Abstract
Purpose
Patients with Fuchs endothelial corneal dystrophy (FECD) often notice poor vision in the morning that improves as the day progresses. In this study, we determined changes in corneal optical properties associated with induced corneal edema.
Methods
Twenty-three phakic eyes (23 participants) with FECD (grades 1 to 6, modified Krachmer scale) and 8 normal eyes (8 participants) were examined by Scheimpflug photography. Central corneal thickness, high-order aberrations from anterior and posterior corneal surfaces, and backscatter from the anterior, mid, and posterior cornea were determined from the Scheimpflug images. A low-oxygen permeable contact lens was placed on the eye and eyes were closed for 2 hours, after which the lens was removed and Scheimpflug photography was repeated for up to 5 hours to determine changes in backscatter and high-order aberrations.
Results
Corneas swelled by 10% (95% CI, 9–10). Backscatter from the anterior cornea increased by 416 SU (scatter units; 95% CI, 344–488; p < 0.001) independent of the presence and severity FECD. Recovery of anterior backscatter was slower in advanced FECD (81 SU/hour, 95% CI, 60–120) compared to normal (123 SU/hour, 95% CI 95–150; p = 0.019). Anterior and posterior corneal high-order aberrations, and mid and posterior backscatter, did not increase with induced swelling.
Conclusion
Inducing corneal edema increases anterior corneal backscatter but not high-order aberrations. Subjective poor vision in the morning in FECD is probably caused by scattered light rather than by high-order aberrations, suggesting these patients experience more disability glare than decreased visual acuity.
Keywords: Fuchs dystrophy, endothelial function, edema, scatter
Introduction
Fuchs endothelial corneal dystrophy (FECD) is characterized by progressively degenerating corneal endothelium with the development of guttae, corneal haze, and edema. As the disease progresses, patients’ visual performance gradually decreases because of increased forward scatter and optical aberrations from the cornea.1–4
As FECD advances, many patients subjectively describe poor vision on awakening that resolves or improves as the day progresses. Because normal corneas swell during prolonged eyelid closure by an estimated 4–5%,5–8 it is assumed that FECD patients are susceptible to similar or exaggerated edema overnight and the effects of swelling explain their poor vision in the morning.9, 10 However, the specific changes in vision and corneal optical properties in this situation are poorly characterized. Light attenuation with corneal thickening is minimal and unlikely the cause of visual fluctuation.11 In contrast, corneal edema could degrade vision by increasing forward scatter or high-order aberrations. Increased intraocular forward light scatter reduces image contrast on the retina and is associated with increased disability glare, whereas increased aberrations cause widening of the retinal image point-spread function and are associated with decreased visual acuity.2, 12–14
In this study, we explored changes in corneal optical properties as possible sources of transient poor vision in FECD patients after purposefully inducing corneal edema by placing a low-oxygen permeable contact lens. We measured changes in backscatter and high-order aberrations from the anterior and posterior corneal surfaces as the cornea recovered from swelling. Although corneal backscatter does not affect the retinal image, it can be isolated from the cornea, whereas corneal forward scatter, which does affect vision, cannot be measured independently of other scattering sources within the eye, especially cataract. Because light scatters in all directions, the same structural changes in the cornea that produce backscatter also produce forward scatter, and thus corneal backscatter is a reasonable surrogate measure for corneal forward scatter.
Materials and Methods
Participants
Participants were recruited from the Cornea Service at Mayo Clinic, Rochester, MN. Severity of FECD was graded on a scale of 1 to 6 by the widest diameter and confluence of guttae and presence of edema by using slit-lamp biomicroscopy (modified Krachmer scale).15, 16 All eyes were phakic without a history of ocular surgery, contact lens use, or topical or systemic medication known to affect the cornea; eyes had no abnormalities other than FECD (in the FECD group only) or cataract. Control participants had normal corneas and were restricted to age 50 years or above to match the expected age range of FECD. All participants gave written consent to participate after discussion of the possible benefits and consequences of the study. This study was approved by the Institutional Review Board at Mayo Clinic and conformed to the tenets of the Declaration of Helsinki.
