Abstract
Background
Postpartum is a period of unique psychosocial stress characterized by sleep disturbance, risk for depressed mood, and heightened parenting stress. However, data on effects of these exposures on inflammatory immune function are limited.
Methods
This study examined associations among sleep, psychosocial stress (i.e., parenting stress, general perceived stress), mood (i.e., depressive symptoms), serum cytokine levels, and LPS-stimulated proinflammatory cytokine production among 69 women (32 African American, 37 White) assessed at 7–10 weeks postpartum.
Results
No associations between behavioral measures and serum cytokine levels were observed among women of either race. In African American women, but not Whites, poorer sleep quality, greater parenting stress, and greater depressive symptoms were associated with greater LPS-stimulated IL-6 and IL-8 production (ps ≤ 0.05). Also in African Americans, greater general perceived stress was associated with greater IL-8 production, and greater depressive symptoms with greater stimulated TNF-α production (ps ≤ 0.05). Simple mediation models highlighted the bidirectional relationship between stress and sleep in relation to inflammation among African American women.
Conclusions
Significant effects of both stress/distress and poor sleep quality on proinflammatory cytokine production during postpartum were observed uniquely among African American women. These data are consistent with an allostatic load model which predicts that conditions of chronic stress impart vulnerability to dysregulated responses to novel stressor exposures. The bidirectional nature of the stress-sleep relationship has clinical relevance. Studies examining whether interventions focused on one or both of these psychological factors during postpartum is beneficial for inflammatory profiles would be informative. In addition, examination of these models in relation to maternal health at postpartum, including delivery related wounds and other infections, is warranted.
Keywords: postpartum, women, race, stress, sleep, depressive symptoms, African American, White, perinatal, inflammation
Graphical abstract
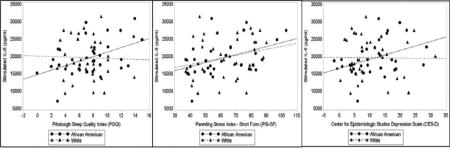
Poorer sleep quality, greater parenting stress, and more depressive symptoms are associated with heightened LPS-stimulated IL-6 production among African American women, but not White women.
INTRODUCTION
Postpartum is a period of unique psychosocial stress characterized by sleep disturbance and heightened stress. Reflecting partial sleep deprivation and fragmentation in response to the newborn’s sleep-wake cycle, women spend an estimated 3 times longer awake after nocturnal sleep onset during the first several weeks postpartum compared to pregnancy or non-postpartum women with children.1–6 Severity of postpartum sleep disruption is predictive of declines in marital satisfaction as well as risk for depression.7–14 Moreover, stress specific to parenting is of particular relevance at postpartum; caring for a newborn entails changes in daily tasks, reductions in personal and partner time, and may introduce new financial challenges.15–17 Further, a mother’s experience of parenting as rewarding versus stressful is affected by her perceptions of both bonding with the child and the child’s temperament.15–17
In the context of these significant psychosocial stressors, women also experience unique physiological vulnerabilities. Infections are the most common cause of serious maternal morbidity at postpartum.18 In the US, an estimated 32.2% of women undergo Cesarean-section, while 11.6% of those delivering vaginally have an episiotomy, which both necessitate wound healing.19, 20 Infections of delivery-related wounds, as well as uterine, bladder, and kidney infections and mastitis are common in postpartum, affecting 6.0-7.4% of women in the first month alone.21 As the majority of US deliveries occur in a hospital setting, exposure to hospital acquired-infectious illnesses including methicillin-resistant Staphylococcus aureus (MRSA) and group A streptococcus (GAS) can be of concern.22, 23 Further, based on epidemiological data, the CDC now recognizes women in the first two weeks postpartum as a high risk group for acquiring seasonal influenza virus infection.24, 25 For these reasons, implications of sleep- and stress-induced immune dysregulation at postpartum have particular relevance.
The psychoneuroimmunology literature has delineated bi-directional associations between behavioral factors and immune function, with extensive research linking both impaired sleep and exposure to stressors with inflammatory dysregulation.26, 27 Various studies link poorer self-reported sleep as well as experimentally-induced sleep restriction with increases in circulating inflammatory mediators and exaggerated ex vivo stimulated cytokine production.28–33 Moreover, data from the past three decades has established that both stressor exposure and depressive symptoms are associated with elevations in circulating inflammatory markers, as well as enhanced stimulated cytokine production.34–37 Although similar effects have been observed in perinatal women, data specific to this population is sparse and has focused primarily on pregnancy rather than postpartum. For example, sleep disturbance in pregnant women has been associated with elevations in serum proinflammatory cytokines, including IL-6 and IL-8.38–40 Moreover, among pregnant women, greater stress or depressive symptoms have been associated with elevations in serum cytokines, exaggerated ex vivo stimulated cytokine production, and exaggerated inflammatory responses to the seasonal flu vaccine.41–43
As described by the allostatic load model, while neuroendocrine and immune responses are adaptive in the face of stressful situations, repeated or prolonged activation of the stress response can impair the body’s ability to maintain allostasis.44 This model predicts that conditions of chronic stress impart vulnerability to dysregulated responses to novel or additional stressor exposures.45 Thus, allostatic load is a pathway by which chronic stress associated with racial minority status may confer risk for poor health outcomes.46, 47 Consistent with this notion, prior data, including that from our group, suggest that African American women may be particularly vulnerable to stress-induced immune dysregulation. For example, our data show that during pregnancy and non-pregnancy, African-American women exhibit more exaggerated increases in serum interleukin(IL)-6 upon exposure to an laboratory acute stressor (Trier Social Stress Test) as compared to White women.48 Moreover, our data have shown that, compared to Whites, African American women show greater increases in serum IL-8 and related increases in risk for preterm birth in the context of poor sleep as measured at mid-gestation.40 However, racial differences in associations between psychosocial factors and inflammation at postpartum remain relatively unexamined.
