all relapse events were defined by four consecutive missing urine samples (71% [132/185] of XR-NTX relapses and 54% [88/163] of BUP-NX relapses).
Figure 2. Relapse-free survival and treatment effect over time for the XR-NTX and BUP-NX treatment groups.
Survival (A) and HRs and corresponding 95% CIs from the non-proportional hazards Cox model (time by treatment interaction included in the model; (C) assessed in the intention-to-treat population (n=570). Survival (B) and HRs by time (D) in the per-protocol population (n=474). XR-NTX=extended-release naltrexone. BUP-NX=buprenorphine-naloxone. HR=hazard ratio.
For the successfully inducted sample (n=474), the proportion of opioid-relapse events was 52% for the XR-NTX group versus 56% for the BUP-NX group, with no difference in the relative hazard of relapse over time (HR 0·92, 95% CI 0·71–1·18; table 2, figure 2B, D). The proportional hazards assumption was not violated in this Cox model and thus, the HR estimate was constant over time (figure 2D). The contrast between relapse events in the intention-to-treat and per-protocol populations was largely accounted for by high occurrence of early relapse among XR-NTX induction failures. For the XR-NTX group, induction failures relapsing on day 21 comprised 70 (25%) of the 283 participants, whereas for BUP-NX induction failures relapsing on day 21 comprised only ten (3%) of the 287 participants.
Treatment effect estimates did not vary by gender. Subgroup analyses by gender did not show a difference in success of induction for either medication or 24 week relapse for either medication between men and women (for both intention-to-treat and per-protocol populations; data not shown).
For the intention-to-treat population, other opioid use outcomes measures (opioid relapse, relapse-free survival, opioid-negative urine samples, and opioid-abstinent days) favoured BUP-NX treatment compared with XR-NTX treatment (table 2). For the per-protocol sample, these same measures did not differ between groups (table 2).
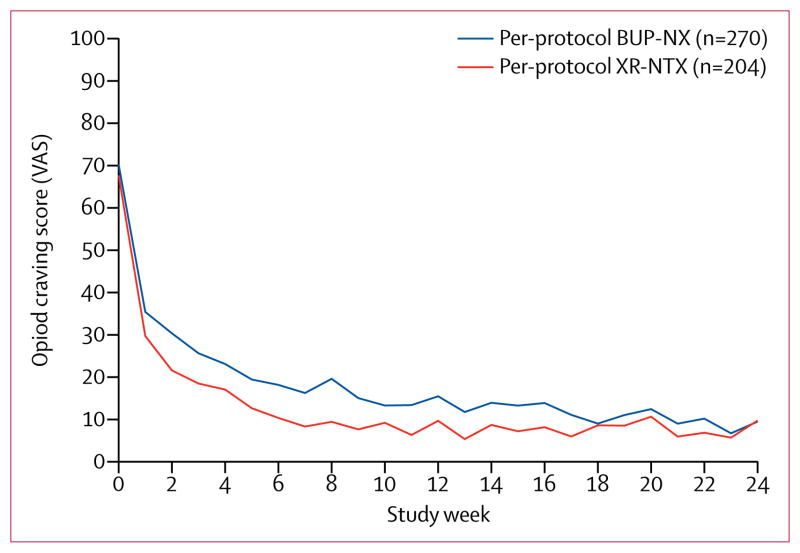
Subjective opioid craving declined rapidly from baseline in both treatment groups (figure 3). Average opioid craving was initially less for the XR-NTX group (p=0·0012 at week 7) than for the BUP-NX group, then converged by week 24 (p=0·20; figure 3).
Figure 3. Opioid craving during the trial.
Craving was self-reported with an opioid craving VAS, range 0–100. VAS=Visual Analogue Scale. XR-NTX=extended-release naltrexone.
BUP-NX=buprenorphine-naloxone.
The proportion of participants reporting adverse events and serious adverse events did not differ between groups, with the exception of injection site reactions among XR-NTX, all of which were of minor to moderate severity (table 3). Altogether, 28 overdose events were reported among 23 participants (table 3). Eighteen (64%) of the 28 events occurred in the group randomised to XR-NTX treatment: eight in participants who had failed induction and never received XR-NTX and ten in participants who had received at least one XR-NTX injection. Ten of the 28 overdose events occurred in the group randomised to BUP-NX treatment: one in a participant who had failed induction and never received BUP-NX and nine in participants who had received at least one dose of BUP-NX (table 3). Five overdose events were fatal, two participants treated with XR-NTX and three participants treated with BUP-NX (table 3). The proportions of participants reporting any overdose event or with a fatal overdose did not differ between treatment groups (table 3).