Scheimpflug photography and measurements
Each participant was seated at the chinrest of a Scheimpflug camera (Pentacam HR; Oculus, Lynnwood, WA). After initial alignment, the standard automatic recording system completed fine alignment and captured 25 Scheimpflug images through the cornea and anterior chamber in one second, while the slit rotated about the optical axis of the eye. Images were captured at evenly spaced angles. The software (Pentacam software version 1.20r29) reconstructed the three-dimensional inner and outer surfaces of the cornea and calculated corneal thickness, corneal image brightness, and wavefront aberrations from the anterior, posterior, and combined corneal surfaces.
The mean corneal backscatter (image brightness) in the anterior 120 μm, the posterior 60 μm, and the mid cornea (region between these limits) was expressed in standardized scatter units (SU) as described previously.17 Scatter units represented the equivalent concentration of a scatter standard (Amco Clear, GFS Chemicals, Powell, OH) that produced the same image brightness as did the cornea. All image brightness data were adjusted for daily variations of instrument sensitivity from measurements of a scatter standard before each exam.17
Wavefront errors across a 6-mm pupil were expressed by the native software as Zernike polynomials to the 6th order for the anterior and posterior corneal surfaces. Total high-order aberrations were calculated by:
where n is the order and m is the frequency of the Zernike coefficient, .
Corneal swelling
On a day prior to the study, each participant was examined in the mid to late afternoon to assure that they qualified for the study and to record a steady-state Scheimpflug image. On the morning of the study, corneas were re-examined by Scheimpflug photography. Then a low-oxygen transmissible contact lens was placed on one cornea and the eyelid was taped closed over the lens. The contact lens was custom-made from polymacon (oxygen permeability, 7.9 x 1011 cm2/sec ml O2/ml mmHg) and was 0.5 mm thick with a 12-mm diameter. Lenses were made in two base curves (8.2 mm and 8.0 mm) and the lens with the base curve that best fitted the mean curvature of each cornea was used. After 2 hours contact lens wear with the eyelid patched closed, the eyelid was opened, the contact lens was removed, and Scheimpflug photography was repeated immediately and every 15 minutes for the first hour and every 30 minutes thereafter for 5 hours or until corneal thickness returned to steady-state.
Central corneal thickness for each examination was determined from Scheimpflug images. The minimum thickness between the screening and the pre-contact lens measurements was used as the non-swollen or steady-state thickness. After removal of the contact lens, the change in thickness at each measurement was defined as the difference between measured central thickness and steady-state thickness.
Statistical Analysis
Linear regression models were used to calculate mean differences of steady-state characteristics between groups (categorical; normal, and mild, moderate, and advanced FECD) with respective 95% confidence intervals (95% CIs). Differences within participant groups for backscatter and high-order aberrations between morning and screening measurements and between minimum and maximum values were assessed with linear regression. Generalized estimating equation models, which account for any correlation between repeated measurements within every participant, were used to assess response patterns over time. A fixed cross-level interaction term between group (categorical) and time was used to examine the relationship between backscatter and high-order aberrations (continuous) and time, and to determine if the strength of that relationship varied as a function of FECD severity. Differences were considered statistically significant if p < 0.05 with two-sided tests.
Results
A total of 23 participants with FECD aged 44 to 89 years (median, 65 years) participated in the study. Corneal swelling was induced in only one eye per participant. For purposes of this study, each eye was classified as mild (grades 1 and 2; 8 eyes), moderate (grades 3 and 4; 4 eyes), or advanced (grades 5 and 6; 11 eyes). Eight control participants without guttae were also studied (ages 54 to 83 years; median, 60 years). Nineteen of the 31 participants were female, with 5 of those in the control group.