The current study examined associations among sleep, psychosocial stress (i.e., parenting stress, general perceived stress), mood (i.e., depressive symptoms), serum cytokine levels, and LPS-stimulated proinflammatory cytokine production among 69 women (32 African American, 37 White) assessed at 7–10 weeks postpartum. It was hypothesized that women reporting poorer sleep, greater stress (e.g., general perceived stress, parenting stress), and/or depressive symptoms would exhibit elevated serum proinflammatory cytokine levels and exaggerated cytokine production. It was also hypothesized that these effects would be exacerbated among African Americans versus Whites. Potential mediating pathways linking sleep, stress/distress, and inflammation were examined.
METHODS
Study Design and Participants
This study enrolled 84 women from The Ohio State University Wexner Medical Center (OSUWMC) and surrounding community of Columbus, Ohio. Exclusion criteria consisted of multi-fetal gestation, diagnosed fetal anomaly, health conditions or use of medications with a clear immunological or endocrinological component (e.g., cancer), illicit drug use other than marijuana, and consumption of >2 alcoholic beverages per week per self-report or medical record at time of enrollment. Women reporting acute illness, such as coldor flu-like symptoms, or antibiotic use within ten days of a study visit were rescheduled.
The full study included three prenatal assessments. However, the current analyses focused only on data from the 7–10 week postpartum visit. At the study visit, women provided a blood sample and completed psychosocial questionnaires. Women were excluded from the current analyses if they did not attend the postpartum visit (n = 11), were missing cytokine data (n = 2), or experienced fetal death or infant mortality (n = 2), resulting in a final analytic sample of 69. The study was approved by The Ohio State University Biomedical Institutional Review Board. Written informed consent was obtained from all participants and modest compensation provided.
Demographic Characteristics
Race, marital status, age, education, annual household income, parity (primiparous/multiparous), and breastfeeding status (yes/no) were determined by self-report. Maternal body mass index (BMI; kg/m2) was calculated using weight and height measured by nursing staff at the study visit.
Psychosocial and Behavioral Measures
The Pittsburgh Sleep Quality Index (PSQI) was used to assess overall sleep quality.49 A score > 5 is indicative of clinically disturbed sleep. This measure includes seven subscales: subjective sleep quality, sleep latency (i.e., time to fall asleep), sleep duration, habitual sleep efficiency (i.e., time asleep/time in bed*100), sleep disturbance, use of sleeping medications, and daytime dysfunction. In the current study, all subscales were reported as sum scores using their original scale (e.g., minutes). The total PSQI score was calculated per guidelines.49 The PSQI has high diagnostic sensitivity and specificity in distinguishing good and poor sleepers.49
The Parenting Stress Index – Short Form (PSI-SF) is a widely used 36-item measure of stress as a result of the parent-child relationship.50, 51 Three subscales comprise the PSI: parental distress, parent-child dysfunctional interaction, and difficult child. The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item measure of depressive symptoms with 4 subscales: depressed mood, somatic symptoms, absence of positive affect, and interpersonal difficulties.52 The 10-item Perceived Stress Scale (PSS), is a well-validated measure of stress and assesses a construct independent of depressive symptomatology.53
Serum Cytokines
Whole blood was collected into vacutainer tubes while participants were in a seated position. Samples were immediately centrifuged, aliquoted, and placed in −80°C freezer storage until analysis. Serum levels of IL-6, TNF-α, and IL-8 were assayed in duplicate on either single spot ultra-sensitive (for IL-6) or multiplex V-Plex (for TNF-α and IL-8) kits from Meso Scale Discovery (MSD, Meso Scale Discovery, 1601 Research Blvd, Rockville, MD). Plates were read by an MSD SECTOR Imager 2400 measuring electrochemiluminescence. Sample concentrations were extrapolated from a standard curve calculated using a four parameter logistic fit using MSD Workbench 3.0 software. The limits of detection (LOD) were 0.31 pg/mL for IL-6, 0.17 pg/mL for TNF-α, and 0.27 pg/mL for IL-8. All samples were above the limit of detection. The inter- and intra- assay coefficients of variation were 8.69% and 5.89% for IL-6, 5.12% and 5.34% for TNF-α, and 5.27% and 3.71% for IL-8, respectively.
LPS-Stimulated Proinflammatory Cytokine Production
PBMCs at a concentration of 1×106 cells/ml were stimulated with 1ug/ml LPS in RPMI-1640 supplemented with 10% human male serum for 24 hours. A non-LPS media control was incubated simultaneously. Next, samples were centrifuged and aliquots removed and frozen at −80°C until assayed. Media samples were assayed neat; LPS samples were diluted 1:6. Samples were assayed in duplicate for IL-6, TNF-α, IL-1β, and IL-8 (pg/ml) using human ProInflammatory II multiplex tissue culture kits from Meso Scale Discovery (MSD; 1601 Research Blvd., Rockville, MD). Plates were read by an MSD Sector Imager 2400 measuring electrochemiluminescence. The inter-assay coefficients of variation were 8.28%, 6.02%, 8.59%, and 9.23%, for IL-6, TNF-α, IL-1β, and IL-8, respectively. The intra-assay coefficients of variation were 3.2%, 2.36%, 1.91%, and 2.93%, for IL-6, TNF-α, IL-1β, and IL-8, respectively.
Statistical Analyses
Only one woman endorsed use of sleep medication per the PSQI and 81.2% of women scored a zero on the interpersonal difficulties subscale of the CES-D; thus, these subscales were not included in examinations beyond those with total scores as outcomes. To examine group differences on demographic characteristics and psychosocial factors, chi-square tests, t-tests, or Mann-Whitney U tests were used, when appropriate. Pearson’s or Spearman’s correlation coefficients were used to assess relationships among psychosocial factors, serum cytokine levels, and LPS-stimulated proinflammatory cytokine production. Mediation was tested using bias-corrected 90% confidence intervals for the indirect effects, based on 10,000 bootstrap samples. A significant indirect effect is present when the confidence interval does not include 0 (Hayes, 2013). All other tests were evaluated at the p < 0.05 level of significance. No adjustments were made for multiple comparisons. Partial correlations adjusting for BMI were depicted graphically by obtaining residuals from 2 regression models: the LPS-stimulated cytokine regressed on BMI and the psychosocial measure regressed on BMI. The mean value of each outcome (cytokine and psychosocial measure) was added to each residual to return to the range of the original scale. The values of the residuals-plus-means were displayed as scatterplots with least-squares regression lines. These figures display graphically the associations adjusting for BMI. All analyses were performed using SPSS 24.0 and PROCESS macro v2.16.
