Abstract
Purpose
Langerhans cell histiocytosis (LCH) is a rare malignant disease characterized by histiocytic proliferation. We intended to characterize the efficacy and safety of radiation therapy (RT) in a contemporary cohort and to explore if there are sites at higher risk for local recurrence.
Materials/Methods
Between 1995 and 2015, we identified 39 consecutive LCH patients who were treated primarily with radiation therapy. Patients were staged by single/multisystem involvement (SS/MS) and established risk organ criteria. In 46 irradiated lesions, clinical and radiologic responses were evaluated at multiple time points after radiotherapy. Patient demographics, treatment, and local failure were compared by site of lesion.
Results
Median age at RT was 35 years (range 1.5 – 67). Twelve patients had multisystem involvement, and of those, 5 patients had disease in organs considered to be high-risk. The following sites were irradiated: bone (31), brain (6), skin (3), lymph node (3), thyroid (2), and nasopharynx (1). Median dose was 11.4 Gy (7.5 – 50.4). At a median follow-up of 45 months (6 – 199), local recurrence or progression was noted in 5 of 46 (11%) lesions. There were no local failures of the 31 bone lesions evaluated, while the 3-year freedom from local failure in the 15 non-bone lesions was 63% (95% CI 32 – 83%; p = 0.0008). Local failures occurred in 2 of 3 skin lesions, in 2 of 6 brain lesions, and 1 of 3 lymph node lesions. Deaths were recorded in 5 of 39 (13%) patients, all of whom were adults with multisystem disease.
Conclusion
Radiotherapy is a safe and effective measure for providing local control of LCH involving the bone. While bone lesions are well controlled with low doses of radiation, disease in other tissues such as the skin and brain may require higher doses of radiation or additional treatment modalities.
Introduction
Langerhans cell histiocytosis (LCH) is a rare disorder characterized by proliferation of myeloid dendritic cells that express the same antigens as the skin Langerhans cell, CD1a and CD207 (1–3). Its clinical presentation varies widely, from nonfatal single system bone lesions to multisystem disease with mortality rates of 10–20% in some groups (4). Disease may present in various tissues throughout the body, most frequently in bone (approximately 80% of patients) and skin (40% of patients) (5). Other organs involved may include lymph nodes, thyroid, oral mucosa, central nervous system, lung, liver, and spleen (5–7).
Over the last few decades, systemic therapy for children with multisystem LCH involvement has been tested in multiple randomized clinical trials (4, 8–10). However, for adult patients and those with single system disease, data on optimal treatment approach is limited. While radiation therapy (RT) has become less frequently used for LCH lesions over the last several decades, it continues to be an important and effective option for treatment. Indications for radiotherapy include severe pain, enlarging mass, bony destruction with a risk of pathological fracture, and risk of high surgical morbidity (11). Rates of local control following RT are over 90% for bone lesions (12–14). Obviously, the effectiveness of RT must be weighed against the risk of long-term adverse events in the pediatric population, where damage to endochondral ossification centers and secondary malignancies are a concern (11, 15).
In this study, we sought to determine how LCH’s widely variable presentation impacts the effectiveness of radiotherapy. Further, we evaluated the safety and efficacy of radiotherapy for LCH in an era with the widespread availability of more conformal RT techniques, which are not represented in available literature.
Methods and Patients
Patient Selection
An Institutional Review Board authorization was obtained for this retrospective study. Between 1990 and 2015, 325 patients had a pathological diagnosis of LCH at our center. Of these, 42 patients received radiation therapy alone, or in combination with other treatment modalities. After excluding three patients lost to follow-up, a final cohort of 39 patients with biopsy-proven LCH treated with radiotherapy was defined and analyzed. All patients were treated between 1995 and 2015.
Data Abstraction and Disease Characterization
Data on patient demographics, treatment details, and outcome were obtained from our electronic medical record and radiation oncology charts. Treatment details collected included site of irradiation, dose, fractionation, energy, and technique as well as presence of prior or concurrent surgery and chemotherapy, when applicable. Surgery was considered concurrent if within three months of radiation therapy. Disease was staged according to the guidelines set forth by the Histiocyte Society (16, 17). Patients were defined to have single system LCH or multisystem LCH if one or greater than one organ system, respectively, was affected. Risk organs were defined as liver, spleen, hematopoietic system, and lung in patients under 18 years of age. In patients older than 18, central nervous system (CNS) tumors were considered high risk and lung disease was considered low risk, as recommended by Euro-Histio-Net’s expert panel (18). For bone lesions, extension to adjacent soft tissue and multiple bone involvement was noted. Treatment toxicities were noted and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (19).