Central corneal thickness increased during contact lens wear and eyelid closure by 10% (95% CI, 9–10) or 53 μm (95% CI, 52–54) and returned to steady-state over several hours in both FECD and control participants. Mean steady-state thickness was 544 μm (95% CI, 520–569; p = 0.8) in mild, 567 μm (95% CI, 534–602; p = 0.2) in moderate, and 583 μm (95% CI, 562–604; p = 0.1) in advanced FECD compared to normal (541 μm; 95% CI, 517–565), as previously reported in these eyes investigating the question of corneal hydration control.18
Steady-state anterior backscatter was higher in advanced FECD (1599 SU; 95% CI, 1522–1677) compared to normal (1309 SU; 95% CI 1286–1331; p <0.001), and was not statistically different in mild (1374 SU; 95% CI, 1283–1465; p = 0.3) and moderate (1453 SU; 95% CI, 1324–1581; p = 0.07) FECD compared to normal. After lens removal, backscatter in the anterior cornea increased by 37% (95% CI, 27–47) or 416 SU (95% CI, 344–488, p < 0.001), was independent of the presence and severity of FECD (p = 0.6), and decreased during the next hours (p < 0.001) (Figure 1). Recovery per hour of anterior backscatter was slower in advanced FECD (slope, 81 SU/hour, 95% CI, 60–120) compared to normal (123 SU/hour, 95% CI 95–150; p = 0.019); it was not significantly different from normal in mild (159 SU/hour, 95% CI, 131–188; p = 0.07) and moderate (117 SU/hour, 95% CI, 89–145; p = 0.7) FECD.
Figure 1. Changes in anterior corneal backscatter with induced corneal edema.
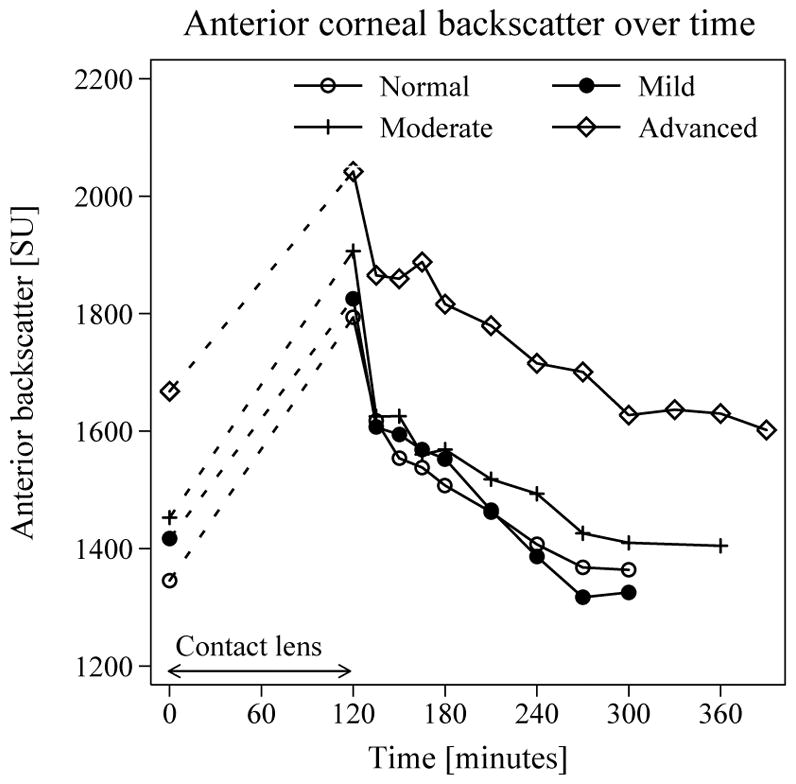
Mean anterior corneal backscatter after contact lens removal increased by 416 SU (95% CI, 344–488; p < 0.001) independent of the presence and severity FECD. Decrease in backscatter over time was slower in advanced FECD (81 SU/hour; 95% CI, 60–120) compared to normal corneas (123 SU/hour, 95% CI, 95–150; p = 0.02).