RESULTS
Sample Characteristics
The sample was 46% African American (n = 32) and 54% White (n = 37). Groups did not differ in age, body mass index, weeks postpartum, breastfeeding status, marital status, income, education or employment (Table 1). In addition, no racial differences were observed in psychosocial factors assessed (Table 2). Overall, women exhibited poor sleep quality, with 71% (n = 49) meeting criteria for clinically disturbed sleep (PSQI > 5). In comparison to African Americans, White women exhibited greater stimulated IL-1β production (U = 368.0, z = −2.70, p = 0.007; Table 3). No differences by race were observed for production of IL-6, IL-8, or TNF-α.
Table 1.
Demographic characteristics by race and for the full sample, reported as mean (SD) or count (%).
| African American | White | t-test or χ2 | |
|---|---|---|---|
| Variable (units) | (n = 32) | (n = 37) | p value |
| Age (years) | 26.5 (4.6) | 25.9 (3.7) | 0.60 |
| BMI (kg/m2) | 29.1 (5.5) | 29.4 (6.0) | 0.83 |
| Parity | |||
| Primiparous | 6 (18.8%) | 17 (45.9%) | 0.02 |
| Multiparous | 26 (81.2%) | 20 (54.1%) | |
| Weeks postpartum | 8.4 (0.8) | 8.5 (0.6) | |
| Currently breastfeeding | Yes = 16 (50.0%) | Yes = 20 (45.9%) | 0.74 |
| No = 16 (50.0%) | No = 17 (54.1%) | ||
| Marital status | |||
| Single | 6 (18.8%) | 2 (5.4%) | 0.11 |
| In a relationship, not married | 16 (50.0%) | 16 (43.2%) | |
| Married | 10 (31.3%) | 19 (51.4%) | |
| Annual household income | |||
| less than $15,000 | 12 (37.5%) | 7 (18.9%) | 0.11 |
| $15,000 – $29,999 | 6 (18.8%) | 16 (43.2%) | |
| $30,000 – $49,999 | 8 (25.0%) | 6 (16.2%) | |
| $50,000 and above | 6 (18.8%) | 8 (21.6%) | |
| Education | |||
| High school graduate or less | 5 (15.6%) | 10 (27.0%) | 0.13 |
| Some college | 18 (56.3%) | 12 (32.4%) | |
| College graduate | 9 (34.8%) | 15 (40.5%) | |
| Currently employed | Yes = 22 (68.8%) | Yes = 25 (67.6%) | 0.92 |
| No = 10 (31.3%) | No = 12 (32.4%) |
BMI: Body Mass Index
Table 2.
Descriptive statistics for psychosocial factors by race and for the full sample, reported as mean (SD).
| African American | White | p values | |
|---|---|---|---|
| Variable (units) | (n = 32) | (n = 37) | |
| Pittsburgh Sleep Quality Inventory | 7.5 (3.7) | 7.2 (2.7) | 0.69 |
| Subjective quality1 | 1.2 (0.9) | 1.3 (0.7) | 0.61 |
| Sleep latency (min) 1 | 18.4 (16.0) | 15.4 (19.2) | 0.28 |
| Sleep duration (hrs) 1 | 5.8 (1.4) | 6.2 (1.3) | 0.28 |
| Sleep efficiency (%) 1 | 67.6 (18.9) | 65.6 (21.6) | 0.80 |
| Sleep disturbance 1 | 4.5 (2.9) | 6.1 (3.5) | 0.07 |
| Daytime dysfunction1 | 1.5 (1.5) | 1.5 (1.4) | 0.97 |
| Parenting Stress Index – Short Form | 62.1 (18.0) | 62.2 (17.0) | 0.99 |
| Parental distress | 26.3 (9.0) | 25.3 (9.2) | 0.65 |
| Difficult child | 19.5 (6.6) | 20.1 (6.8) | 0.72 |
| Parent-child dysfunctional interaction1 | 16.3 (6.0) | 16.8 (5.0) | 0.26 |
| Perceived Stress Scale | 14.6 (6.0) | 14.4 (6.9) | 0.89 |
| CES-D Scale | 11.8 (7.8) | 9.6 (7.6) | 0.23 |
| Depressed mood1 | 3.1 (3.1) | 2.2 (2.6) | 0.21 |
| Somatic symptoms | 5.8 (3.7) | 5.1 (3.1) | 0.38 |
| Absence of positive affect 1 | 2.7 (3.1) | 2.0 (2.5) | 0.43 |
Mann-Whitney U Test ; CES-D: Center for Epidemiologic Studies Depression Scale
Note: Independent samples t-tests were used to test for group differences, unless otherwise noted.
Table 3.
Descriptive statistics for LPS-stimulated cytokines by race and for the full sample, reported as mean (SD).
| African American | White | p values | |
|---|---|---|---|
| (n = 32) | (n = 37) | ||
|
|
|||
| IL-1β (pg/ml)1 | 4,248.7 (2,284.8) | 5,703.9 (2,969.0) | 0.007 |
| IL-6 (pg/ml) | 19,589.0 (5,111.0) | 19,604.7 (6,069.8) | 0.99 |
| IL-8 (pg/ml) | 180,938.7 (25,989.2) | 181,337.8 (25,719.4) | 0.95 |
| TNF-α (pg/ml) | 3,056.8 (1,011.0) | 2,885.2 (695.5) | 0.41 |
Mann-Whitney U Test; LPS: lipopolysaccharide; IL: interleukin; TNF: tumor necrosis factor
Note: Independent samples t-tests were used to test for group differences, unless otherwise noted.