Table 3.
Adverse events and serious adverse events
| XR-NTX group (n=283) | BUP-NX group (n=287) | |
|---|---|---|
| Treatment-emergent adverse events | ||
|
| ||
| Participants with one or more treatment-emergent adverse event* | 111 (54%) | 141 (52%) |
| Number of treatment-emergent adverse events | 247 | 334 |
| Study medication discontinued due to adverse event | 6 | 8 |
| Type of treatment-emergent adverse event | ||
| Injection site reaction, mild or moderate | 46 | NA |
| Gastrointestinal | 34 | 59 |
| Psychiatric disorders | 30 | 29 |
| Injury, poisoning, and procedural complications | 23 | 25 |
| Infections and infestations | 22 | 27 |
| Nervous system disorders | 22 | 28 |
|
| ||
| Treatment-emergent serious adverse events | ||
|
| ||
| Participants with one or more serious adverse event | 29 (14%) | 29 (11%) |
| Number of treatment-emergent serious adverse events | 39 | 35 |
| Type of treatment-emergent serious adverse event | ||
| Psychiatric disorders | 9 | 11 |
| Infections and infestations | 5 | 6 |
| Pregnancy | 3 | 4 |
| Death | 3 | 4 |
|
| ||
| Overdose events | ||
|
| ||
| Participants with one or more overdose event (all)† | 15 | 8 |
| Participants with one or more overdose event (per protocol)‡ | 9 | 7 |
| Number of overdose events (all)§ | 18 | 10 |
| Number of overdose events (per protocol) | 10 | 9 |
|
| ||
| Fatal overdose events | ||
|
| ||
| Number of fatal overdose events (all) | 2 | 3 |
| Number of fatal overdose events (per protocol) | 2 | 3 |
Data are n (%) or N. NA=not applicable. XR-NTX=extended-release naltrexone. BUP-NX=buprenorphine-naloxone.
Treatment emergent is defined as any adverse events that occurred after the study day of induction for those participants inducted onto study medication.
p=0·14 (Fisher’s exact).
p=0·31 (Fisher’s exact).
Four participants reported more than one overdose event. Three of the four participants were randomly assigned to XR-NTX (two of these induction failures, one successfully inducted); each reported two overdose events. One of the four was randomly assigned to BUP-NX (successfully inducted) and reported three overdose events. None of these nine overdoses were fatal.
Most overdose events occurred at times quite distal to the last dose of study medication (days 25, 33, 42, 49, 54, 66, 73, 76, 87, 88, 90, 110, 117, 141, 149, 170, 190, 227, and 318), or, for those participants who were never inducted, distal to discharge from detoxification programmes (days 10, 21, 37, 76, 86, 167, 174, 238, and 255). This outcome makes it difficult to attribute an association between study medication and overdose.
Discussion
This large multicentre, randomised, controlled, comparative effectiveness trial had five major findings. First, it was more difficult to start XR-NTX treatment than BUP-NX treatment: 28% dropped out of treatment before XR-NTX induction versus only 6% before BUP-NX induction. Second, nearly all induction failures had early relapse. Third, in the intention-to-treat population of all patients who were randomly assigned, XR-NTX had lower relapse-free survival than BUP-NX, directly related to early induction failure. Fourth, for the per-protocol population, who successfully initiated medication, XR-NTX and BUP-NX were similarly effective. Finally, fatal overdose, non-fatal overdose, and other serious adverse events did not differ between treatment groups. Thus, if induction to either medication is successful, XR-NTX and BUP-NX were comparably effective and safe options. These findings afford providers, patients, and families a choice between agonist and antagonist therapies. The risk of XR-NTX induction failure should be considered, and agonist treatments for those individuals unable to complete detoxification should be encouraged.
Clinically, ease of induction is a well known limitation of naltrexone and an advantage of buprenorphine. Study sites varied in detoxification approaches and lengths of stay, but all had to wait, per protocol, for a negative-opioid urine sample before XR-NTX induction, which favoured both longer lengths of stay and non-agonist detoxification. Published strategies23,24 to increase successful XR-NTX induction with single or minimal dosing of buprenorphine and oral naltrexone, and not dependent on a negative urine sample, might be more effective than some of the induction protocols used by our sites.