Backscatter from the mid-cornea did not change after contact lens removal (p = 0.2) in any group (p = 0.2). Steady-state posterior corneal backscatter was 833 SU (95% CI, 714–954; p = 0.2) in mild, 742 SU (95% CI, 573–912; p = 0.8) in moderate, and 981 SU (95% CI, 879–1083; p = 0.003) in advanced FECD compared to normal (727 SU; 95% CI 697–757). Backscatter from the posterior cornea did not change after lens removal compared to steady-state in any group (p = 0.4) and did not change over time (p = 0.7).
Mean total high-order aberrations of the anterior surface were 0.47 μm (95% CI, 0.36–0.58) in normal corneas compared to 0.65 μm (95% CI, 0.55–0.77; p = 0.1) in mild, 0.52 μm (95% CI, 0.36–0.67; p = 0.6) in moderate, and 0.58 μm (95% CI, 0.48–0.67; p = 0.1) in advanced FECD before contact lens placement. Total anterior corneal high-order aberrations decreased by 0.08 μm (95% CI, 0.00–0.15; p = 0.03) immediately after contact lens removal and no significant change was observed over time (p = 0.3). The difference between individual minimum and maximum high-order aberrations was 0.18 μm (95% CI, 0.13–0.23) and was not different between groups (p = 0.8, Figure 2). Total high-order aberrations of the posterior cornea did not change after contact lens removal (p = 0.7).
Figure 2. Changes in anterior corneal high-order aberrations with induced corneal edema.
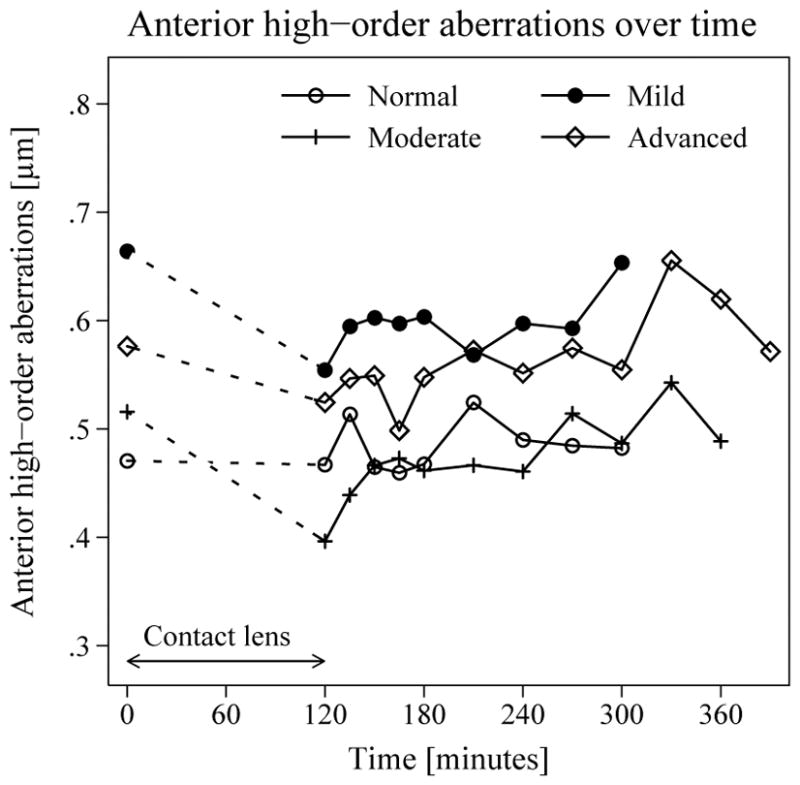
Changes in anterior corneal total high-order aberrations after contact lens removal were compared to before lens placement and analyzed with generalized estimating equation models. Mean total high-order aberrations (6 mm-diameter optical zone) were slightly lower after lens removal (0.06 μm, 95% CI, 0.02–0.10; p = 0.002) and did not change over the course of the study.