Response Criteria
Clinical and radiographic responses were assessed from reviewing radiologic reports and oncologist notes at baseline and after radiotherapy. Response data was gathered at multiple time points: 6 weeks (4 – 8 weeks), 12 weeks (8 – 16 weeks), 6 months (16– 30 weeks), 1 year (30 weeks – 15 months), 3 years (15 months – 42 months) and 5 years (42 months – 6 years) after completion of radiotherapy. Local response of each irradiated lesion was defined as complete response (CR), partial response (PR), stable disease (SD), or progression of disease (POD). A PR was defined as radiologic improvement including decrease in lesion size and development of sclerosis in a previously non-sclerotic bone lesion. POD was defined as any increase in lesion size from prior scans or recurrence of disease after complete response. Patient signs and symptoms at presentation and after therapy were obtained from oncologist notes. Local clinical response was defined as CR, PR, SD, or POD based on resolution or worsening of patient-reported symptoms reasonably attributable to the irradiated lesion. Lesions without symptoms recorded were excluded from clinical response analysis. Response at time points prior to first follow up and after last follow up were not included in radiographic or clinical response analyses. If response at a time point was unknown and responses were recorded before and after the time point, it was assumed to be the response of the prior time point unless the future response was POD. Responses at all time points after POD were analyzed as POD to prevent confounding from salvage therapies.
Endpoints
The primary endpoint of this study was freedom from local failure (FFLF), defined as time from end of radiotherapy to local progression or recurrence of the lesion. Overall survival (OS) was calculated from end of radiotherapy to time of death. Distant progression-free survival was defined as time from end of a patient’s first course of radiotherapy to progression or development of disease outside of the irradiated area.
Statistical Analysis
The Fisher’s exact test and Wilcoxon rank-sum test were used to compare bone and non-bone lesions for categorical and continuous variables, respectively. The Kaplan-Meier method was used to estimate FFLF, OS, and distant progression-free survival rates, and the log-rank test was used to compare curves of bone and non-bone lesions. Only the first treated lesion was included in calculation of distant progression-free survival to avoid counting one distant progression more than once. Comparisons with p < 0.05 were considered statistically significant. Statistical analysis was performed in GraphPad Prism 7.0a (GraphPad Software, San Diego, California) and IBM SPSS Statistics v21 (IBM, Armonk, New York), and SAS 9.4 (SAS, Cary, North Carolina).
Results
Patient Demographics and Presentation
In the cohort of 39 patients treated with radiation for LCH, there were 22 males and 17 females. The median age at diagnosis of LCH was 31 years (range 1.3 to 65.9), and the median age at receipt of radiotherapy was 33.4 (mean 1.5 – 67.5). Fifteen patients (38%) were under 18 years (Table 1).
Table 1.
Patient, Tumor, and Treatment Characteristics by Site of Lesion
| Patient Characteristics | Total | Bone Only | Non-Bone | p | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | 39 | 26 | 13 | |||||