Once participants were successfully inducted to either XR-NTX or BUP-NX, they achieved similarly favourable and important clinical outcomes: relapse-free survival, overall relapse, retention in treatment, negative urine samples, days of opioid abstinence, and self-reported cravings. These findings align with results of non-inferiority from the concurrent Norwegian study,25 which was also a randomised comparison of XR-NTX to BUP-NX treatment after a longer detoxification run-in, which minimised induction failure. Few participants in the Norwegian trial26 were not able to induct onto either XR-NTX or BUP-NX treatment, and retention, opioid use, or adverse event outcomes did not differ between treatment groups, similar to what was observed in our per-protocol population. Forthcoming analyses from our trial will examine cost-effectiveness, individual-level clinical and genetic moderators of treatment effects, and comparative effects on other drug use, HIV risk behaviours, and cognitive function.
Importantly, this large study found no differences between treatment groups for overall death or overdose events. Overdose events and overdose fatalities were observed in both groups, nearly all of them following failed medication induction or discontinuation and dropout from either medication. These outcomes were consistent with observational analyses showing overdose risk increases substantially after discontinuation of methadone and buprenorphine.27 Although our study was not powered to detect significant differences in overdose events and did not include a no-medication control condition, these results are similar to another large XR-NTX trial,4 in which no overdose deaths were observed among 153 participants treated with XR-NTX over 18 months versus seven among treatment-as-usual controls. So far, no large trial has given a clear signal that XR-NTX treatment increases overdose events or death compared with placebo treatment, treatment as usual, and now, BUP-NX treatment.
A challenge to both treatment groups of this study was overall retention in treatment. Study treatment retention for 24 weeks was between 43% and 47%, which was modestly lower than retention in other 24 week trials2–4 of either medication. A defining feature of this trial was recruitment from inpatient detoxification units, as opposed to outpatient settings. The risk of early opioid treatment dropout is likely to be greater among participants actively using heroin and initially admitted to acute detoxification units than opioid patient cohorts initiating outpatient medication treatment.28 Detoxification admissions typically represent a spectrum of motivation and treatment-seeking; many patients are in crisis and unclear of further treatment options, while other patients are highly motivated to begin a thoughtfully considered new treatment programme. The early randomisation group in this trial, who were more recently admitted and more recently using heroin (or other opioids) than the late randomisation group, had higher overall relapse events in both treatment groups than the late randomisation group. This finding might have shown a higher risk of early dropout, leaving against medical advice, or ambivalence towards chronic medication treatment with either medication among the early randomisation group versus the late randomisation group who are more likely to be a motivated and adherent group, having already survived the initial detoxification days.
Regarding the limitations of our study, the core trial design choices, particularly the acute detoxification setting, flexible randomisation, and the varied induction protocols, which were likely to have had a substantial effect on XR-NTX induction, limit interpretation and generalisability.16,17 An entirely outpatient study would possibly have inducted even fewer people to the XR-NTX group, and would have been consistent with standard BUP-NX induction in the USA, which is largely office-based. Alternatively, recruitment of previously detoxified people or randomisation only of participants able to immediately induct to XR-NTX treatment, the design of an earlier randomised controlled trial,4 would have probably favoured XR-NTX treatment compared with BUP-NX treatment. Site differences in detoxification protocols and lengths of stay contributed to induction and relapse events, and showed substantial variability in standard opioid detoxification approaches. Finally, open-label, real-world effectiveness trials include more sources of bias than tightly controlled efficacy studies, including the absence of placebo control or masking, but potentially increase generalisability. The analyses of the per-protocol population might be affected by confounding because this group is defined on the basis of an after randomisation factor (induction success); however, the intention-to-treat and per-protocol populations, and both treatment groups within each population, were similar with respect to baseline demographic and clinical characteristics.