Psychosocial Factors and LPS-Stimulated Proinflammatory Cytokine Production
Partial correlations between stimulated levels of each cytokine and psychosocial factors were examined separately by race, after controlling for BMI. Key results are depicted in Figures 1 & 2 and Tables 4 & 5. In relation to serum markers, no significant associations between psychosocial factors and serum cytokine levels were observed among women of either race (ps > 0.05). Moreover, among White women, no significant relationships between psychosocial factors and stimulated production of IL-6, IL-8, or IL-1β (ps ≥ 0.06) were observed. However, greater general stress (PSS) was associated with lower TNF-α production (r = −0.33, p = 0.05).
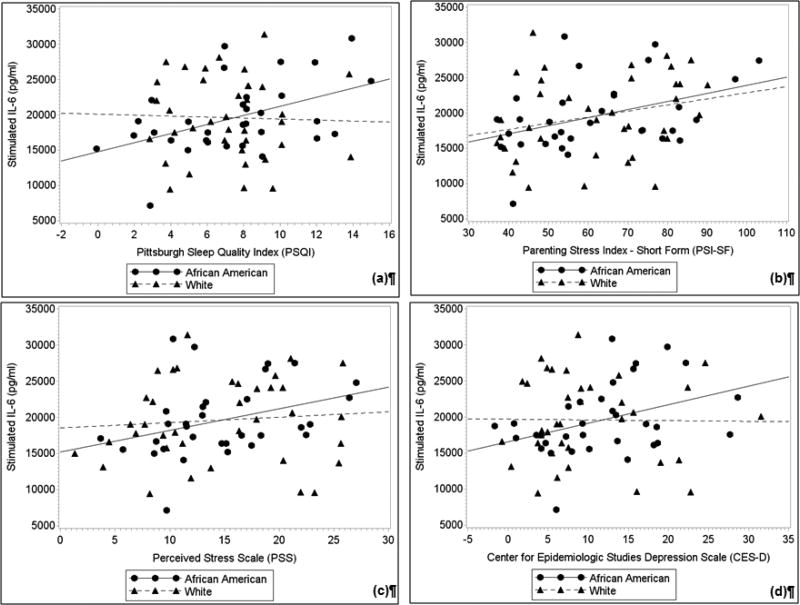
Fig. 1. Partial correlations between (a) sleep quality, (b) parenting stress, (c) perceived stress, and (d) depressive symptoms with LPS-stimulated IL-6 after adjusting for body mass index.
In African American women, but not Whites, poorer sleep quality, greater parenting stress, and greater depressive symptoms were associated with greater LPS-stimulated IL-6 production (ps < 0.05). In addition, a trend was observed between greater perceived stress and greater LPS-stimulated IL-6 among African American women (p < 0.10). Plots represent the values of the residuals-plus-means with least-squares regression lines.
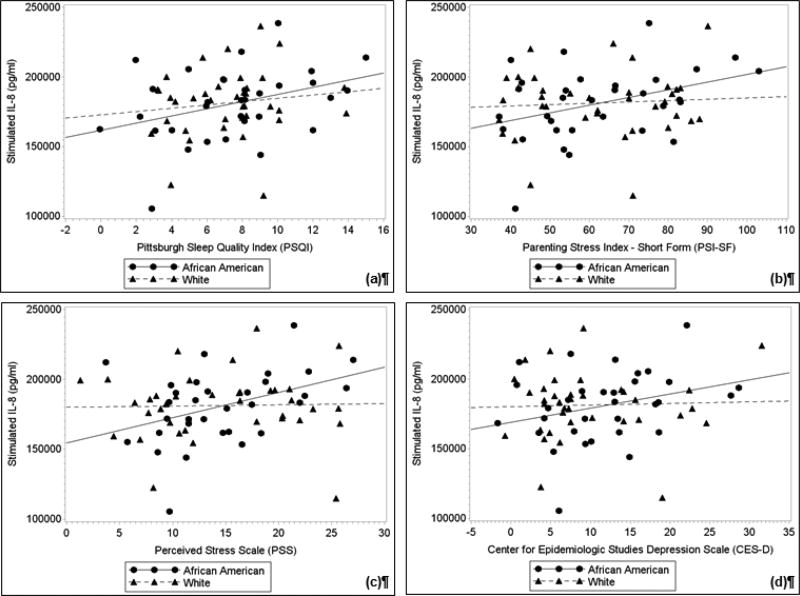
Fig. 2. Partial correlations between (a) sleep quality, (b) parenting stress, (c) perceived stress, and (d) depressive symptoms with LPS-stimulated IL-8 after adjusting for body mass index.
In African American women, but not Whites, poorer sleep quality, greater parenting stress, greater perceived stress, and greater depressive symptoms were associated with greater LPS-stimulated IL-8 production (ps < 0.05). Plots represent the values of the residuals-plus-means with least-squares regression lines.
Table 4.
Correlations between stimulated interleukin-6 and psychosocial factors by race and for the full sample.
| African American | White | Total | |
|---|---|---|---|
| (n = 32) | (n = 37) | (N = 69) | |
| Pittsburgh Sleep Quality Inventory - Total | 0.46** | −0.02 | 0.22† |
| Subjective quality1 | 0.33† | −0.05 | 0.09 |
| Sleep latency1 | 0.25 | −0.09 | 0.05 |
| Sleep duration1 | −0.51** | −0.19 | −0.29* |
| Sleep efficiency1 | −0.38* | 0.03 | −0.12 |
| Sleep disturbance1 | 0.47** | 0.10 | 0.25* |
| Daytime dysfunction1 | 0.29 | 0.15 | 0.27* |
| Parenting Stress Index -Total | 0.41* | 0.26 | 0.30* |
| Parental distress | 0.48** | 0.22 | 0.30* |
| Difficult child | 0.35† | 0.29† | 0.28* |
| Parent-child dysfunctional interaction1 | 0.18 | 0.14 | 0.16 |
| Perceived Stress Scale | 0.33† | 0.08 | 0.14 |
| CES-D Scale - Total | 0.37* | −0.02 | 0.13 |
| Depressed mood1 | 0.46** | −0.01 | 0.17 |
| Somatic symptoms | 0.29 | 0.04 | 0.14 |
| Absence of positive affect1 | 0.36* | 0.09 | 0.17 |
All analyses control for BMI.
