Abstract
Objective
To assess appropriate pantoprazole dosing for obese children, we conducted a prospective pharmacokinetics (PK) investigation of pantoprazole in obese children, a patient population that is traditionally excluded from clinical trials.
Study design
A total of 41 obese children (6–17 years of age), genotyped for CYP2C19 variants *2, *3, *4, and *17, received a single oral dose of pantoprazole, ~1.2 mg/kg lean body weight (LBW), with LBW calculated via a validated formula. Ten post-dose pantoprazole plasma concentrations were measured, and PK variables generated via noncompartmental methods (WinNonlin). Linear and nonlinear regression analyses and analyses of variance were used to explore obesity, age, and CYP2C19 genotype contribution to pantoprazole PK. PK variables of interest were compared with historic nonobese peers treated with pantoprazole.
Results
Independent of genotype, when normalized to dose per kg total body weight, pantoprazole apparent clearance and apparent volume of distribution were significantly lower (P < .05) and systemic exposure significantly higher (P < .01) in obese vs nonobese children. When normalized per kg LBW, these differences were not evident in children ≥12 years of age and markedly reduced in children <12 years of age.
Conclusions
LBW dosing of pantoprazole led to pantoprazole PK similar to nonobese peers. Additional factors, other than body size (eg, age-related changes in CYP2C19 activity), appear to affect pantoprazole PK in children <12 years of age.
Childhood obesity has reached epidemic proportions.1 Currently, 1 in 6 children in the US meets body mass index (BMI) criteria for obesity (BMI ≥95% for age).1,2 The pediatric obesity epidemic brings a variety of comorbidities traditionally attributed to adult patients (eg, hypertension, type II diabetes mellitus).3,4Many of these obesity-related comorbidities represent life-long medical conditions that require pharmacotherapy; yet, limited guidelines exist for the appropriate dose-selection of medications in obese children.5,6 An example of this challenge occurs with treatments for gastroesophageal reflux disease (GERD).
Obese children are 6 times more likely than normal-weight peers to suffer from GERD,7 a condition for which proton pump inhibitors (PPIs) have become the mainstay of therapy.8 In 2010, over 500 000 pediatric prescriptions were filled for 1 PPI agent alone,9 and PPIs remain in the top 12 drugs prescribed in the US.10 Pantoprazole data published in adults suggest that dose escalation may be appropriate for obese patients receiving PPI therapy.11 However, this strategy may be problematic and inappropriate in the pediatric obese population, where increasing the conventional weight-based dosing of PPIs (ie, mg/kg dosing based on total body weight, TBW) could lead to unnecessary systemic exposure that does not enhance efficacy, but rather predisposes patients to adverse events (AEs) associated with high-dose PPI therapy (eg, osteopenic fractures, gastrointestinal infections, pneumonia).12–15 A better approach may be to first assess any pharmacokinetic differences between obese and nonobese peers, especially as the linearity of the relationship between PPI pharmacokinetics (eg, area under the concentration time curve, AUC) and pharmacodynamics (eg, intragastric pH) is less clear at higher PPI doses.16 As substrates for the hepatic cytochrome (CYP) P450 2C19 (CYP2C19), the dose-exposure relationship for PPIs is markedly influenced by allelic variants in CYP2C19.17–20 To further explore the association between obesity and CYP2C19 genotype in pediatric patients, we conducted a controlled, open-label prospective trial in obese children with GERD, using pantoprazole as a model CYP2C19 substrate.17 As 99% of metabolic processes in the human body, including drug clearance, take place in lean body tissues,21 we hypothesized that pantoprazole dosing based on lean body weight (LBW) in obese children would lead to pantoprazole pharmacokinetics (PK) (eg, drug clearance and drug exposure) comparable to those previously reported in nonobese peers.22
Methods
This study was conducted in accordance with current US Food and Drug Administration (FDA) regulations and good clinical practice guidelines. It was approved by the Institutional Review Boards at The Children’s Mercy Hospital, Duke University, Arkansas Children’s Hospital, and East Carolina University (ClinicalTrials.gov: NCT02186652). Informed permission/assent and consent was obtained before the conduct of any study-related procedures.