|
| ||||||||
| Sex | Male | 22 (56) | 17 (65) | 5 (38) | 0.17 | |||
| Female | 17(44) | 9 (35) | 8 (62) | |||||
|
| ||||||||
| Age | <18 | 15 (38) | 14 (54) | 1 (8) | 0.006 | |||
| >18 | 24 (62) | 12 (46) | 12 (92) | |||||
|
| ||||||||
| Tumor and Treatment Characteristics | Total | Bone | CNS | Skin | Lymph Node | Other | p (Bone vs. All Others) | |
|
| ||||||||
| No. of lesions | 46 | 31 | 6 | 3 | 3 | 3 | ||
|
| ||||||||
| Recurrent/Refractory | Yes | 9 (20) | 3 (10) | 3 (50) | 2 (67) | 0 | 1 (33) | 0.04 |
| No | 37 (80) | 28 (90) | 3 (50) | 1 (33) | 3 (100) | 2 (67) | ||
|
| ||||||||
| Treatment | RT Alone | 30 (65) | 21 (68) | 3 (50) | 3 (100) | 2 (67) | 1 (33) | 0.22 |
| RT + Surgery | 10 (22) | 8 (26) | 1 (17) | 0 | 1 (33) | 0 | ||
| RT + Chemo | 4 (9) | 1 (3) | 2 (33) | 0 | 0 | 1 (33) | ||
| RT + Surgery + Chemo | 2 (4) | 1 (3) | 0 | 0 | 0 | 1 (33) | ||
|
| ||||||||
| RT Total Dose (cGy) | Median (range) | 1200 (750 – 5040) | 1080 (750 – 2400) | 1050 (1000 – 1800) | 1500 (1200 – 3150) | 5000 (5000 – 5040) | 3780 (2520 – 5040) | 0.009 |
|
| ||||||||
| RT Dose per Fraction | Median (range) | 180 (150 – 200) | 190 (150 – 200) | 180 (150 – 200) | 150 (150 – 150) | 200 (180 – 200) | 180 (180 – 180) | 0.04 |
|
| ||||||||
| Technique | IMRT/IGRT/3DCRT | 17 (37) | 13 (42) | 1 (17) | 0 | 1 (33) | 2 (67) | 0.07 |
| Conventional | 18 (39) | 13 (42) | 3 (50) | 0 | 2 (67) | 1 (33) | ||
| Electron | 3 (7) | 0 | 0 | 3 (100) | 0 | 0 | ||
| Unknown | 8 (17) | 5 (16) | 2 (33) | 0 | 0 | 0 | ||
The majority (31/46; 67%) of irradiated lesions were in the bone. Other irradiated tumor sites included CNS (6), skin (3), lymph node (3), thyroid (2), and nasopharynx (1; Figure 1). Patients treated only for bone lesions were younger, with 14/26 (54%) patients under 18 years of age compared to 1/13 (8%) patients with non-bone lesions (p = 0.006). The most common presenting symptom in the cohort was pain in 22 (48%) lesions, followed by local swelling in 10 (22%) lesions. Of 5 patients with pituitary mass lesions, 4 patients experienced symptomatic endocrine abnormalities such as hypothyroidism and panhypopituitarism and 2 patients complained of visual deficits. Other presenting symptoms included fatigue (4), headache (4), painful or pruritic skin lesions (3), and proptosis (2).
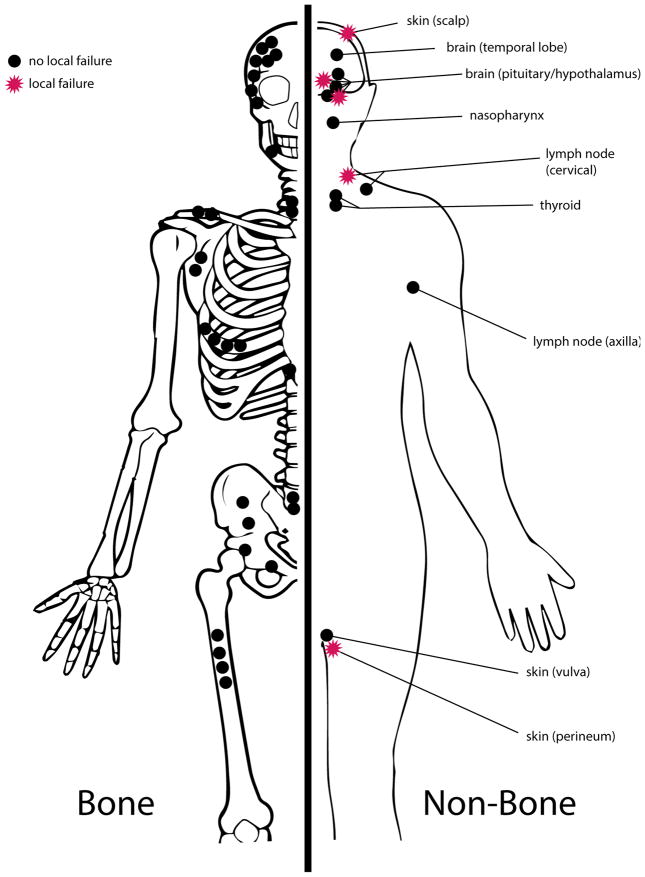
Figure 1.
Distribution of 46 treated Langerhans cell histiocytosis lesions. Bone lesions are represented on the left, and non-bone lesions on the right. Lesions that underwent local failure (11%) are represented as red stars.
At the time of initial radiotherapy, 31 of 39 (79%) patients had single system disease (SS-LCH) and 8 patients had multisystem involvement (MS-LCH). Of the 8 patients with MS-LCH, 3 had disease in high-risk organs. Four patients with SS-LCH at time of initial radiotherapy eventually had progression to MS-LCH. Fifteen of 31 (48%) bone lesions extended into the adjacent soft tissue, and 5 patients had multifocal bone involvement. Nine of 46 (20%) lesions were refractory to treatment prior to RT, with non-bone lesions more likely to be refractory compared to bone lesions (p = 0.04; Table 1). Prior treatments for the 9 refractory lesions were as follows: 6 lesions were refractory to 1–3 systemic regimens, 2 lesions were incompletely excised, and 1 patient had failed multiple prior topical skin creams.