Discussion
In this study, induced corneal edema was associated with increased anterior corneal backscatter. Resolution of induced anterior corneal backscatter was slower in advanced FECD than normal, and was additive to an already higher than normal steady-state anterior backscatter. Despite these changes in corneal backscatter, total high-order aberrations from the anterior and posterior surfaces of the cornea did not increase over the course of the study. Our results suggest that visual symptoms related to mild corneal edema may be attributed to increased backscatter rather than increased high-order aberrations.
In clinical practice, many patients with FECD and other types of chronic corneal edema describe subjective impairment in vision on awakening, a degradation in vision that improves as the day progresses. These symptoms have been associated with increased corneal thickness in the morning and are believed to be secondary to hypoxic swelling from overnight eyelid closure.9, 19 In this study we investigated the specific optical factors that change with induced edema. The simple attenuation of light from increased corneal thickness is insufficient to explain vision changes because light transmittance decreases by only 4% when corneal thickness is doubled (Lambert-Beer law).11
Anterior corneal backscatter is known to be higher than normal in FECD, including in mild disease,3 and can be explained by ultrastructural changes including subepithelial fibrosis and the presence of corneal edema.4, 20 That corneal edema is only partially responsible for increased backscatter in FECD3, 20 is evidenced by partial improvement in anterior backscatter after restoring endothelial function by endothelial keratoplasty with resolution of edema; in contrast, subepithelial fibrosis persists after restoring endothelial function.21, 22 In this study, we induced edema in normal and FECD corneas to simulate low-oxygen conditions that might be experienced during eyelid closure with sleep and measured the changes in the optical properties of the cornea. The predominant finding was that anterior corneal backscatter increased, independent of the presence or severity of FECD. Corneal edema produces irregular expansion in lamellar collagen interspacing that increases light scattering.23–25 Although the increase in anterior backscatter with induced edema did not differ between normal and FECD corneas, in advanced FECD, steady-state anterior backscatter was higher than normal,3 and this was compounded by slower resolution of induced backscatter by approximately one third. Backscatter per se has no effect on vision because it is scattered away from the retina; nevertheless, the same corneal changes that produce increased backscatter also cause increased forward scatter, which does degrade vision by reducing contrast perception and creating disability glare.2, 14 Forward scatter can be measured from the entire ocular media, and is increased in eyes with lenticular opacity,14 but isolated measurements from the cornea are not possible, and thus corneal backscatter is the best surrogate measurement for corneal light scatter.
Visual acuity is more sensitive to changes in high-order aberrations that degrade the center of the retinal image point-spread function, and minimally affected by increased forward scatter.2, 26 In a large study across a range of severity of FECD, we identified that high-order aberrations were higher than normal in FECD.3 In the present study, we did not find an increase in aberrations from the anterior or posterior corneal surfaces after inducing corneal edema. In fact, we found a small but significant decrease in anterior total high-order aberrations after removing the contact lens; one explanation for this is that induction of high-order aberrations may have been limited by using a soft contact lens on the anterior corneal surface.
Our results suggest that mildly increased corneal edema on awakening in eyes with FECD affects vision by increasing scattered light rather than by increasing aberrations. This implies that patients probably experience increased glare and poor contrast on awakening rather than impaired visual acuity. This is consistent with increased glare being identified as an early symptom of FECD, especially in younger subjects, and with glare being a potential clinical indicator of when endothelial keratoplasty might be considered.1 Although this study was limited by not directly measuring straylight (forward scatter) or visual acuity to confirm these findings, the impact of scattered light and aberrations on the retinal image point-spread function and vision are well known.14, 27 The latter measurements would also be confounded by cataract, which cannot easily be controlled for given the age of the population studied, and thus we limited our measurements to changes in corneal optical properties. Also, while our experimental conditions may have induced more hypoxic stress and edema than normal overnight conditions, which produce approximately 4–5% swelling in normal corneas,28 the model helped determine the relative changes in corneal optical properties in FECD.