In summary, for the intention-to-treat population, XR-NTX treatment was less effective than BUP-NX treatment for the prevention of opioid relapse following admission for inpatient detoxification. This outcome was primarily due to fewer XR-NTX inductions and high occurrence of relapse among induction failures. Both medications were similar in effectiveness and safety once treatment was initiated.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, MEDLINE, and Cochrane Reviews for clinical trials and systematic reviews evaluating extended-release naltrexone (XR-NTX) for opioid use disorders, with no restrictions by date or language. Naltrexone oral daily tablets have not been shown to be effective treatment for opioid use disorders in adults, as summarised in a 2011 Cochrane Systematic Review. A 2008 Cochrane Systematic Review on sustained-release formulations of naltrexone for opioid dependence concluded that evidence to evaluate effectiveness was insufficient on the basis of too few studies. Since 2008, XR-NTX, or naltrexone for extended-release injectable suspension, has been approved in the USA for prevention of opioid relapse following detoxification on the basis of a placebo-controlled, industry phase 3 trial done in Russia. A 2016 US evaluation of XR-NTX versus treatment as usual among adults with opioid use disorders and criminal justice involvement, which was ongoing during the start of our current study, found XR-NTX to be effective at preventing opioid relapse; relapse was reduced by about 30% (odds ratio 0·43, 95% CI 0·28–0·65). To our knowledge, no previous studies have compared XR-NTX with a standard of care for opioid-agonist maintenance with either methadone or buprenorphine. This study was done in parallel with a Norwegian randomised trial also evaluating XR-NTX versus buprenorphine.
Added values of this study
To our knowledge, this study is the first US trial and the larger and longer of the two US and Norwegian trials to evaluate XR-NTX versus buprenorphine-naloxone (BUP-NX) among adults with opioid use disorders admitted to community detoxification and treatment programmes. In our trial, most participants were actively using heroin at baseline and are likely to represent the current US opioid epidemic. Study sites varied in timing of treatment assignment and specific detoxification protocols, allowing real-world estimates of XR-NTX induction success. We aimed to replicate usual community outpatient conditions across the 24 week outpatient treatment phase in this open-label, comparative effectiveness trial.
Implications of all the available evidence
Both the US and Norwegian studies found that for those participants able to begin treatment, XR-NTX and BUP-NX were equally safe and effective in preventing relapse. Induction to XR-NTX remains a challenge, which was quantified in the US study and which limited effectiveness in the overall population because those participants not initiating treatment relapsed quickly. Induction success varied with different detoxification approaches. The Norwegian study bypassed the induction hurdle by assigning the treatment after detoxification was largely completed. Conversely, BUP-NX has no induction hurdle. Patients, families, and providers now have data to help them make complex treatment decisions involving personal preferences, detoxification options and risks, and long-term outcomes.
Acknowledgments
This study was sponsored by the National Institute on Drug Abuse (NIDA). We thank all the site research teams, clinical staff, and most importantly the study participants for their effort and time. We thank the Protocol Development Committee, the Study Executive Committee, and Data Safety Monitoring Board for their support and counsel. This research was supported by grants from the NIDA National Drug Abuse Treatment Clinical Trials Network (U10DA013046, UG1/U10DA013035, UG1/U10DA013034, U10DA013045, UG1/U10DA013720, UG1/U10DA013732, UG1/U10DA013714, UG1/U10DA015831, U10DA015833, HHSN271201200017C, and HHSN271201500065C) and K24DA022412 (EVN Jr).
Funding NIDA Clinical Trials Network.
Footnotes
Contributors
The study Clinical Trials Network (CTN) Greater New York Node (JDL, JR, EVN Jr, PN, PG, and SF), Emmes Corporation (DSt, JM), and the National Institute on Drug Abuse Center for the CTN (CCTN; DL) designed the study and wrote the protocol. All authors implemented the study protocol and contributed to data collection. Emmes (RL) and the CCTN (DL, GS) coordinated the Data Safety Monitoring Board and study monitoring. The CTN Greater New York Node (JDL, JR, EVN Jr, PN), Emmes Corporation (JM, AGM, JK, DS-B, DSa, DSt), and CCTN (DL, GS) had access to study data, and statistically analysed and interpreted the data. JDL wrote the first draft of the manuscript. All authors contributed to and approved the final manuscript.
See Online for appendix
Declaration of interests
All authors report grant or contract funding from the National Institute of Drug Abuse (NIDA) for this study. JDL and JR received other research support from NIDA and National Institute on Alcohol Abuse and in-kind study drug from Alkermes for another trial. EVN Jr received other research support from NIDA, Brainsway, Braeburn Pharma, and Alkermes, unpaid consulting fees from Alkermes, and consulting fees from the University of Arkansas. MF received other research support from Alkermes, US World Meds, MediaRez, and the Laura and John Arnold Foundation, and consulting fees from Alkermes and US World Meds. SR received other research support from NIDA, the Heffter Research Institute, Council on Spiritual Practices, and the Sarlo Foundation, and travel support from the Multidisciplinary Association for Psychedelic Studies. All other authors declare no competing interests.