Treatment Details
Median radiation dose delivered was 12 Gy (interquartile range 10.5 – 20 Gy) over a median of 7 fractions (interquartile range 6 – 10). Patients with bone lesions were treated with lower total doses of radiation than soft tissue lesions (p = 0.0003; Table 1). The three patients treated for skin were treated with electron beam radiotherapy. Of 35 treatment plans with recorded treatment technique, 18 (51%) were with conventional external beam therapy, and 17 were with conformal techniques such as intensity modulated RT, image-guided RT, and/or 3-D conformal RT.
Radiation was the only treatment modality for 30/46 (65%) lesions, was combined with surgery in 10 (22%), chemotherapy in 4 (9%) and both surgery and chemotherapy in 2 (4%). Three patients were treated with RT after curettage of a bone lesion, and the remaining nine surgeries were excisions. The chemotherapy used was a variety of combinations that included vinblastine, cladribine, methotrexate, and 6-mercaptopurine. Three patients with bone lesions were also treated with zolendronate after radiotherapy.
Clinical and Radiographic Response
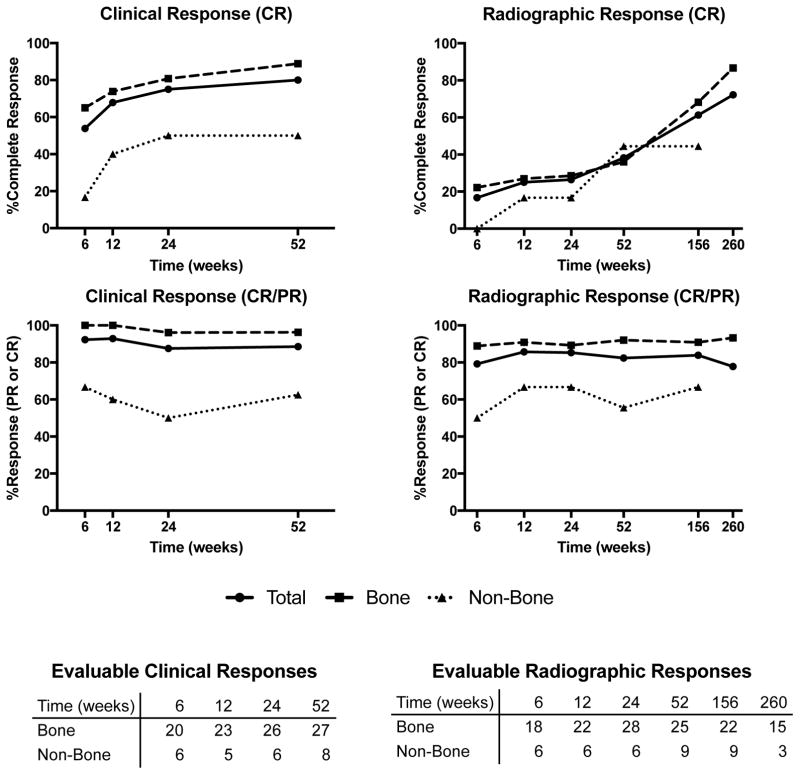
Clinical and radiographic response of the cohort is plotted in Figure 2. At all time points up to one-year, the most common radiographic response was PR. At three years and five years, the most common response was CR. At one-year follow up, there were 13 CR, 15 PR, 3 SD, and 3 POD. No imaging of bone lesions showed progression of disease. Bone lesions showed slower progression from partial to complete response than non-bone lesions; by one year after treatment, 14/25 bone lesions had PR and 9/25 CR compared to 1/9 PR and 4/9 CR in soft tissue lesions. Bone lesions began to show signs of improvement at early follow up within a few months after therapy, but frequently did not show complete healing until three to five years after treatment.
Figure 2.
Clinical and radiographic response rates over time. Evaluable clinical and radiographic responses at each time point are enumerated below the graphs. Five-year radiographic response for non-bone lesions was not displayed due to lack of follow-up at this time point.
Clinically, the majority (19/28, 68%) of symptomatic lesions with evaluable responses had complete symptomatic resolution by 12 weeks. All bone lesions with evaluable clinical responses at 6 weeks (20 patients) and 12 weeks (23 patients) had at least partial improvement of symptoms. At one-year follow up, 28/35 evaluable responses were CR, 3 PR, 1 SD, and 3 had POD. Complete symptomatic response was higher in bone lesions than soft tissue lesions, with 24/27 (89%) complete symptomatic responses for bone lesions at one year compared to 4/8 (50%) for soft tissue lesions.
