Synopsis
The concept of cognitive reserve (CR) was proposed to account for the discrepancy between levels of brain pathology or damage and clinical and cognitive function. This article provides a detailed review of prospective longitudinal studies that have investigated the interaction between CR and Alzheimer’s disease (AD) biomarkers on clinical and cognitive outcomes among individuals with preclinical AD. Current evidence is consistent with the view that higher levels of CR are associated with a delay in the onset of symptoms of mild cognitive impairment and that there may be multiple pathways by which CR exerts its protective effects.
Keywords: Cognitive reserve, Alzheimer’s disease, mild cognitive impairment, biomarkers, amyloid, tau, atrophy
Overview: The Concept of Cognitive Reserve
The aging of the population, which is accompanied by an increasing prevalence of Alzheimer’s disease (AD), makes it imperative to identify factors that reduce risk of onset of dementia. Cognitive reserve is increasingly being studied as one potential mechanism for reducing the risk of cognitive decline and dementia among older adults. The concept of cognitive reserve grew out of observations that there can be a marked discrepancy between an individual’s clinical symptomatology and estimates of the amount of neuropathology in the brain For example, an early study by Stern et al. (1992) that began investigating this issue reported that among individuals with probable AD, and matched for clinical severity, those with more years of education had more advanced pathology, as indicated by less cerebral blood flow in AD-vulnerable regions1.
It has been proposed that lifetime experiences that are associated with cognitive stimulation (such as years of education, occupational attainment, and engagement in mentally stimulating leisure activities) modify the brain in a way that allows individuals to tolerate greater levels of neuropathology or injury before showing symptoms of functional decline2. Although the concept of CR has primarily been studied within the context of Alzheimer’s disease, it is hypothesized to apply to any brain disease or condition that results in brain damage, and an increasing number of studies support this proposal3–5. It has also been proposed that CR moderates the relationship between brain changes and age-related cognitive decline2,6.
In this review, we first briefly summarize the major lines of evidence in support of the concept of CR within the context of AD. We then provide a detailed review of longitudinal biomarker studies that have examined the relationship between measures of cognitive reserve, AD pathology, and subsequent cognitive change or impairment among individuals who were cognitively normal when first evaluated. We have focused on studies of individuals with normal cognition at baseline because it is now recognized that AD pathology begins to develop when individuals are cognitively normal, a phase of the disease commonly referred to as preclinical AD7. As such, these types of studies provide insight into how and to what extent cognitive reserve delays the onset of the symptomatic phase of the disease, which has major public health implications; it has been estimated that interventions that delay the onset of dementia by 5 years would reduce the prevalence of dementia by 50%8.
Evidence in Support of Cognitive Reserve
Supporting the concept of cognitive reserve (CR), many large prospective epidemiological studies of initially non-demented individuals have shown that more years of education9, greater occupational breadth and complexity9,10, and greater lifetime engagement in cognitively stimulating activities11 are associated with a reduced risk of dementia. The evidence regarding the relationship between measures of CR and rates of change in cognition is more mixed, with many recent studies reporting little or no association between CR and rates of cognitive decline, despite evidence that individuals with higher CR have a higher performance on cognitive tests12. It has been suggested that the differences in findings among these studies likely reflect methodological and cohort differences and, taken together, the evidence indicates that CR primarily influences baseline levels of cognitive performance.12,13 Thus, epidemiological studies strongly support the notion that higher levels of CR are associated with better cognitive performance, as well as a reduced risk of developing dementia later in life, while the impact of CR on the trajectory of cognitive decline is less clear. Epidemiological research on CR, however, has generally been limited by a lack of measures of underlying AD pathology. As such, these types of studies cannot directly examine whether and how measures of CR affect the association between levels of neuropathology and cognitive performance.