In summary, we found that contact lens-induced corneal swelling, simulating hypoxic overnight conditions, increased anterior backscatter in FECD, and this resolved more slowly in advanced FECD compared to normal corneas. Our results suggest that subjective poor vision on awakening in FECD is caused by scattered light rather than by aberrations, indicating that these patients probably experience increased disability glare rather than decreased acuity. Measurement of psychophysical vision parameters and subjective symptoms would better determine the type of visual degradation, and this might in turn help surgeons understand corneal endothelial function when counseling patients about surgery.
Acknowledgments
Funding Source: Research to Prevent Blindness, New York, NY (an unrestricted department grant and SVP as Olga Keith Wiess Special Scholar), Dr. Werner Jackstaedt Foundation, Wuppertal, Germany (Research Fellowship to K.W.), Mayo Clinic Center for Translational Science Activities (grant no. UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, Bethesda, MD), and Mayo Foundation, Rochester, MN.
Abbreviations and Acronyms
- FECD
Fuchs endothelial corneal dystrophy
- SU
Scatter units
Footnotes
Conflict of Interest: none, all authors.
Presented in part as a poster at the annual meeting of the Association for Research in Vision and Ophthalmology, Seattle, Washington, May 2, 2016.
References
- 1.van der Meulen IJ, Patel SV, Lapid-Gortzak R, et al. Quality of vision in patients with Fuchs endothelial dystrophy and after Descemet stripping endothelial keratoplasty. Arch Ophthalmol. 2011;129:1537–1542. doi: 10.1001/archophthalmol.2011.247. [DOI] [PubMed] [Google Scholar]
- 2.McLaren JW, Patel SV. Modeling the effect of forward scatter and aberrations on visual acuity after endothelial keratoplasty. Invest Ophthalmol Vis Sci. 2012;53:5545–5551. doi: 10.1167/iovs.12-10011. [DOI] [PubMed] [Google Scholar]
- 3.Wacker K, McLaren JW, Amin SR, et al. Corneal high-order aberrations and backscatter in Fuchs endothelial corneal dystrophy. Ophthalmology. 2015;122:1645–1652. doi: 10.1016/j.ophtha.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin SR, Baratz KH, McLaren JW, et al. Corneal Abnormalities Early in the Course of Fuchs’ Endothelial Dystrophy. Ophthalmology. 2014;121:2325–2333. doi: 10.1016/j.ophtha.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmanson JP, Sheldon TM, Goosey JD. Fuchs’ endothelial dystrophy: a fresh look at an aging disease. Ophthalmic Physiol Opt. 1999;19:210–222. doi: 10.1046/j.1475-1313.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandell RB, Polse KA, Brand RJ, et al. Corneal hydration control in Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 1989;30:845–852. [PubMed] [Google Scholar]
- 7.Kiely PM, Carney LG, Smith G. Diurnal variations of corneal topography and thickness. Am J Optom Physiol Opt. 1982;59:976–982. doi: 10.1097/00006324-198212000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Leung BK, Bonanno JA, Radke CJ. Oxygen-deficient metabolism and corneal edema. Prog Retin Eye Res. 2011;30:471–492. doi: 10.1016/j.preteyeres.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamis AP, Filatov V, Tripathi BJ, et al. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 10.Waring GO, 3rd, Rodrigues MM, Laibson PR. Corneal dystrophies. II. Endothelial dystrophies. Surv Ophthalmol. 1978;23:147–168. doi: 10.1016/0039-6257(78)90151-0. [DOI] [PubMed] [Google Scholar]