Outcome
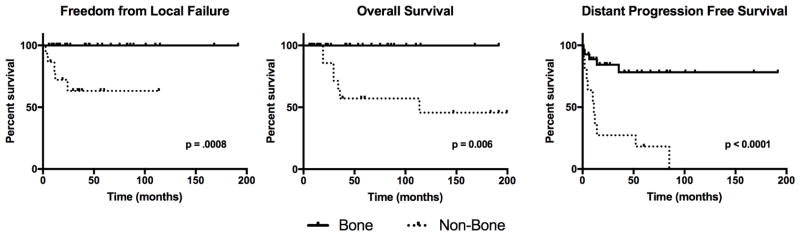
At a median follow up of 45 months (range 6–199), 5 lesions had a local failure after radiotherapy (Figure 3). 5-year freedom from local failure (FFLF) for the entire cohort was 87.4% (95% CI 71.9 – 94.6). Non-bone lesions were more likely to fail treatment, with a 3-year FFLF of 63.2% (32.2 – 83.0), while bone lesions had no local failures during the follow-up time (p = 0.0008). Details of the lesions that locally progressed or recurred are listed in Table 2. Two brain, one lymph node, and one skin lesion had a progression of disease, and one skin patient had a lesion recurrence in the treatment field after complete response. Of note, one skin lesion was re-treated with a higher dose (30 Gy) of electron therapy after local failure, initially responded to re-treatment, and then had second local relapse two months after re-irradiation.
Figure 3.
Kaplan-Meier curves for patients with bone lesions and non-bone lesions. 46 lesions are included in freedom from local failure and 39 patients are included in overall survival. Distant progression free survival calculated from treatment of first lesion for 39 patients.
Table 2.
Characteristics of Patients Experiencing Local Failure
| Age/Gender | Risk Stratum | Lesion | Prior Treatments | Technique | Dose (Gy) | Fractions | Concurrent Treatment | CR Achieved? | Time to Failure (months) |
|---|---|---|---|---|---|---|---|---|---|
| 51M | SS | Skin | none | electrons | 15 | 10 | none | no | 2.5 |
| 32M | SS | Skin | Multiple skin creams (inc. corticosteroids) | electrons | 31.5 | 21 | none | yes | 24.2 |
| 11F | MS/RO+ | Brain | VBL; thioguanine + MTX | conventional whole brain | 10.5 | 7 | none | no | 12.1 |
| 40F | SS | Brain | none | conventional | 10 | 5 | none | no | 4.7 |
| 61M | MS/RO− | Lymph Node | none | unknown | 50 | unknown | none | no | 11.3 |
CR: Complete Response; SS: Single System; MS: Multisystem; RO: Risk Organ; VBL: Vinblastine; MTX: methotrexate
Five patients in the cohort died, with a 5-year OS of 85.5% (65.8 – 94.3; Figure 3). Patients treated for non-bone lesions (3-year OS 63.6%, 95% CI 29.7 – 84.5) had lower OS than those treated for bone lesions (p = 0.006). All five patients who died were adults treated for non-bone lesions and had multisystem disease at the time of RT (Table 3). Two patients had risk organ involvement. One patient died of sepsis and multi-organ failure secondary to disease treatment complications. Details on the other four deaths were unavailable.
Table 3.
Characteristics of Patients who Died of Disease
| Age/Gender | Risk Stratum | Lesion Treated by RT | Other Lesions | Prior Treatments | Technique | Dose (Gy) | Fractions | Concurrent Treatment | Time to Death (months) |
|---|---|---|---|---|---|---|---|---|---|
| 23F | MS/RO+ | Pituitary | Liver, Lungs | 2-CDA | conventional | 18 | 10 | none | 114 |
| 67M | MS/RO− | Lymph Node, Nasopharynx | none | none | IMRT | 50.4 | 28 | VBL | 19 |
| 33F | MS/RO− | Thyroid | Skin | none | unknown | unknown | unknown | surgery, imatinib, MTX | 36 |
| 39M | MS/RO+ | Thyroid | Kidney, bone, pituitary, bone marrow, lungs, mediastinum, heart | VBL, MTX, 6-MPIMRT | 25.2 | 14 | surgery | 34 | |
| 61F | MS/RO− | Lymph Node | Skin | none | conventional | 50 | unknown | none | 29 |
MS: Multisystem; RO: risk organ; 2-CDA: cladribine; MTX: methotrexate; 6-MP: 6-mercaptopurine
There were 16 patients who had progressions outside the treatment field of initial radiotherapy, including in 5 of 27 patients treated for bone lesions (Figure 3). Patients who were treated for non-bone lesions had a higher risk of progression (p < 0.0001), and 11 of 12 patients treated for non-bone lesions eventually had disease progression outside of the field of radiotherapy. Multisystem involvement, age over 21, and treatment with adjuvant chemotherapy were each significantly associated with progression outside radiation fields (p < 0.05 for all).