Thus, studies that have incorporated biomarkers, which are considered an indirect reflection of underlying neuropathology, are of particular importance in clarifying the mechanisms by which CR may be protective. The majority of studies on CR with biomarker measures of AD pathology have been cross-sectional in nature. A common finding of cross-sectional studies is that at similar levels of cognitive functioning, individuals with higher CR tend to have biomarker measures reflecting higher levels AD pathology in the brain. For example, atrophy measures based on magnetic resonance imaging (MRI)14–16 and levels of amyloid and tau, derived from PET imaging17,18, or measured in cerebrospinal fluid (CSF),19 tend to be more abnormal among individuals with higher CR. These findings suggest that the effects of AD pathology on cognition are reduced in individuals with higher reserve. Some cross-sectional studies also suggest that the effects of aging on brain structure, function, and AD pathology may be reduced among individuals with higher CR20–22. An important limitation of cross-sectional studies, however, is that they cannot test whether measures of CR do in fact alter future cognitive trajectories or the risk of cognitive impairment.
For this reason, prospective longitudinal studies that collect both AD biomarkers and cognitive and clinical data are essential for testing the extent to which CR is associated with reduced age-related cognitive decline or a reduced risk of cognitive impairment in the presence of AD pathology. The same factors that have been associated with CR (such as educational and occupational attainment) may also minimize the accumulation of pathology, a concept known as brain maintenance23 that has been proposed as a complimentary mechanism to CR. Thus, longitudinal studies addressing CR may also be relevant to the concept of brain maintenance.
Longitudinal AD Biomarker Studies of Cognitive Reserve Among Individuals with Normal Cognition When First Evaluated
The number of prospective longitudinal studies that have investigated the relationship between measures of CR, AD biomarkers, and longitudinal cognitive or clinical outcomes among individuals who were cognitively normal at baseline is relatively limited (see Table 1). These studies have examined three major themes: (1) the association between baseline measures of CR and baseline AD biomarker levels in relation to the time to progress to cognitive impairment24–28, (2) the association between baseline measures of CR and baseline AD biomarker levels in relation to the rate of change in cognition13,29, and (3) the association between baseline measures of CR and the rate of change in AD biomarkers over time25,26,30,31.
Table 1.
Longitudinal studies of the association between CR and AD biomarkers among individuals with normal cognition at baseline
| Study | Outcome Variable(s) |
AD-Biomarkers | CR measures | Mean clinical follow-up time in years (SD) |
Number of cognitively normal subjects at baseline |
Baseline CR- biomarker association |
CR associated with delayed clinical progression/ better cognitive performance accounting for baseline biomarker levels |
Longitudinal CR- biomarker association |
Relationship between biomarker and clinical/cognitive outcome modified by CR |
|---|---|---|---|---|---|---|---|---|---|
| Soldan, et al. (submitted) | Change in cognitive composite z-score | Composite z-score (CSF Aβ1–42, p-tau, entorhinal cortex thickness, hippocampal volume, cortical thickness in ADvulnerable regions) | Composite score (education, NART-IQ, WAIS-R vocabulary) | 12.1 (4.2) max=20 | 303 with clinical/cognitive data; 170 with baseline biomarker data | No | Yes, better baseline cognitive performance and faster decline after MCI symptom onset | – | No. |
| Pettigrew et al. (2017) | Time to onset of clinical symptom of MCI | Cortical thickness in AD vulnerable regions | Composite score (education, NART-IQ, WAIS-R vocabulary) | 11.8 (3.6) max=20 | 232 48 progressed |
No | Yes Delayed clinical progression | – | Yes, for those who progressed 7+ years after baseline only. |
| Soldan et al. (2015) | Time to onset of clinical symptom of MCI; Change in AD biomarkers | Volumes of hippocampus, entorhinal cortex, amygdala; entorhinal cortex thickness | Composite score (education, NART-IQ, WAIS-R vocabulary) | 11.1 (3.6), max=18 | 245 57 progressed |
No | Yes Delayed clinical progression | No | Yes, for left entorhinal cortex volume only. |
| Vemuri et al. (2015) | Change in cognitive composite z-score | Cortical PiB-PET (dichotomous); white matter hyperintensity volume, brain infarcts on FLAIR-MRI (dichotomous) | Education/occup ation score and self reported mid/late-life cognitive activity score | 2.7 | 393 | No | Yes, better baseline cognitive performance, but no difference in slope | – | No. |