- 11.Wacker K, Bourne WM, Patel SV. Effect of Graft Thickness on Visual Acuity After Descemet Stripping Endothelial Keratoplasty: A Systematic Review and Meta-Analysis. Am J Ophthalmol. 2016;163:18–28. doi: 10.1016/j.ajo.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg TJ, van Rijn LJ, Michael R, et al. Straylight effects with aging and lens extraction. Am J Ophthalmol. 2007;144:358–363. doi: 10.1016/j.ajo.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Seery LS, McLaren JW, Kittleson KM, et al. Retinal point-spread function after corneal transplantation for Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2011;52:1003–1008. doi: 10.1167/iovs.10-5375. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg TJ, Franssen L, Coppens JE. Straylight in the human eye: testing objectivity and optical character of the psychophysical measurement. Ophthalmic Physiol Opt. 2009;29:345–350. doi: 10.1111/j.1475-1313.2009.00638.x. [DOI] [PubMed] [Google Scholar]
- 15.Krachmer JH, Purcell JJ, Jr, Young CW, et al. Corneal endothelial dystrophy. A study of 64 families Arch Ophthalmol. 1978;96:2036–2039. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 16.Repp DJ, Hodge DO, Baratz KH, et al. Fuchs’ endothelial corneal dystrophy. Subjective grading versus objective grading based on the central-to-peripheral thickness ratio Ophthalmology. 2013;120:687–694. doi: 10.1016/j.ophtha.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaren JW, Wacker K, Kane KM, et al. Measuring Corneal Haze by Using Scheimpflug Photography and Confocal Microscopy. Invest Ophthalmol Vis Sci. 2016;57:227–235. doi: 10.1167/iovs.15-17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wacker K, McLaren JW, Kane KM, et al. Corneal Hydration Control in Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2016;57:5060–5065. doi: 10.1167/iovs.16-20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe S, Oie Y, Fujimoto H, et al. Relationship between Corneal Guttae and Quality of Vision in Patients with Mild Fuchs’ Endothelial Corneal Dystrophy. Ophthalmology. 2015 doi: 10.1016/j.ophtha.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Patel SV, McLaren JW. In vivo confocal microscopy of Fuchs endothelial dystrophy before and after endothelial keratoplasty. JAMA Ophthalmol. 2013;131:611–618. doi: 10.1001/jamaophthalmol.2013.799. [DOI] [PubMed] [Google Scholar]
- 21.Baratz KH, McLaren JW, Maguire LJ, et al. Corneal haze determined by confocal microscopy two years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol. 2012;130:868–874. doi: 10.1001/archophthalmol.2012.73. [DOI] [PubMed] [Google Scholar]
- 22.Wacker K, Baratz KH, Maguire LJ, et al. Descemet Stripping Endothelial Keratoplasty for Fuchs’ Endothelial Corneal Dystrophy: Five-Year Results of a Prospective Study. Ophthalmology. 2016;123:154–160. doi: 10.1016/j.ophtha.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Freund DE, McCally RL, Farrell RA. Effects of fibril orientations on light scattering in the cornea. J Opt Soc Am A. 1986;3:1970–1982. doi: 10.1364/josaa.3.001970. [DOI] [PubMed] [Google Scholar]
- 24.Hsueh C-M, Lo W, Chen W-L, et al. Structural Characterization of Edematous Corneas by Forward and Backward Second Harmonic Generation Imaging. Biophys J. 2009;97:1198–1205. doi: 10.1016/j.bpj.2009.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SV, Baratz KH, Maguire LJ, et al. Anterior corneal aberrations after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Ophthalmology. 2012;119:1522–1529. doi: 10.1016/j.ophtha.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Freund DE, McCally RL, Farrell RA. Direct summation of fields for light scattering by fibrils with applications to normal cornea. Appl Opt. 1986;25:2739–2746. doi: 10.1364/ao.25.002739. [DOI] [PubMed] [Google Scholar]
- 28.Leung BK, Bonanno JA, Radke CJ. Oxygen-deficient metabolism and corneal edema. Prog Retin Eye Res. 2011;30:471–492. doi: 10.1016/j.preteyeres.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