Adverse Events
Grade 1 dysphagia, dry mouth, or mucositis was noted in three patients treated to the head and neck area (mandible, nasopharynx, and thyroid). There were no acute adverse events greater than grade 1 noted. One pediatric patient with a left orbital lesion was noted to have mild chronic asymmetry of the upper eyelids beginning three months after radiation therapy. Another patient with a lesion in the left frontal bone developed grade 3 chronic pain and headache. Of note, this patient was treated with surgical resection that was complicated by surgical site infection followed by adjuvant radiotherapy. No secondary malignancies or post-treatment bone fractures were noted.
Discussion
LCH is a rare disease entity with a wide range of clinical presentations and treatments. Here, we show that outcome after radiotherapy depends on location of the irradiated lesion. Bone lesions were exquisitely radio-responsive, while all treatment failures were observed in non-bone lesions. Skin lesions and brain lesions were particularly difficult to control with radiotherapy.
The 5-year local control rate of 87% in our cohort is similar to those reported in prior studies of LCH, which range from 80–100% (11–15, 20–22). However, it has been unclear in past literature which lesions respond to radiotherapy and which ones fail treatment. Part of this uncertainty is due to the wide variety of doses, fractionation schemes, beam energy, and other treatment parameters that are used to treat the disease. Total doses reported for treatment of bone lesions range from 2 Gy to over 30 Gy (11, 13–15, 20, 22), and therefore it is difficult to determine whether prior treatment failures were due to inherent tumor resistance to radiotherapy or lack of sufficient treatment dose.
Our study, however, is the first to demonstrate that the radiosensitivity of this disease is impacted by the organ involved with LCH. In our cohort, low doses of radiation (median dose 10.8 Gy) were sufficient to locally control bone disease and produce excellent symptom relief, with 24/27 (89%) lesions having complete resolution of symptoms at one year after treatment. Despite being treated with higher doses of radiation, non-bone lesions had a higher rate of local failure and lower clinical response rates. These findings are supported by trends apparent in other available studies. A study of 22 patients by Selch et al. had local control in 35 of 40 (88%) bone lesions and 11/16 (69%) soft tissue lesions, though no direct statistical comparisons were made (13). Another study of 40 patients showed poor response to soft tissue lesions which presented multifocally, including no responses in several patients treated to the liver, spleen, lung, and/or pituitary (23).
The efficacy of radiotherapy to the brain has similarly been called into question, though most studies report primarily on resolution of diabetes insipidus. Greenberger et al. reported treatment to the supra-sellar region in 21 patients, with 4 patients subsequently having complete cessation of need for vasopressin and 4 patients having a reduction in vasopressin requirements (15). However, more recent data has questioned the radiotherapy’s effectiveness in treating diabetes insipidus (11, 23). In a study of 69 patients treated with RT between 1956 and 2009, Kotecha et al. reported that none of eight patients treated to the pituitary had a reduction of vasopressin requirements (11). Data from our cohort support the findings of Kotecha et al., as none of the five patients treated for pituitary mass lesions had a reduction in vasopressin required, and two patients required more vasopressin after treatment. However, in patients whose symptoms are secondary to mass effect, radiotherapy may be more effective; two patients in our study with bilateral hemianopsia had an improvement in symptoms after multi-modality treatment with radiotherapy and either surgery or chemotherapy.
Patients in our study who were treated with electrons for cutaneous LCH had a particularly high rate of failure. Of three patients, two patients had initial response but eventual in-field recurrence and the third patient responded initially but was lost to follow up after six months. Selch et al. similarly noted difficulty controlling skin lesions in the long term, with five local failures of mucocutaneous lesions, including 4 in one patient. Further, two cases in adults treated for cutaneous LCH with electron beam radiotherapy have been described, with both having a “marked but temporary” response (24, 25). In combination with these reports, our study suggests that radiotherapy may be useful to provide a rapid initial response but does not have long-term efficacy in cutaneous LCH. For these patients, higher doses of radiation or consolidation with systemic or topical chemotherapies may be beneficial.
Recent advances in the understanding of this disease’s pathogenesis provide context for its clinical diversity. Pioneering genomic analyses have determined that virtually all LCH lesions have over-activation of the MEK/ERK pathway, including approximately 60% patients with the targetable BRAF V600E mutation (3, 26–29). Furthermore, a mouse model has shown activation of the MEK/ERK pathway in early myeloid dendritic cell precursors is associated with a phenotype similar to high-risk multisystem disease, while mutations in differentiated progenitors caused low-risk or single system disease (30). This has given rise to the hypothesis that disease presentation and severity is dependent on the development stage of the mutated myeloid precursor (2, 3). Molecular differences in pathway activation may also impact clinical presentation: BRAF V600E mutations are less frequent in children with bone lesions and are associated with resistance to chemotherapy and higher risk disease (31). These differences in cell of origin and BRAF V600E mutation status may partially explain the disparities in radiosensitivity observed in our study. Further studies pairing LCH’s genomic profile and response to radiotherapy are warranted.