Note: Higher Total PSQI scores, subjective quality rating, and sleep latency reflect greater severity of sleep problems
CES-D: Center for Epidemiologic Studies - Depression Scale
Spearman correlation. Pearson correlations were used unless otherwise noted.
p ≤ 0.10;
p ≤ 0.05;
p ≤ 0.01
Table 5.
Correlations between stimulated interleukin-8 and psychosocial factors by race and for the full sample.
| African American (n = 32) |
White (n = 37) |
Total (N = 69) |
|
|---|---|---|---|
| Pittsburgh Sleep Quality Inventory – Total | 0.36* | 0.14 | 0.27* |
| Subjective quality1 | 0.30 | 0.01 | 0.12 |
| Sleep latency1 | 0.07 | 0.13 | 0.10 |
| Sleep duration1 | −0.47** | −0.10 | −0.28* |
| Sleep efficiency1 | −0.18 | −0.04 | −0.12 |
| Sleep disturbance1 | 0.37* | 0.30† | 0.35** |
| Daytime dysfunction1 | 0.34† | −0.15 | 0.17 |
| Parenting Stress Index – Total | 0.39* | 0.07 | 0.22† |
| Parental distress | 0.39* | 0.10 | 0.22† |
| Difficult child | 0.39* | 0.21 | 0.27* |
| Parent-child dysfunctional interaction1 | 0.16 | −0.12 | 0.02 |
| Perceived Stress Scale | 0.42* | 0.02 | 0.17 |
| CES-D Scale - Total | 0.32† | 0.02 | 0.16 |
| Depressed mood1 | 0.47** | 0.07 | 0.23† |
| Somatic symptoms | 0.14 | 0.11 | 0.12 |
| Absence of positive affect1 | 0.31† | 0.11 | 0.15 |
All analyses control for BMI.
Note: Higher Total PSQI scores, subjective quality rating, and sleep latency reflect greater severity of sleep problems.
CES-D: Center for Epidemiologic Studies - Depression Scale
Spearman correlation. Pearson correlations were used unless otherwise noted.
p ≤ 0.10;
p ≤ 0.05;
p ≤ 0.01
Among African American women, controlling for BMI, poorer sleep quality, greater parenting stress, and greater depressive symptoms (per CES-D total score and/or subscales) were associated with both greater stimulated IL-6 and IL-8 production (ps ≤ 0.05; Tables 4 & 5). Greater stimulated IL-8 production was also observed in relation with greater general perceived stress (p ≤ 0.05) Table 5). In addition, greater depressive symptoms (per CES-D depressed mood subscale), were associated with greater LPS-stimulated TNF-α production (r = 0.42, p = 0.02). No associations between psychosocial factors and IL-1β production were observed among African Americans (ps ≥ 0.29).
Mediating Models
Expected associations between stimulated cytokine production and psychosocial factors were observed exclusively among African American women in the cohort and most consistently in relation to IL-6 and IL-8 production. Thus, subsequent mediation analyses focused on these markers among African American women. Correlations among key psychosocial variables are shown in Table 6. In a series of simple mediation models, we first tested overall sleep quality (PSQI total score), as a potential mediator linking psychosocial stress/distress (PSI-SF, PSS, and CES-D) with stimulated IL-6 and IL-8 production (Table 7). As shown, controlling for BMI, sleep quality played a mediating role in the relationship of both parenting stress and depressive symptoms with stimulated production of both cytokines.
Table 6.
Correlations between sleep quality and psychological measures for African American women (n = 32).
| PS QI Total |
Sleep duration1 |
Sleep efficiency1 |
Sleep disturbance1 |
PSI Total |
PSI Parental Distress |
PSI Difficult Child |
PSS Total |
CES-D Total |
CES-D Depressed1 |
CES-D Positive Affect1 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| PSQI Total | −0.80*** | −0.62*** | 0.29 | 0.35* | 0.50** | 0.26 | 0.21 | 0.24 | 0.11 | 0.29 | |
| Sleep duration1 | 0.74*** | −0.08 | −0.29 | −0.28 | −0.42* | −0.04 | 0.01 | −0.03 | −0.15 | ||
| Sleep efficiency1 | 0.05 | −0.21 | −0.22 | −0.22 | −0.00 | 0.03 | 0.06 | −0.11 | |||
| Sleep disturbance1 | 0.20 | 0.25 | 0.15 | 0.16 | 0.30† | 0.18 | 0.24 | ||||
| PSI Total | 0.84*** | 0.89*** | 0.65*** | 0.47** | 0.61*** | 0.49** | |||||
| PSI Parental Distress | 0.58*** | 0.67*** | 0.63*** | 0.63*** | 0.52** | ||||||
| PSI Difficult Child | 0.51** | 0.28 | 0.42* | 0.36* | |||||||
| PSS Total | 0.66*** | 0.62*** | 0.60*** | ||||||||
| CES-D Total | 0.82*** | 0.64*** | |||||||||
| CES-D Depressed1 | 0.39* | ||||||||||
| CES-D Positive Affect1 |
PSQI: Pittsburgh Sleep Quality Inventory; PSI: Parental Stress Inventory; PSS: Perceived Stress Scale; CES-D: Center for Epidemiologic Studies - Depression Scale
Spearman correlations; Pearson correlations used unless otherwise noted.
p ≤ 0.10;
p ≤ 0.05;
p < 0.01;
p ≤ 0.001
Table 7.