| Soldan et al. (2013) | Time to onset of clinical symptom of MCI; Change in AD biomarkers | CSF Aβ1–42, t-tau, p-tau | Composite score (education, NART-IQ, WAIS-R vocabulary) | 8.0 (3.4), max=17 | 239 53 progressed |
No | Yes Delayed clinical progression | No | Yes, for CSF t-tau and p-tau. |
| Suo et al. (2012) | Change in hippocampal volume | Hippocampal volume, whole-brain volume (VBM) | Lifetime Experiences Questionnaire (LEQ) | 2–3 max = 3 | 151 | Yes, midlife LEQ/occupational complexity and bilateral hippocampus, and left amygdala | – | Yes, high supervisory experiences associated with less hippocampal atrophy (N=91) |
– |
| Lo & Jagust (2013) | Change AD biomarkers | CSF Aβ1-42, t-tau, p-tau, FDG-PET metabolism in 5 AD-vulnerable regions, hippocampal volume | Education (tertiles), occupation (3 levels), NART errors (tertiles) | 2–3, max = 3 | 229; 35 (CSF) 103 (FDG) 228 (HCV) |
No | – | Yes, higher CR associated with less decline in CSF Aβ1–42. | – |
| Roe et al. (2011a) | Time to CDR>=0.5; Change in CDR-SB, Short Blessed Test, MMSE | CSF Aβ1-42, t-tau, p-tau | Education | 3.3 (2.0) | 197 26 progressed |
– | Yes, delayed clinical progression after accounting for Aβ1-42, but not sig. among those with low tau/p-tau; Sig. among those with high tau/p-tau | – | Yes, among those with high tau or p-tau and low education, WBV was associated with faster progression. In low tau/p-tau group, neither education nor WBV associated with progression. Similar results obtained for CDR-SB and Blessed Test, but not for MMSE. |
| Roe et al. (2011b) | Time to CDR >=0.5 | CSF Aβ1-42, t-tau, p-tau | Education, occupational attainment (6 levels) | 3.2 (1.6) | 213 14 progressed |
– | Yes Delayed Clinical Progression | – | – |
Cognitive Reserve, AD Biomarkers and Risk of Cognitive Impairment
An important question that has been addressed by studies examining the first question - the combined effects of CR and AD biomarkers on the risk of progression to cognitive impairment - is whether CR and AD biomarkers are independent predictors of risk or whether they interact to alter future risk of progression. The presence of such an interaction is very important because it would indicate that measures of CR modify the association between the biomarker in question and risk of progression, or that the protective effects of CR on the risk of progression differ for individuals with high vs. low levels of the biomarker.
Two studies24,26 addressed this question by testing whether the association between structural MRI measures of brain atrophy and the time to symptom onset of MCI is modified by CR, as quantified by a composite measure of CR (i.e., a composite z-score composed of years of education, and measures of vocabulary and reading ability). Soldan et al. (2015) found that the baseline volumes of three medial-temporal lobe structures (hippocampus, entorhinal cortex and amygdala), and the rate of change in these structures over time, were associated with the time to progress from normal cognition to symptom onset of MCI, independently of the baseline CR composite score, which was associated with a reduced risk of progression (i.e., delayed symptom onset)26. Only one structure, the left entorhinal cortex volume, interacted with CR, such that smaller baseline volumes were associated with faster time to clinical symptom onset in individuals with low CR, but not in individuals with high CR. Similar results were reported by Pettigrew et al. (2017) who found that both CR and mean cortical thickness in ‘AD vulnerable regions’ were independently associated with risk of progression from normal cognition to MCI within 7 years of baseline24. In contrast there was an interaction between baseline CR score and cortical thickness for risk of progression more than 7 years form baseline, reflecting a stronger association between low cortical thickness and risk of symptom onset among individuals with lower CR. Additionally, Pettigrew reported that the reduction in the risk of progression associated with higher CR was greater for progression after 7 years from baseline than for progression within 7 years, suggesting that the protective effect of CR decreases as AD pathology levels increase. Taken together, the results from these two studies suggest that MRI measures of atrophy in brain regions commonly affected by AD and measures of CR have relatively independent and additive effects on the risk of progression to MCI. However, these studies also provided some evidence for interactions between CR and atrophy in some brain regions, suggesting a stronger association between atrophy and risk among individuals with lower CR than higher CR.