There were few adverse events noted in our cohort, of which several may be attributable to disease or other concurrent treatments. This may be partially attributable to the widespread availability of conformal techniques in our study period. A study by Willis et al. which included 37 pediatric patients treated with radiotherapy noted 42% of all LCH patients developed late skeletal sequelae, and 2 patients developed secondary malignancies (12). Further, Kotecha et al. noted 33% of children treated to the facial bones developed long-term anatomic malformations as well as jaw hypoplasia in another pediatric patient (11). While adverse events were rare in our cohort, one pediatric patient treated for an orbital LCH lesion was noted to have subtle hypoplasia several years following treatment. This highlights the importance of limiting radiation dose in skeletally immature patients, especially to cosmetically sensitive areas such as the face.
In summary, while radiotherapy remains an effective option for treatment of LCH, the effectiveness of radiotherapy differs by the involved organ. While bone lesions are eradicated and well controlled with low doses of RT, lesions in other organs commonly involved in LCH, such as brain and skin, remain difficult to control with standard LCH low dose RT range. For these locations, higher RT dose and additional or alternative modalities should be considered. Finally, more work to characterize the biology of this highly variable disease may further elucidate the basis for these disparate responses and help guide further therapy.
Summary.
Langerhans cell histiocytosis is a rare histiocytic malignancy associated with a wide range of clinical presentations and outcomes. We explored treatment patterns and outcomes in a cohort of 39 patients with 46 radiation-treated lesions. We found the rate of local failure was significantly higher in patients with non-bone lesions, especially of the skin and brain, compared to excellent local control in bone lesions with relatively low doses of radiation.
Acknowledgments
Funding: This work was supported by the Lymphoma Foundation, the Connecticut Cancer Foundation, the National Cancer Institute (P30 CA008748 to J.Y.), the Conquer Cancer Foundation of ASCO, the Lung Cancer Research Foundation, Radiological Society of North America, and the Clinical and Translational Science Center at Weill Cornell Medical Center and MSKCC (UL1TR00457 to B.H.L.). The funding sources had no involvement in study design, data collection and analysis, writing of the report, or the decision to submit this article for publication.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howarth DM, Gilchrist GS, Mullan BP, et al. Langerhans cell histiocytosis: Diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278–2290. doi: 10.1002/(sici)1097-0142(19990515)85:10<2278::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Zinn DJ, Chakraborty R, Allen CE. Langerhans cell histiocytosis: Emerging insights and clinical implications. Oncology (Williston Park) 2016;30:122–132. 139. [PubMed] [Google Scholar]
- 3.Berres ML, Merad M, Allen CE. Progress in understanding the pathogenesis of langerhans cell histiocytosis: Back to histiocytosis x? Br J Haematol. 2015;169:3–13. doi: 10.1111/bjh.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem langerhans cell histiocytosis. Blood. 2013;121:5006–5014. doi: 10.1182/blood-2012-09-455774. [DOI] [PubMed] [Google Scholar]
- 5.Grois N, Potschger U, Prosch H, et al. Risk factors for diabetes insipidus in langerhans cell histiocytosis. Pediatr Blood Cancer. 2006;46:228–233. doi: 10.1002/pbc.20425. [DOI] [PubMed] [Google Scholar]
- 6.Malpas JS, Norton AJ. Langerhans cell histiocytosis in the adult. Medical and pediatric oncology. 1996;27:540–546. doi: 10.1002/(SICI)1096-911X(199612)27:6<540::AID-MPO6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Islinger RB, Kuklo TR, Owens BD, et al. Langerhans' cell histiocytosis in patients older than 21 years. Clinical orthopaedics and related research. 2000:231–235. doi: 10.1097/00003086-200010000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Gadner H, Grois N, Arico M, et al. A randomized trial of treatment for multisystem langerhans' cell histiocytosis. J Pediatr. 2001;138:728–734. doi: 10.1067/mpd.2001.111331. [DOI] [PubMed] [Google Scholar]
- 9.Gadner H, Grois N, Potschger U, et al. Improved outcome in multisystem langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. doi: 10.1182/blood-2007-08-106211. [DOI] [PubMed] [Google Scholar]
- 10.Gadner H, Heitger A, Grois N, et al. Treatment strategy for disseminated langerhans cell histiocytosis. Dal hx-83 study group. Medical and pediatric oncology. 1994;23:72–80. doi: 10.1002/mpo.2950230203. [DOI] [PubMed] [Google Scholar]