Mediation models testing overall sleep quality per PSQI total score as a mediator.
| Effect | SE | 90%CI estimates |
Effect | SE | 90%CI estimates |
||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PSI → PSQI → IL-6 | 35.33 | 25.22 | 6.71, 97.93* | PSI → PSQI → IL-8 | 128.57 | 107.83 | 7.77, 399.53* |
| PSS → PSQI → IL-6 | 70.53 | 72.26 | −13.31, 221.44 | PSS → PSQI → IL-8 | 251.57 | 291.27 | −53.67, 899.90 |
| CES-D → PSQI → IL-6 | 60.06 | 47.95 | 3.95, 166.79* | CES-D → PSQI → IL-8 | 237.97 | 194.62 | 10.63, 697.10* |
Significant effect; PSI: Parenting Stress Index; PSQI: Pittsburgh Sleep Quality Inventory; IL: interleukin; PSS Perceived Stress Scale; CES-D: Center for Epidemiologic Studies – Depression Scale
Next, models were tested whereby sleep quality (PSQI total score) served as the primary causal factor (Table 8). As shown, models linking PSQI scores to stimulated IL-6 and IL-8 production via parenting stress (PSI-SF) were significant. In addition, the model linking sleep quality (PSQI) with stimulated IL-6 production via depressive symptoms (CES-D) was significant, while this model for IL-8 approached significance.
Table 8.
Mediation models testing psychological factors as mediators
| Effect | SE | 90%CI estimates |
Effect | SE | 90%CI estimates |
||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PSQI → PSI→ IL-6 | 135.52 | 126.09 | 4.08, 435.30* | PSQI → PSI → IL-8 | 752.78 | 618.01 | 39.39, 2142.06* |
| PSQI → PSS → IL-6 | 66.47 | 86.69 | −14.52, 288.21 | PSQI → PSS → IL-8 | 530.26 | 596.59 | −128.11, 1889.77 |
| PSQI → CES-D → IL-6 | 87.88 | 81.26 | 3.78, 287.60* | PSQI → CES-D → IL-8 | 408.18 | 453.65 | −19.17, 1524.33 |
Significant effect; PSI: Parenting Stress Index; PSQI: Pittsburgh Sleep Quality Inventory; IL: interleukin; PSS Perceived Stress Scale; CES-D: Center for Epidemiologic Studies – Depression Scale
DISCUSSION
In the current study, we examined associations between sleep quality and psychosocial stress/distress with serum proinflammatory cytokines and LPS-stimulated proinflammatory cytokine production among women at 7–10 weeks postpartum. Our results showed effects of parenting stress, general perceived stress, depressive symptoms, and poor sleep quality on enhanced proinflammatory cytokine production, uniquely among African American women. This is consistent with our prior data which show greater inflammatory dysregulation in response to acute stressor exposures as well as poor sleep among African American versus White women during pregnancy.40, 48 These findings are also in keeping with the concept of allostatic load, which posits that exposure to repeated or chronic stress impairs the ability to most adaptively respond to new or additional stressors.44
In the current investigation, we did not observe any associations between psychosocial parameters and serum levels of proinflammatory cytokines. This may, in part, reflect greater sensitivity provided by LPS-stimulation which induces a robust response, greatly enhancing variability within the sample. Thus, it is plausible that associations at the serum level could be observed in a larger cohort. However, it is of note that our prior data on acute stress reactivity demonstrate that African American and White women (in pregnancy and non-pregnancy) exhibit similar levels of serum IL-6 at baseline; racial differences in inflammatory profiles emerged only after the acute stressor exposure.42 In parallel, in the current study, while serum levels represent a “resting” state, ex vivo LPS-stimulation provides a proxy measure for how the cells respond under conditions of bacterial challenge. Thus, both our current and prior findings are in keeping with an allostatic load conceptualization. Together, these studies provide evidence that, compared to White women, African Americans exhibit impaired ability to maintain physiological allostasis upon exposure to both psychological and biological challenges.
Moreover, in the current study, associations were seen primarily in relation to LPS-stimulated production of IL-6 and IL-8. A large literature suggests that IL-6 is particularly responsive to psychosocial stress and sleep disturbance.54, 55 Further, our data and others has previously linked disrupted sleep in perinatal women specifically with IL-8.39, 40 IL-8 is a potent chemoattractant implicated in endothelial dysfunction which plays an important role in the development of atherosclerosis and is implicated in risk of cardiovascular events.56, 57 Thus, dysregulation of these inflammatory markers at postpartum may be of particular relevance.
In mediation analyses, which were conducted only among African American women, we tested two directional pathways by which sleep quality may be linked with psychological stress/distress in affecting inflammatory responses. Results supported both pathways: links from stress to inflammation via sleep quality as well as from sleep quality to inflammation via stress. This is consistent with the literature in that stress and sleep quality are bi-directionally related.58–60 These models assume that psychosocial factors are impacting inflammation, rather than the reverse; in the unique context of postpartum in which new stressors (e.g., parenting responsibilities, financial obligations) and sleep disturbance are introduced, this directional pathway is arguably most robust. However, effects of inflammation on sleep and stress/depressive symptoms cannot be ruled out. In addition, these data did not provide longitudinal assessment at postpartum, which would permit for clearer delineation of mediating pathways linking psychosocial parameters of interest.
The bi-directional nature of the stress and sleep relationship is likely exacerbated at postpartum. Parenting a newborn is itself a substantial stressor exposure, involving changes in daily routines, work schedules, social and partner relationships, and acquisition of new skills (particularly for first time parents). Moreover, caring for a newborn commonly requires multiple sleep/wake cycles. Thus, for women who have difficulty falling or staying asleep, these issues are encountered not once, but multiple times in a given night. These characteristics of postpartum sleep may contribute to increased parenting stress and also exacerbate or enhance vulnerability to depressed mood. Studies examining whether interventions focused on one or both of these psychological factors during postpartum is beneficial for inflammatory profiles would be informative.
These findings may have implications for maternal health at postpartum. As described, this is a period of unique vulnerability in that the experience of delivery-related wounds which require healing is common. In addition, uterine, bladder, kidney infections and mastitis may occur. Via effects on immune function, stress and sleep disruption may contribute to poor healing and risk for infection. Studies examining stress, inflammation, and specific health outcomes including occurrence of delivery-related wounds and other infections would inform our understanding of the health implications of the current findings.