Three other studies addressed this same question by investigating the relationship between measures of CR and CSF measures of amyloid beta (abeta), total tau (t-tau), and phosphorylated tau (p-tau) in relationship to the risk of progression to cognitive impairment25,27,28 For the findings regarding the relationship between CR and CSF abeta, two of these studies reported that CR and CSF abeta measures predicted time to progress from normal cognition to MCI, but that there was no interaction between baseline levels of CSF abeta and CR (as measured by years of education27 or a composite score25). Similarly, the third study reported that fewer years of education and lower (i.e., more abnormal) CSF abeta levels were significantly associated with a faster time to onset of cognitive impairment, however, the interaction between the two measures was not examined28. Taken together, these findings suggest that the protective effects of CR on the risk of progression are equivalent across the observed range of CSF abeta levels and that CR and abeta have additive and independent effects on the risk of progression. This is noteworthy because CSF abeta is widely accepted as a biomarker for amyloid plaques, one of the primary pathological hallmarks of AD.
The findings regarding the relationship between CR and CSF p-tau and t-tau suggest that there may be an interaction between CR and degree of neuronal injury, as measured by these biomarkers. Soldan et al. (2013) found an interaction between the baseline CR composite score and both t-tau and p-tau in relationship to the time to onset of symptoms of MCI25. Among participants with higher baseline levels of t-tau or p-tau, the degree to which CR modified the risk of symptom onset was less than that in participants with lower levels of t-tau and p-tau, though higher CR was still associated with a delay in symptom onset in both the low and high t-tau or p-tau groups. This suggests that as levels of neuronal injury increase in the brain, the protective effects of CR decrease, consistent with the findings by Pettigrew et al. (2017) using MRI measures of neuronal injury24. This may occur because CR is unable to compensate for increasing levels of neuronal injury, or because the neural mechanisms that underlie CR break down with increasing levels of neuronal injury. The results by Soldan et al. (2013)25 also indicated that CSF t-tau and p-tau levels were more strongly associated with the risk of progression among individuals with higher CR than lower CR. This was due to the fact that individuals with lower CR were at significantly elevated risk of progressing (because of their low CR), even when t-tau/p-tau levels were low, and thus higher t-tau/p-tau levels were associated with less additional risk. By comparison, those with higher CR, whose overall risk of developing cognitive impairment is much lower, elevated tau/p-tau levels were more predictive of progression.
The findings by Roe et al. (2011)27 were somewhat different, as they reported a threeway interaction between CR (as measured by years of education), t-tau/p-tau levels, and whole brain volume in relationship to the time to cognitive impairment. Among individuals with low t-tau or p-tau levels, there was no association between years of education and risk of progression; whereas among individuals with high t-tau or p-tau levels, more education was associated with a delayed time to incident cognitive impairment, particularly among those with lower brain volumes. The two-way interaction between t-tau/p-tau levels and education (collapsed across whole brain volume) was not reported. The absence of an association between education and risk of progression among those with low t-tau or p-tau levels may reflect the somewhat smaller sample size and smaller number of individuals who became symptomatic over the course of the study (which reduces statistical power) and the relatively short follow-up duration of 3 years (compared to 8 years in Soldan et al., 2013). Additionally, years of education alone tends to be less predictive of future cognitive impairment than composite CR measures that incorporate measures of literacy or vocabulary in addition to education24,32,33. The second study by Roe et al. (2011)28 reported that both years of education and baseline tau or p-tau levels were predictive of incident cognitive impairment in the same model, although their possible interaction was not examined. Overall, the results of studies that have investigated the combined effects of CR and CSF AD biomarkers in relation to the risk of progression to MCI indicate that even after accounting for levels of these biomarkers at baseline, higher CR is associated with a reduced risk of symptom onset of MCI. While the effects of abeta and CR on the time to symptom onset appear to be independent of one another, there is some evidence that the protective effects of CR are modified by CSF t-tau and p-tau levels.