References
- 1.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 4.Lee JD, Friedmann PD, Kinlock TW, et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374:1232–42. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and State Treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105:e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IMS Institute for Healthcare Informatics. [accessed Aug 1, 2017];Use of opioid recovery medications. 2016 https://www.imshealth.com/files/web/IMSH%20Institute/Reports/Healthcare%20Briefs/IIHI_Use_of_Opioid_Recovery_Medications.pdf.
- 7.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2017 doi: 10.1016/j.jsat.2017.07.001. published online July 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comer SD, Sullivan MA, Yu E, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–18. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JD, McDonald R, Grossman E, et al. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction. 2015;110:1008–14. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- 10.Alkermes Inc. [accessed Aug 1, 2017];Highlights of prescribing information: VIVITROL (naltrexone for extended-release injectable suspension) Revised July, 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021897s020s023lbl.pdf.
- 11.Reckitt Benckiser Pharmaceuticals Inc. [accessed Aug 1, 2017];Highlights of prescribing information: SUBOXONE (buprenorphine and naloxone) sublingual film. Revised August, 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022410s000lbl.pdf.
- 12.Gibson AE, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug Alcohol Rev. 2007;26:405–10. doi: 10.1080/09595230701373834. [DOI] [PubMed] [Google Scholar]
- 13.Lobmaier PP, Kunøe N, Gossop M, Katevoll T, Waal H. Naltrexone implants compared to methadone: outcomes six months after prison release. Eur Addict Res. 2010;16:139–45. doi: 10.1159/000313336. [DOI] [PubMed] [Google Scholar]
- 14.Mokri A, Chawarski MC, Taherinakhost H, Schottenfeld RS. Medical treatments for opioid use disorder in Iran: a randomized, double-blind placebo-controlled comparison of buprenorphine/naloxone and naltrexone maintenance treatment. Addiction. 2016;111:874–82. doi: 10.1111/add.13259. [DOI] [PubMed] [Google Scholar]
- 15.Kelty E, Hulse G. Fatal and non-fatal opioid overdose in opioid dependent patients treated with methadone, buprenorphine or implant naltrexone. Int J Drug Policy. 2017;46:54–60. doi: 10.1016/j.drugpo.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Lee JD, Nunes EV, Mpa PN, et al. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): study design and rationale. Contemp Clin Trials. 2016;50:253–64. doi: 10.1016/j.cct.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes EV, Lee JD, Sisti D, et al. Ethical and clinical safety considerations in the design of an effectiveness trial: a comparison of buprenorphine versus naltrexone treatment for opioid dependence. Contemp Clin Trials. 2016;51:34–43. doi: 10.1016/j.cct.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobell LC, Sobell MB. Alcohol Timeline Followback users’ manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 19.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–46. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss RD, Potter JS, Griffin ML, et al. Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug Alcohol Dep. 2015;150:112–19. doi: 10.1016/j.drugalcdep.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hser YI, Evans E, Huang D, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111:695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hser YI, Huang D, Saxon AJ, et al. Distinctive trajectories of opioid use over an extended follow-up of patients in a multisite trial on buprenorphine + naloxone and methadone. J Addict Med. 2017;11:63–69. doi: 10.1097/ADM.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannelli P, Wu LT, Peindl KS, Swartz MS, Woody GE. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: a very low dose naltrexone and buprenorphine open label trial. Drug Alcohol Depend. 2014;138:83–88. doi: 10.1016/j.drugalcdep.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459–67. doi: 10.1176/appi.ajp.2016.16050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunøe N, Opheim A, Solli KK, et al. Design of a randomized controlled trial of extended-release naltrexone versus daily buprenorphine-naloxone for opioid dependence in Norway (NTX-SBX) BMC Pharmacol Toxicol. 2016;17:18. doi: 10.1186/s40360-016-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanum L, Solli KK, Latif ZE, et al. The effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.3206. Published online Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes EV, Gordon M, Friedmann PD, et al. Relapse to opioid use disorder after inpatient treatment: Protective effect of injection naltrexone. J Subst Abuse Treat. 2017 doi: 10.1016/j.jsat.2017.04.016. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.