This was a prospective, multicenter, open-label study of the PK and tolerability of pantoprazole in obese children (6–11 years of age) and adolescents (12–17 years of age) who, based on clinical criteria, required treatment with an acid-modifying agent for GERD. Obesity (BMI ≥95th percentile for age) and clinical diagnosis of GERD were confirmed at a screening visit, which included collection of blood for safety laboratory studies and DNA genotyping for CYP2C19 activity. All participants fasted at least 8 hours before study drug administration. On day 1 of the study, participants received a single oral dose of pantoprazole (commercially purchased, pantoprazole sodium, PROTONIX Delayed-Release Tablets, single-lot numbers: 228037AN for 20 mg tablets and 224201AN for the 40 mg tablets; Wyeth Pharmaceuticals, Inc, Philadelphia, Pennsylvania), according to a fixed-dose scheme based on LBW, so as not to exceed the maximum recommended pantoprazole dose. LBW was calculated using an established equation (Janmahasatian equation) for male and female children.23 Children with LBW 20–25 kg received 20 mg pantoprazole, LBW 26–45 kg 40 mg, LBW 46–65 kg 60 mg, and LBW ≥66 kg 80 mg. Repeated blood samples, 1.0 mL each, were collected from participants via an indwelling intravenous catheter pre-dose (within 30 minutes of receiving pantoprazole) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, and 8 hours (±10 minutes) after pantoprazole dosing. For those participants with the poor metabolizer (PM) CYP2C19 genotype and to better characterize the pantoprazole disposition profile in PM individuals, an additional PK sample was collected 12 hours after dosing. On day 10–13 of study, a follow-up phone call was conducted to assess the participant’s general well-being and to collect any AEs.
All participants had a clinical diagnosis of GERD (ie, clinical symptoms consistent with GERD, evidence of erosive esophagitis on endoscopy, histopathology on esophageal biopsies consistent with reflux esophagitis, abnormal pH-metry consistent with acid reflux, or other tests and procedures consistent with GERD established at least 7 days before receipt of study drug), but were otherwise healthy. Specifically, those participants with diabetes mellitus, hepatic dysfunction (serum aspartate transaminase ≥150 IU/L, alanine transaminase ≥150 IU/L, total bilirubin ≥2.0 mg/dL, or alkaline phosphatase ≥600 IU/L), renal dysfunction (serum creatinine ≥2.0 mg/dL), infection with Hepatitis B or C, or pregnancy were excluded from the study. Those participants who received a dose of pantoprazole, lansoprazole, omeprazole, esomeprazole, or rabeprazole within 48 hours of receipt of study drug were excluded. Also excluded were participants who received the following concomitant medications (ie, known inhibitors or inducers of CYP2C19) within 7 days of study drug administration: fluoxetine, fluvoxamine, ketoconazole, ticlopidine, felbamate, topiramate, valproic acid, phenobarbital, carbamazepine, erythromycin, clarithromycin, grape-fruit juice, verapamil, diltiazem, cimetidine, St. John’s Wort, rifampin, or rifapentine.
PK variables relevant to drug disposition of pantoprazole were assessed as the study’s primary endpoint (apparent peak plasma concentration [Cmax], time of Cmax [Tmax], AUC, apparent terminal-phase volume of distribution [Vd/F], apparent total plasma clearance, and parent:metabolite ratios). Secondary endpoints included assessment of the relationship of nongenetic factors (eg, age, BMI) and genetic factors (eg, sex, CYP2C19 genotype) to variability in drug disposition (ie, PK variables), as well as the safety of pantoprazole in obese children and adolescents.
To generate PK variables, all plasma samples were run in duplicate and the mean value used for analysis. Pantoprazole and pantoprazole-sulfone concentrations in plasma were quantified by the Pediatric Trials Network’s central laboratory (OpAns, LLC, Durham, North Carolina) using a validated high-performance liquid chromatography tandem mass spectrometry assay. During method validation, accuracy and precision of all sample runs were within the FDA bioanalytical assay validation criteria (eg, ±15%). The lower limit of quantification for both pantoprazole and pantoprazole sulfone were 10 ng/mL.