Cognitive Reserve, AD Biomarker and Rate of Cognitive Decline
Only two longitudinal studies have examined the second question mentioned above - the rate of change in neuropsychological measures of cognition in relationship to CR and AD biomarkers among individuals with normal cognition at baseline13,29. In both studies, cognitive performance was quantified with a composite z-score composed of measures from multiple cognitive domains. Vemuri et al. (2015)29 operationalized CR in two ways, with one score reflecting educational and occupational attainment and the other indexing mid and late-life cognitive leisure activities. The results showed that higher scores on the measure of educational and occupational attainment were associated with higher cognitive scores, independent of the amount of amyloid, as measured by PiB-PET imaging, and independent of cerebrovascular disease, as measured by white matter hyperintensities and brain infarcts on FLAIR-MRI. Importantly, there was no interaction between the measures of CR and the MRI measures, suggesting similar rates of change in cognition over the follow-up period among those with higher and lower CR scores (mean follow-up 2.7 years). Consistent with these findings, Soldan et al. (under review)13 also reported that, independent of AD biomarker levels, higher CR (as indexed by a composite score) was associated with better cognitive performance but did not alter the rates of cognitive change while individuals were asymptomatic (mean follow-up = 11 years). In this study, AD pathology was quantified by a composite score combining several major biomarker types (CSF abeta and p-tau, as well as MRI measures of the hippocampus, entorhinal cortex, and AD-vulnerable cortical regions).
Due to the long follow-up period in this latter study, and the fact that a substantial number of participants had developed cognitive impairment on follow-up (n=66), Soldan et al. (submitted) also examined rates of change in cognition after the onset of symptoms of MCI. In line with theoretical predictions2, individuals with higher CR showed faster rates of cognitive decline than those with lower CR after they became symptomatic13. Additionally, the mean age of onset of symptoms of MCI was strongly associated with the baseline CR score: subjects with CR scores above the median had a mean age of symptom onset that was approximately 7 years later than for those with CR scores below the median of the group13. It is important to note that the subjects in this study were highly educated (mean of 17 years of education), so this study may underestimate the degree to which individual differences in CR may delay the symptomatic phase of AD. Taken together, the results from studies investigating the combined effects of CR and AD biomarkers on cognitive change and time to symptom onset suggest that after accounting for baseline pathology levels, CR does not alter cognitive trajectories prior to symptom onset, but it does significantly delay the onset of symptoms by several years.
Cognitive Reserve and Rate of Change in AD Biomarkers
Currently, there is weak evidence for the proposal that measures of CR are directly associated with the rate of change in AD biomarkers among individuals who were cognitively normal at baseline – the third question mentioned above. This is largely because the available data is limited by relatively short follow-up periods (2–4 years of longitudinal biomarker data, on average). Lo and Jagust (2013)30 reported that among a group of 35 cognitively normal individuals, higher scores on CR proxy variables (i.e., measures of education, occupation, and reading/vocabulary) were associated with less longitudinal decline in CSF abeta, but not with change in MRI hippocampal volume or FDG PET metabolism. Suo et al. (2012)31 found that high self-reported supervisory experience in midlife (a measure assumed to reflect occupational complexity) was associated with less hippocampal atrophy over time in a sample of 91 older adults. However, self-reported general cognitive activities in early, mid, or late life did not modulate rates of brain atrophy. In two studies with larger samples (N=239 and N=245), there was no relationship between a baseline CR composite and rates of change in CSF abeta, t-tau, and p-tau25, or MRI measures of the hippocampus, amygdala, or entorhinal cortex26. Studies with large samples and more longitudinal biomarker data will be needed to determine to what degree CR alters the trajectories of AD biomarkers and other aspects of brain health.