- 11.Kotecha R, Venkatramani R, Jubran RF, et al. Clinical outcomes of radiation therapy in the management of langerhans cell histiocytosis. American journal of clinical oncology. 2014;37:592–596. doi: 10.1097/COC.0b013e318281d6ce. [DOI] [PubMed] [Google Scholar]
- 12.Willis B, Ablin A, Weinberg V, et al. Disease course and late sequelae of langerhans' cell histiocytosis: 25-year experience at the university of california, san francisco. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14:2073–2082. doi: 10.1200/JCO.1996.14.7.2073. [DOI] [PubMed] [Google Scholar]
- 13.Selch MT, Parker RG. Radiation therapy in the management of langerhans cell histiocytosis. Medical and pediatric oncology. 1990;18:97–102. doi: 10.1002/mpo.2950180203. [DOI] [PubMed] [Google Scholar]
- 14.Olschewski T, Seegenschmiedt MH. Radiotherapy of langerhans' cell histiocytosis : Results and implications of a national patterns-of-care study. Strahlenther Onkol. 2006;182:629–634. doi: 10.1007/s00066-006-1630-9. [DOI] [PubMed] [Google Scholar]
- 15.Greenberger JS, Cassady JR, Jaffe N, et al. Radiation therapy in patients with histiocytosis: Management of diabetes insipidus and bone lesions. International journal of radiation oncology, biology, physics. 1979;5:1749–1755. doi: 10.1016/0360-3016(79)90556-x. [DOI] [PubMed] [Google Scholar]
- 16.Abla O, Egeler RM, Weitzman S. Langerhans cell histiocytosis: Current concepts and treatments. Cancer Treat Rev. 2010;36:354–359. doi: 10.1016/j.ctrv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (lch): Guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175–184. doi: 10.1002/pbc.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with langerhans cell histiocytosis: Recommendations from an expert panel on behalf of euro-histio-net. Orphanet journal of rare diseases. 2013;8:72. doi: 10.1186/1750-1172-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute NC. Nci, nih, dhhs. May 29, 2009. Common terminology criteria for adverse events v4.0. Nih publication # 09–7473. [Google Scholar]
- 20.el-Sayed S, Brewin TB. Histiocytosis x: Does radiotherapy still have a role? Clinical oncology (Royal College of Radiologists (Great Britain)) 1992;4:27–31. doi: 10.1016/s0936-6555(05)80770-8. [DOI] [PubMed] [Google Scholar]
- 21.Jahraus CD, Russo S, Penagaricano J, et al. Radiotherapy dose fractionation in pediatric langerhans cell histiocytosis. South Med J. 2004;97:1268–1269. doi: 10.1097/01.SMJ.0000146548.89694.8A. [DOI] [PubMed] [Google Scholar]
- 22.Kriz J, Eich HT, Bruns F, et al. Radiotherapy in langerhans cell histiocytosis - a rare indication in a rare disease. Radiat Oncol. 2013;8:233. doi: 10.1186/1748-717X-8-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gramatovici R, D'Angio GJ. Radiation therapy in soft-tissue lesions in histiocytosis x (langerhans' cell histiocytosis) Medical and pediatric oncology. 1988;16:259–262. doi: 10.1002/mpo.2950160407. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenwald DJ, Jakubovic HR, Rosenthal D. Primary cutaneous langerhans cell histiocytosis in an adult. Archives of dermatology. 1991;127:1545–1548. [PubMed] [Google Scholar]
- 25.Moravvej H, Yousefi M, Barikbin B. An unusual case of adult disseminated cutaneous langerhans cell histiocytosis. Dermatol Online J. 2006;12:13. [PubMed] [Google Scholar]
- 26.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent braf mutations in langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berres ML, Allen CE, Merad M. Pathological consequence of misguided dendritic cell differentiation in histiocytic diseases. Adv Immunol. 2013;120:127–161. doi: 10.1016/B978-0-12-417028-5.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond EL, Durham BH, Haroche J, et al. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer discovery. 2016;6:154–165. doi: 10.1158/2159-8290.CD-15-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with braf v600 mutations. The New England journal of medicine. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berres ML, Lim KP, Peters T, et al. Braf-v600e expression in precursor versus differentiated dendritic cells defines clinically distinct lch risk groups. J Exp Med. 2014;211:669–683. doi: 10.1084/jem.20130977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heritier S, Emile JF, Barkaoui MA, et al. Braf mutation correlates with high-risk langerhans cell histiocytosis and increased resistance to first-line therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34:3023–3030. doi: 10.1200/JCO.2015.65.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]