Of note, in the current study, we utilized the CES-D as our measure of depressive symptoms, rather than the Edinburgh Postnatal Depression Scale (EPDS). The CES-D is commonly administered and shows predictive validity in perinatal studies.61–65 However the EPDS was specifically designed for use in postpartum.66 A key differentiating feature of these two scales is that the EPDS purposefully excludes assessment of somatic symptoms (e.g., sleep difficulties, fatigue, changes in appetite) with the rationale of avoiding overlap with typical perinatal experiences. This is problematic for studies of immune underpinnings of depression, because sickness behaviors elicited by inflammatory exposure are largely somatic in nature.67–69 In keeping with the larger literature in non-perinatal adults, our prior data suggest that somatic symptoms are of importance in predicting immune and neuroendocrine dysregulation in perinatal women.70 While somatic symptoms were not the primary driver of observed associations between depressive symptoms and inflammatory profiles in the current investigation, assessment using the CES-D permitted for this determination. Thus, we propose that, despite some possible overlap with “normal” perinatal experiences, assessing somatic symptoms is critical for validity and clarity in studies linking depressive symptoms with biological factors in perinatal women.
The current study focused on predominately lower income African American and White women. Thus, generalizability to other groups is unknown. It is plausible that the observed effects would not be present among women with greater socioeconomic resources. In addition, although African American and White women were generally demographically similar, we did not assess characteristics such as family wealth; at the same education and income levels, Whites have markedly greater wealth than African Americans which substantially affects economic security.71 Groups may differ in wealth, or in other meaningful ways that were not captured herein. Also, the sample size of this study introduced limitations with regard to statistical modeling; while we observed differential effects in African Americans versus Whites when examined separately, the study was not sufficiently powered to test formal moderation, or moderated mediation which would provide stronger evidence. The current analyses do not “rule out” the possibility that these effects are also applicable among White women, albeit effects may be smaller and thus require a larger sample to detect. These results should be considered within these limitations.
In sum, the current study demonstrates that among postpartum women, poorer sleep quality, as well as greater parenting stress, perceived stress, and depressive symptoms are predictive of enhanced stimulated proinflammatory cytokine production. These effects were uniquely observed among African American women in this cohort. Mediation models within African American women supported sleep as both a mediator and initiator linking stress/distress with enhanced inflammatory responding, highlighting the bidirectionality of these constructs at postpartum. Future studies incorporating longitudinal design and specific health outcomes (e.g., postpartum infections) would further our understanding of the health implications of stress and sleep-induced immune dysregulation at postpartum.
Highlights.
Data on psychosocial factors and inflammatory immune function at postpartum are limited
We observed no associations of serum cytokines with sleep or psychosocial distress
In African Americans, poor sleep and stress predicted greater stimulated cytokine production
These effects were not observed among White women
Stress-induced immune dysregulation may affect health at postpartum
Acknowledgments
We appreciate the contributions of our Clinical Research Assistants to data collection. We would like to thank our study participants and the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
ROLE OF FUNDING SOURCES
This study was supported by NICHD (R21HD067670, LMC). The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources (UL1TR001070). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Yamazaki A, Lee KA, Kennedy HP, Weiss SJ. Sleep-wake cycles, social rhythms, and sleeping arrangement during Japanese childbearing family transition. J Obstet Gynecol Neonatal Nurs. 2005;34(3):342–8. doi: 10.1177/0884217505276156. [DOI] [PubMed] [Google Scholar]
- 2.Doering JJ. The physical and social environment of sleep in socioeconomically disadvantaged postpartum women. J Obstet Gynecol Neonatal Nurs. 2013;42(1):E33–43. doi: 10.1111/j.1552-6909.2012.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203(5):465, e1–7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishihara K, Horiuchi S. Changes in sleep patterns of young women from late pregnancy to postpartum: Relationships to their infants' movements. Perceptual and Motor Skills. 1998;87:1043–56. doi: 10.2466/pms.1998.87.3.1043. [DOI] [PubMed] [Google Scholar]
- 5.Swain AM, O'Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90(3):381–6. doi: 10.1016/s0029-7844(97)89252-6. [DOI] [PubMed] [Google Scholar]
- 6.Gay CL, Lee KA, Lee S-Y. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5(4):311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina AM, Lederhos CL, Lillis TA. Sleep Disruption and Decline in Marital Satisfaction Across the Transition to Parenthood. Fam Syst Health. 2009;27(2):153–60. doi: 10.1037/a0015762. [DOI] [PubMed] [Google Scholar]
- 8.Okun ML. Sleep and postpartum depression. Curr Opin Psychiatry. 2015;28(6):490–6. doi: 10.1097/YCO.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 9.Bhati S, Richards K. A Systematic Review of the Relationship Between Postpartum Sleep Disturbance and Postpartum Depression. Jognn-J Obst Gyn Neo. 2015;44(3):350–7. doi: 10.1111/1552-6909.12562. [DOI] [PubMed] [Google Scholar]
- 10.Hiscock H, Bayer J, Gold L, Hampton A, Ukoumunne O, Wake M. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child. 2006 doi: 10.1136/adc.2006.099812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer JK, Hiscock H, Hampton A, Wake M. Sleep problems in young infants and maternal mental and physical health. J Paediatr Child Health. 2007;43(1–2):66–73. doi: 10.1111/j.1440-1754.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiscock H, Bayer JK, Hampton A, Ukoumunne OC, Wake M. Long-term mother and child mental health effects of a population-based infant sleep intervention: cluster-randomized, controlled trial. Pediatrics. 2008;122(3):e621–e7. doi: 10.1542/peds.2007-3783. [DOI] [PubMed] [Google Scholar]
- 13.Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth. 2005;32(3):187–93. doi: 10.1111/j.0730-7659.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 14.Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130(3):378–84. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abidin RR. The Determinants of Parenting Behavior. Journal of Clinical Child Psychology. 1992;21(4):407–12. [Google Scholar]
- 16.East PL, Barber JS. High Educational Aspirations Among Pregnant Adolescents Are Related to Pregnancy Unwantedness and Subsequent Parenting Stress and Inadequacy. J Marriage Fam. 2014;76(3):652–64. doi: 10.1111/jomf.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang Y, Fine MA, Ispa J, Thornburg KR, Sharp E, Wolfenstein M. Understanding Parenting Stress Among Young, Low-income, African-American, First-Time Mothers. Early Education & Development. 2004;15(3):265–82. [Google Scholar]