All participants had CYP2C19 genotyping performed using validated sequencing and restriction fragment length polymorphism polymerase chain reaction methodologies (ILS Genomics, Morrisville, North Carolina). Those individuals with 2 functional alleles (*1 or *17) were classified as CYP2C19 extensive metabolizers (EMs), 1 functional and 1 nonfunctional allele (*2) as intermediate metabolizers (IMs), and 2 nonfunctional alleles as PMs. No ultra-rapid metabolizers (ie, *17/*17) were identified.
All participants were monitored for AEs and serious AEs throughout the study. Monitoring included (1) observation and self-report of AEs and/or changes in health during the study visit; (2) repeat vital signs after the last PK sample collection; and (3) a follow-up phone call to participants, 10–13 days after receipt of study drug, to assess participants’ general well-being and to collect any additional AE information or information regarding changes in health. Laboratory studies obtained before study drug administration were performed for screening purposes only and were, therefore, not repeated. All AEs were coded using the Medical Dictionary for Regulatory Activities v 18.0.
Statistical Analyses
For the comparative PK analysis, plasma pantoprazole concentration–time data were analyzed using noncompartmental methods. Cmax, Tmax, AUC from time zero to the time of the last measurable concentration (AUClast) and to infinity (AUCinf), apparent terminal-phase disposition half-life (t1/2), apparent oral clearance (CL/F), and Vd/F, where F was a bioavailability factor reflecting the fraction of the dose absorbed, were estimated using WinNonlin. Apparent terminal elimination rate constant (λz) was determined as the slope of a log-linear least squares of at least 3 concentration-time points judged, by visual inspection, to be in the apparent terminal elimination phase. AUCT was the AUC from the time of dosing to time of last measureable plasma concentration, and was calculated using the linear trapezoidal method. Half-life was calculated as t1/2 = ln2/λz, and AUCinf was estimated using AUCinf = AUClast + CT/λz. CL/F and Vd/F were calculated as CL/F = dose/AUCinf and Vd/F = (CL/F)/λz.Values for CL/F and Vd/F were normalized by body weight. No value for λz -related variables (eg, AUC0-inf, t1/2, CL/F) were reported for concentration profiles that did not exhibit a terminal elimination phase in the concentration vs time profile.
Pantoprazole metabolite concentrations (ie, pantoprazole-sulfone) in serum were plotted as a function of time and their respective AUC determined as described for the parent compound. Parent:metabolite AUC ratios were then generated for each participant and compared based on obesity, age group, and sex.
Using the value at the baseline visit, the following descriptive statistics were calculated for demographic variables: mean, SD, coefficient of variation, median, and range. For dosing variables, median and range were calculated. The range of variables denotes the minimum and maximum values in the data set. For participants 6–11 years of age and participants 12–17 years of age, the mean, geometric mean, median, SD, coefficient of variation, and range of PK variable estimates were calculated. Intergroup comparisons (by age group, sex, obesity status, liver function tests, resting energy expenditure level, waist:hip ratio, and genotype) were conducted using a 2-tailed Student t test or Mann-Whitney U test, as appropriate. All statistical analyses were performed using the software Stata v 13.1 (StataCorp, LLC, College Station, Texas) with significance set at α <0.05.