Pathways Linking Cognitive Reserve to Cognitive and Clinical Outcomes
Despite the strong evidence that proxy measures of CR are associated with delayed clinical symptom onset, the mechanism(s) underlying these effects remain poorly understood. Figure 1 illustrates four possible pathways by which CR may alter longitudinal cognitive and clinical outcomes. (1) First, CR may reduce the risk of MCI or dementia via mechanisms that are independent of the level of specific AD-related pathologic brain changes. For example, current evidence suggests that measures of CR and levels of brain amyloid independently predict the time to symptom onset25,27. (2) Second, CR may interact with markers of pathology or brain health to influence future cognitive decline or risk of progression. For instance, smaller volumes or thickness in some AD vulnerable brain regions appear to be a stronger risk factor for developing cognitive impairment among individuals with low CR than those with higher CR24,26. Also, the protective effects of CR on clinical outcomes appear to diminish as levels of neuronal injury increase25, suggesting that the neural mechanisms of CR become overwhelmed by pathology. (3) A third pathway by which CR may influence future cognitive and clinical outcomes is by delaying the onset of age-related or AD-related brain changes, or reducing the rate of AD pathology accumulation. Although current evidence for this pathway is limited, future studies with longer follow-up periods will be able to investigate this pathway. For example, recent evidence suggests that midlife vascular risk factors, including obesity, high cholesterol, hypertension, and smoking are associated with late-life amyloid accumulation34. To the extent that these midlife vascular risk factors are associated with CR proxy measures, such as educational or occupational attainment35–37, CR may influence the accumulation of AD pathology indirectly via health-related behaviors in early and mid-life. (4) A fourth pathway that has been proposed is that CR alters the association between genetic factors or aging on clinical and cognitive outcomes. Older age is the greatest risk factor for AD and both amyloid and tau pathology increase with age. Preliminary evidence from cross-sectional studies suggests that the association between age and AD pathology levels20 or age-related structural brain changes21,22 may be attenuated among individual with higher CR.
Figure 1.
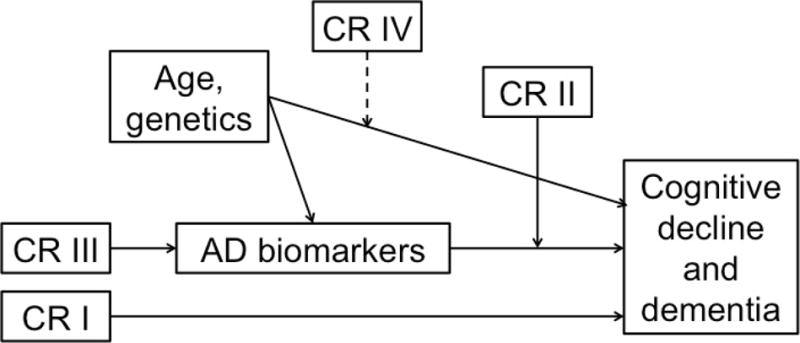
Illustration of four possible pathways by which cognitive reserve (CR) may influence rates of cognitive decline and risk of dementia in later life. Here, CR refers to proxy measures such as educational or occupational attainment, as well as their neural implementation(s), which are not well understood.
I) CR is linked to outcomes in a way that is unrelated to biomarker levels.
II) CR moderates the relationship between biomarkers and outcomes.
III) CR has a direct effect on biomarker levels (i.e., onset or rate of accumulation).
IV) CR modifies* the relationship between age/genetics and outcomes.
*dashed line indicates that although illustrated as a moderation effect, it could in fact be a mediation effect (or the relationship may depend on a specific demographic factor, gene, etc.)
An important limitation of current longitudinal studies is that they have not yet fully explored the neural mechanisms of CR. Evidence from cross-sectional suggests that CR may be implemented in the brain in the form of greater neural efficiency and speed38–40, neural capacity, neural compensation39,41, and greater functional connectivity42. Longitudinal studies will be necessary to test whether these putative mechanisms of CR are associated with better clinical outcomes, in the same way as proxy measures of CR. If so, it might be possible to devise interventions that specifically target these neural mechanisms, thereby increasing reserve and resilience of the brain.