- 18.Hebert PR, Reed G, Entman SS, Mitchel EF, Berg C, Griffin MR. Serious maternal morbidity after childbirth: Prolonged hospital stays and readmissions. Obstet Gynecol. 1999;94(6):942–7. doi: 10.1016/s0029-7844(99)00419-6. [DOI] [PubMed] [Google Scholar]
- 19.Friedman AM, Ananth CV, Prendergast E, D'Alton ME, Wright JD. Variation in and Factors Associated with Use of Episiotomy. Journal of the American Medical Association. 2015;313(2):197–9. doi: 10.1001/jama.2014.14774. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2014. National Vital Statistics Reports. 2015;64(12):1–64. [PubMed] [Google Scholar]
- 21.Yokoe DS, Christiansen CL, Johnson R, et al. Epidemiology of and surveillance for postpartum infections. Emerg Infect Dis. 2001;7(5):837–41. doi: 10.3201/eid0705.010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiman L, O'Keefe M, Graham PL, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37(10):1313–9. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 23.Chuang I, Van Beneden C, Beall B, Schuchat A. Population-based surveillance for postpartum invasive group a streptococcus infections, 1995–2000. Clin Infect Dis. 2002;35(6):665–70. doi: 10.1086/342062. [DOI] [PubMed] [Google Scholar]
- 24.Louie JK, Jamieson DJ, Rasmussen SA. 2009 pandemic influenza A (H1N1) virus infection in postpartum women in California. Am J Obstet Gynecol. 2011;204(2):144, e1–6. doi: 10.1016/j.ajog.2010.08.057. [DOI] [PubMed] [Google Scholar]
- 25.Grohskopf LA, Shay DK, Shimabukuro TT, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices - United States, 2013–2014. Morbidity and Mortality Weekly Report. 2013;62(7):1–48. [PubMed] [Google Scholar]
- 26.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev. 2012;36 doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Leeuwen WMA, Lehto M, Karisola P, et al. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. Plos One. 2009;4(2) doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey DJ, Fleshner M, Wright KP. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 31.Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated proinflammatory cytokine production. Biol Psychol. 2009;82(1):12–7. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14(6):560–7. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 33.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: Sex differences. Brain Behav Immun. 2010;24(1):54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behav, Immun. 2007;21(7):901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Dowlati Y, Herrmann N, Swardfager W, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2010;67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76(3):181–9. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 38.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: Implications for pregnancy complications. Reproductive Sciences. 2007;14(6):560–7. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 39.Okun ML, Kiewra K, Luther JF, Wisniewski SR, Wisner KL. Sleep Disturbances in Depressed and Nondepressed Pregnant Women. Depress Anxiety. 2011;28(8):676–85. doi: 10.1002/da.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair LM, Porter K, Leblebicioglu B, Christian LM. Poor Sleep Quality and Associated Inflammation Predict Preterm Birth: Heightened Risk among African Americans. Sleep. 2015;38(8):1259–67. doi: 10.5665/sleep.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behav, Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Christian LM, Porter K, Karlsson E, Schultz-Cherry S, Iams JD. Serum proinflammatory cytokine responses to influenza virus vaccine among women during pregnancy versus non-pregnancy. Am J Reprod Immunol. 2013;70(1):45–53. doi: 10.1111/aji.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23(6):750–4. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 45.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Williams DR. Race, socioeconomic status, and health the added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896(1):173–88. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen XL, Redline S, Shields AE, Williams DR, Williams MA. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24(8):612–9. doi: 10.1016/j.annepidem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christian LM, Glaser R, Porter K, Iams JD. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med. 2013;75(7):658–69. doi: 10.1097/PSY.0b013e31829bbc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 50.Abidin RR. Parenting Stress Index:(Short Form) Pediatric Psychology Press; 1990. [Google Scholar]
- 51.Haskett ME, Ahern LS, Ward CS, Allaire JC. Factor structure and validity of the Parenting Stress Index-Short Form. Journal of Clinical Child and Adolescent Psychology. 2006;35(2):302–12. doi: 10.1207/s15374424jccp3502_14. [DOI] [PubMed] [Google Scholar]
- 52.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 53.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 54.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boekholdt SM, Peters RJ, Hack CE, et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vac Biol. 2004;24(8):1503–8. doi: 10.1161/01.ATV.0000134294.54422.2e. [DOI] [PubMed] [Google Scholar]
- 57.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398(6729):718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 58.Kahn M, Sheppes G, Sadeh A. Sleep and emotions: Bidirectional links and underlying mechanisms. Int J Psychophysiol. 2013;89(2):218–28. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Minkel JD, Banks S, Htaik O, et al. Sleep Deprivation and Stressors: Evidence for Elevated Negative Affect in Response to Mild Stressors When Sleep Deprived. Emotion. 2012;12(5):1015–20. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM. Bidirectional, Temporal Associations of Sleep with Positive Events, Affect, and Stressors in Daily Life Across a Week. Ann Behav Med. 2017;51(3):402–15. doi: 10.1007/s12160-016-9864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2009;24(1):146–53. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- 62.Phillips GS, Wise LA, Rich-Edwards JW, Stampfer MJ, Rosenberg L. Prepregnancy depressive symptoms and preterm birth in the Black Women's Health Study. Ann Epidemiol. 2010;20(1):8–15. doi: 10.1016/j.annepidem.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 64.Christian LM, Franco A, Glaser R, Iams J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behav, Immun. 2009;23(6):750–4. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behav, Immun. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox JL, Holden JM, Sagovsky R. Detection of Postnatal Depression - Development of the 10-Item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 67.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behav, Immun. 2007;21(2):153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9(1):46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christian LM, Mitchell AM, Gillespie SL, Palettas M. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology. 2016;74:69–76. doi: 10.1016/j.psyneuen.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Killewald A, Pfeffer FT, Schachner JN. Wealth Inequality and Accumulation. Annual Review of Sociology. 2017;(0) doi: 10.1146/annurev-soc-060116-053331. [DOI] [PMC free article] [PubMed] [Google Scholar]