Results
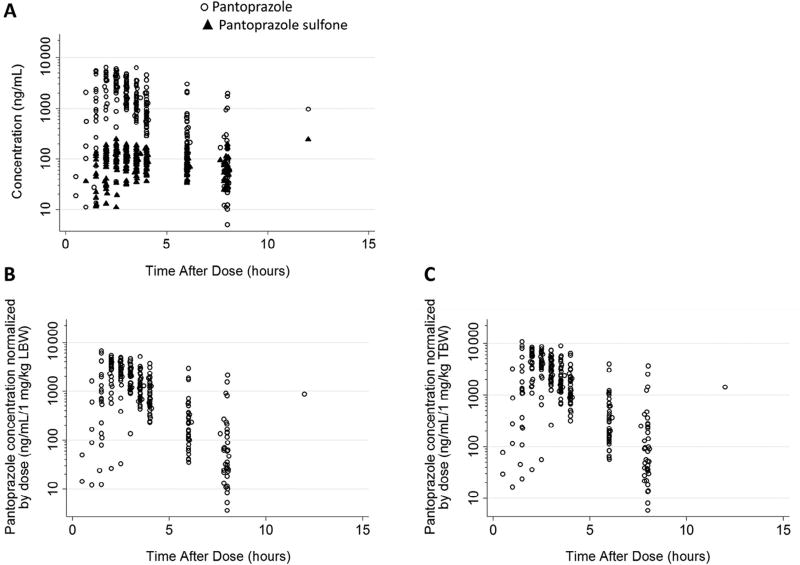
Forty-one obese children with GERD (6–17 years of age, inclusive) were enrolled and completed the study. A total of 438 plasma PK samples (452 anticipated), including predose samples, were collected; plasma concentration-time data are summarized in the Figure. A median of 7 (1–9) quantifiable samples were collected per participant. All participants with at least 1 evaluable PK sample were included in the PK analysis (n = 40). One participant, with all PK samples below the lower limit of quantification, was excluded. Patient demographics and characteristics for the 40 evaluable participants are shown in Table I.
Figure.
Concentration vs time curve for pantoprazole (circles) and pantoprazole sulfone (minor, inactive CYP3A4 metabolite; triangles) after a single oral administration of pantoprazole in 40 pediatric patients with GERD.
Table I.
Patient characteristics
| Characteristics* | Age 6–11 y (n = 19) |
Age 12–17 y (n = 21) |
|---|---|---|
| Age (y) | 10 (6–11) | 14 (12–17) |
| TBW (kg) | 54.5 (32.4–123.4) | 97.5 (67.2–131.6) |
| LBW (kg) | 35.0 (21.0–60.2) | 55.5 (43.1–81.4) |
| BMI (kg/m2) | 25.3 (22.1–42.0) | 36.5 (26.8–41.5) |
| BMI percentile | 98 (95–99) | 98 (95–99) |
| Waist:hip ratio | 0.90 (0.81–1.01) | 0.88 (0.77–1.09) |
| Alanine aminotransferase (U/L) | 25 (10–55) | 33 (18–73) |
| Aspartate aminotransferase (U/L) | 28 (19–40) | 28 (13–48) |
| Serum creatinine (mg/dL) | 0.50 (0.37–0.81) | 0.60 (0.40–0.90) |
| Female | 12 (63) | 10 (48) |
| Race | ||
| White | 8 (42) | 12 (57) |
| Black or African American | 7 (37) | 5 (24) |
| Asian | 1 (5) | 0 (0) |
| Other (eg, Hispanic, Native American) | 3 (16) | 4 (19) |
| CYP2C19 activity based on genotype | ||
| PM (2 nonfunctional alleles) | 1 (5) | 0 (0) |
| IM (1 nonfunctional allele) | 9 (47) | 9 (43) |
| EM (2 functional alleles) | 9 (47) | 12 (57) |
| Study drug | ||
| Pantoprazole dose (mg) | ||
| Mean (SD) | 42 (13) | 64 (10) |
| Median (min, max) | 40 (20, 80) | 60 (40, 80) |
| Pantoprazole dose (mg/kg LBW) | ||
| Mean (SD) | 1.1 (0.2) | 1.1 (0.1) |
| Median (min, max) | 1.1 (0.8, 1.4) | 1.1 (0.9, 1.3) |
Unless otherwise indicated, data are presented as median (range) for continuous variables, or N (%) for categorical variables.
Comparison of the pantoprazole PK variable estimates observed in our prospective cohort of obese children and adolescents to values reported in historical controls from a previously published pediatric study by Ward et al is shown in Table II.22 All historical controls 6–11 years of age were nonobese (33.6 ± 10.8 kg) and the majority of historical controls 12–17 years of age were nonobese (75.7 ± 29.1 kg). Cmax and AUC0-∞ of pantoprazole, corrected for TBW-normalized pantoprazole dose, were higher in obese children and adolescents, compared with historical controls. CL/F and Vd/F of pantoprazole, normalized by TBW, in obese children and adolescents were lower than in historical controls.