Cognitive Reserve and Public Health: Practical Implications
The study of CR and its neural implementation has important implications for public health. To the extent that higher CR protects against the clinical manifestations of AD by delaying the onset of the symptomatic phase of the disease, it provides an important mechanism for preserving cognitive function in old age, even while brain pathology levels are rising. Current evidence suggests that higher CR is associated with approximately a 50% reduction in the risk of symptom onset of MCI24–26,33 and may delay the onset of symptoms by several years13. As such, CR provides far greater potential benefits to individuals than any drug that is currently on the market for treating the symptoms of MCI or dementia. Moreover, by delaying the onset of the symptomatic phase of AD, CR allows older individuals to maximize daily functioning and minimize reliance on caregivers. Caring for someone with dementia is associated with enormous stress, financial strain, and negative health outcomes. Therefore, the goal of any intervention for AD should be to prolong the time that older adults are able to live independently and be active and engaged members of their family and community. Moreover, the current findings regarding CR suggest that health policies aimed at improving educational and occupational opportunities for individuals may have far-reaching consequences for future rates of cognitive decline and dementia.
Key Points.
Evidence indicates that higher levels of cognitive reserve (CR) (as measured by proxy variables like educational and occupational attainment) delay the onset of symptoms of mild cognitive impairment due to Alzheimer’s disease (AD).
Recent findings suggest that the protective effects of CR may be independent of amyloid pathology, but interact with measures of neuronal injury to alter risk of cognitive impairment.
It is unclear whether CR alters future risk of cognitive decline by directly affecting brain pathology.
Prospective longitudinal biomarker studies are needed to investigate the mechanisms by which CR alters future risk of cognitive decline.
Acknowledgments
The preparation of this manuscript was supported in part by grants from the National Institute on Aging: U19-AG03365, P50-AG005146.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Marilyn Albert is a consultant to Eli Lilly and has funding from Avid Radiopharmaceuticals. Anja Soldan and Corinne Pettigrew have nothing to disclose.
Contributor Information
Anja Soldan, Assistant Professor, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA, 1620 McElderry Street, Reed Hall West - 1, Baltimore, MD 21205
Corinne Pettigrew, Research Associate, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA
Marilyn Albert, Professor, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA
References
- 1.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of neurology. 1992 Sep;32(3):371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 2.Stern Y. Cognitive reserve. Neuropsychologia. 2009 Aug;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perneczky R, Drzezga A, Boecker H, et al. Activities of daily living, cerebral glucose metabolism, and cognitive reserve in Lewy body and Parkinson’s disease. Dementia and geriatric cognitive disorders. 2008;26(5):475–481. doi: 10.1159/000167791. [DOI] [PubMed] [Google Scholar]
- 4.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014 May 20;82(20):1776–1783. doi: 10.1212/WNL.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathias JL, Wheaton P. Contribution of brain or biological reserve and cognitive or neural reserve to outcome after TBI: A meta-analysis (prior to 2015) Neuroscience and biobehavioral reviews. 2015 Aug;55:573–593. doi: 10.1016/j.neubiorev.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in cognitive sciences. 2013 Oct;17(10):502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American journal of public health. 1998 Sep;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994 Apr 06;271(13):1004–1010. [PubMed] [Google Scholar]
- 10.Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. (Series B, Psychological sciences and social sciences).The journals of gerontology. 2005 Sep;60(5):P251–258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Jama. 2002 Feb 13;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 12.Zahodne LB, Glymour MM, Sparks C, et al. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. Journal of the International Neuropsychological Society: JINS. 2011 Nov;17(6):1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soldan A, Pettigrew C, Cai Q, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. doi: 10.1016/j.neurobiolaging.2017.09.002. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Julkunen V, Paajanen T, et al. Education increases reserve against Alzheimer’s disease-evidence from structural MRI analysis. Neuroradiology. 2012 Sep;54(9):929–938. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querbes O, Aubry F, Pariente J, et al. Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain: a journal of neurology. 2009 Aug;132(Pt 8):2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sole-Padulles C, Bartres-Faz D, Junque C, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiology of aging. 