Table II.
Comparison of pantoprazole plasma PK in obese children and adolescents vs nonobese historical controls
| Obese pediatric patients | Historical nonobese peers21 | |||
|---|---|---|---|---|
|
|
|
|||
| PK parameters*,† | Age 6–11y‡y (n = 18) |
Age 12–17 y‡ (n = 21) |
Age 6–11 y (n = 13) |
Age 12–17 y (n = 11) |
| Tmax (h) | 2.3 (1.5–3) | 2.5 (1.5–8.0) | 2.0 (1.0–4.0) | 2.0 (1.0–12.0) |
| tlag (h) | 1.5 (1.0–3) | 2.0 (1.0–8.0) | 0.8 (0–1.0) | 1.0 (0–8) |
| t1/2 (h) | 0.90 ± 0.20 | 1.08 ± 0.26 | 0.7 ± 0.2 | 0.9 ± 0.3 |
| (P < .05) | (P < .01) | |||
| Uncorrected Cmax (mcg/mL) | 4.27 ± 1.43 | 4.10 ± 1.18 | 2.1 ± 1.3 | 2.2 ± 1.4 |
| (P < .01) | (P < .01) | |||
| Cmax‡ (mcg/mL/1 mg/kg TBW) | 5.99 ± 2.13 | 6.23 ± 1.73 | 2.1 ± 1.1 | 3.7 ± 1.8 |
| (P < .01) | (P < .01) | |||
| Cmax‡ (mcg/mL/1 mg/kg LBW) | 3.87 ± 1.39 | 3.70 ± 0.99 | - | - |
| (P < .01) | (P = 1.00) | |||
| Uncorrected AUC0-last (mcg*h/mL) | 6.17 ± 2.17 | 7.47 ± 2.80 | - | - |
| Uncorrected AUC0-∞ (mcg*h/mL) | 6.26 ± 2.26 | 7.69 ± 3.45 | 3.8 ± 1.8 | 4.3 ± 3.1 |
| (P < .01) | (P < .05) | |||
| % AUC0-∞ extrapolated from AUC0-last | 1.2 ± 1.6 | 2.5 ± 3.3 | - | - |
| AUC0-∞¶ (mcg*h/mL/1 mg/kg TBW) | 8.87 ± 4.00 | 11.56 ± 4.81 | 3.1 ± 1.4 | 6.9 ± 3.4 |
| (P < .01) | (P < .01) | |||
| AUC0-∞¶ (mcg*h/mL/1 mg/kg LBW) | 5.73 ± 2.48 | 6.82 ± 2.70 | - | - |
| (P < .01) | (P = .9) | |||
| CL/F§ (L/h/kg TBW) | 0.14 ± 0.07 | 0.10 ± 0.04 | 0.40 ± 0.22 | 0.18 ± 0.08 |
| (P < .01) | (P < .01) | |||
| CL/F§ (L/h/kg LBW) | 0.21 ± 0.11 | 0.17 ± 0.06 | - | - |
| (P < .01) | (P = .7) | |||
| Vd/F§ (L/kg TBW) | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.40 ± 0.27 | 0.21 ± 0.06 |
| (P < .01) | (P < .01) | |||
| Vd/F§ (L/kg LBW) | 0.25 ± 0.09 | 0.25 ± 0.07 | - | - |
| (P < .05) | (P = .1) | |||
Tlag, lag time.
Uncorrected and corrected data are provided.
Parameters are given as mean ± SD, except Tmax and tlag, which are given as median (range).
For each age group, P values indicate comparison of PK parameters between obese participants and those reported for nonobese historic controls in a pediatric study by Ward et al.22
Cmax, and AUC0-∞ were corrected for TBW-normalized-dose (1 mg/TBW kg) and LBW-normalized-dose pantoprazole (1 mg/LBW kg).
CL/F and Vd/F were normalized to TBW and LBW.