2009 Jul;30(7):1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Rentz DM, Mormino EC, Papp KV, Betensky RA, Sperling RA, Johnson KA. Cognitive resilience in clinical and preclinical Alzheimer’s disease: the Association of Amyloid and Tau Burden on cognitive performance. Brain imaging and behavior. 2017 Apr;11(2):383–390. doi: 10.1007/s11682-016-9640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of neurology. 2008 Nov;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumurgier J, Paquet C, Benisty S, et al. Inverse association between CSF Abeta 42 levels and years of education in mild form of Alzheimer’s disease: the cognitive reserve theory. Neurobiology of disease. 2010 Nov;40(2):456–459. doi: 10.1016/j.nbd.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Almeida RP, Schultz SA, Austin BP, et al. Effect of Cognitive Reserve on Age-Related Changes in Cerebrospinal Fluid Biomarkers of Alzheimer Disease. JAMA neurology. 2015 Jun;72(6):699–706. doi: 10.1001/jamaneurol.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiology of aging. 2016 Apr;40:138–144. doi: 10.1016/j.neurobiolaging.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habeck C, Razlighi Q, Gazes Y, Barulli D, Steffener J, Stern Y. Cognitive Reserve and Brain Maintenance: Orthogonal Concepts in Theory and Practice. Cereb Cortex. 2016 Jul 11; doi: 10.1093/cercor/bhw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends in cognitive sciences. 2012 May;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Pettigrew C, Soldan A, Zhu Y, et al. Cognitive reserve and cortical thickness in preclinical Alzheimer’s disease. Brain imaging and behavior. 2017 Apr;11(2):357–367. doi: 10.1007/s11682-016-9581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldan A, Pettigrew C, Li S, et al. Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiology of aging. 2013 Dec;34(12):2827–2834. doi: 10.1016/j.neurobiolaging.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soldan A, Pettigrew C, Lu Y, et al. Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Human brain mapping. 2015 Jul;36(7):2826–2841. doi: 10.1002/hbm.22810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roe CM, Fagan AM, Grant EA, et al. Cerebrospinal fluid biomarkers, education, brain volume, and future cognition. Archives of neurology. 2011 Sep;68(9):1145–1151. doi: 10.1001/archneurol.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roe CM, Fagan AM, Williams MM, et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology. 2011 Feb 8;76(6):501–510. doi: 10.1212/WNL.0b013e31820af900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain: a journal of neurology. 2015 Mar;138(Pt 3):761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo RY, Jagust WJ. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer disease and associated disorders. 2013 Oct-Dec;27(4):343–350. doi: 10.1097/WAD.0b013e3182900b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suo C, Leon I, Brodaty H, et al. Supervisory experience at work is linked to low rate of hippocampal atrophy in late life. NeuroImage. 2012 Nov 15;63(3):1542–1551. doi: 10.1016/j.neuroimage.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Manly JJ, Schupf N, Tang MX, Stern Y. Cognitive decline and literacy among ethnically diverse elders. Journal of geriatric psychiatry and neurology. 2005 Dec;18(4):213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]
- 33.Pettigrew C, Soldan A, Li S, et al. Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cognitive neuroscience. 2013;4(3–4):136–142. doi: 10.1080/17588928.2013.831820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottesman RF, Schneider AL, Zhou Y, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. Jama. 2017 Apr 11;317(14):1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JP. The impact of socioeconomic status on health over the life-course. The Journal of Human Resources. 2007;42(4):739–764. [Google Scholar]
- 36.Devaux M, Sassi F, Church J, Cecchini M, Borgonovi F. Exploring the relationship between education and obesity. OECD Journal: Economic Studies. 2011;2011(1) [Google Scholar]
- 37.Non AL, Gravlee CC, Mulligan CJ. Education, genetic ancestry, and blood pressure in African Americans and Whites. American journal of public health. 2012 Aug;102(8):1559–1565. doi: 10.2105/AJPH.2011.300448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speer ME, Soldan A. Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiology of aging. 2015 Mar;36(3):1424–1434. doi: 10.1016/j.neurobiolaging.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steffener J, Reuben A, Rakitin BC, Stern Y. Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain imaging and behavior. 2011 Sep;5(3):212–221. doi: 10.1007/s11682-011-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habeck C, Hilton HJ, Zarahn E, Flynn J, Moeller J, Stern Y. Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of nonverbal memory. NeuroImage. 2003 Nov;20(3):1723–1733. doi: 10.1016/j.neuroimage.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 41.Stern Y, Zarahn E, Habeck C, et al. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008 Apr;18(4):959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arenaza-Urquijo EM, Landeau B, La Joie R, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. NeuroImage. 2013 Dec;83:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]