When Cmax and AUC0-∞ of pantoprazole were corrected for LBW-normalized dose in obese children (6–11 years of age), they remained higher (3.87 ± 1.39 and 5.73 ± 2.48, respectively) but more closely approximated the values reported in historical controls (2.1 ± 1.1 and 3.1 ± 1.4, respectively). When normalized to LBW, CL/F and Vd/F of pantoprazole remained lower in obese children (0.21 ± 0.11 and 0.25 ± 0.09, respectively), but approximated closer the values reported in nonobese peers (0.40 ± 0.22 and 0.40 ± 0.27, respectively). In adolescents (12–17 years of age), correction of pantoprazole Cmax and AUC0-∞ for LBW-normalized dose (3.7 ± 0.99 and 6.82 ± 2.70, respectively), and pantoprazole CL/F and Vd/F for LBW (0.17 ± 0.06 and 0.25 ± 0.07, respectively), resulted in values comparable with those reported in nonobese adolescents (Table II). Pantoprazole Tmax and t1/2 were comparable between obese and nonobese pediatric patients in both age groups (Table II).
Pantoprazole t1/2 was significantly shorter in obese children than adolescents (P = .02), with trends toward higher CL/F and lower AUC0-∞ observed in obese children vs adolescents (Table III). The only PM in this study was excluded from these comparisons, as her pantoprazole AUC0-∞ was greater than 2-fold higher than all the non-PMs in the study.
Table III.
| AUC0-∞‡ (mcg*h/mL/1 mg/kg LBW) |
CL/F§ (L/h/kg LBW) |
Vd/F§ (L/kg LBW) |
t1/2 (h) |
Parent:metabolite AUC0-∞ ratio |
|
|---|---|---|---|---|---|
| Age | |||||
| 6–11 y | 5.73 (2.48) | 0.21 (0.11) | 0.25 (0.09) | 0.90 (0.20) | 6.24 (1.46) |
| 12–17 y | 6.82 (2.70) | 0.17 (0.06) | 0.25 (0.07) | 1.08 (0.26) | 6.13 (1.35) |
| P value | .17 | .17 | .92 | .02 | .83 |
| Obesity status | |||||
| Obese (BMI ≥95%) | 6.36 (2.97) | 0.20 (0.11) | 0.25 (0.09) | 0.97 (0.27) | 6.42 (1.43) |
| Morbidly obese (BMI >99%) | 6.17 (1.89) | 0.18 (0.06) | 0.25 (0.05) | 1.03 (0.21) | 5.67 (1.19) |
| P value | .97 | .97 | .40 | .21 | .16 |
| Sex | |||||
| Male | 6.57 (1.88) | 0.17 (0.06) | 0.22 (0.06) | 0.95 (0.14) | 5.99 (1.08) |
| Female | 6.03 (3.20) | 0.21 (0.11) | 0.28 (0.08) | 1.03 (0.32) | 6.35 (1.62) |
| P value | .31 | .31 | .02 | .92 | .47 |
| Waist:hip ratio | |||||
| Low (<Q1) | 5.76 (2.31) | 0.20 (0.09) | 0.28 (0.08) | 1.01 (0.24) | 6.25 (1.42) |
| High (>Q3) | 6.63 (3.57) | 0.20 (0.13) | 0.24 (0.09) | 0.98 (0.35) | 6.80 (1.27) |
| P value | .78 | .78 | .16 | .72 | .39 |
| 2C19 genotype | |||||
| IM | 7.90 (2.72) | 0.14 (0.05) | 0.20 (0.04) | 1.05 (0.27) | 5.82 (1.22) |
| EM | 5.09 (1.81) | 0.23 (0.10) | 0.29 (0.07) | 0.95 (0.23) | 6.40 (1.46) |
| P value | .002 | .002 | <.001 | .16 | .26 |
| ALT | |||||
| Low (<Q1) | 5.75 (1.97) | 0.19 (0.06) | 0.25 (0.04) | 0.97 (0.29) | 5.81 (2.09) |
| High (>Q3) | 6.12 (2.08) | 0.18 (0.07) | 0.25 (0.09) | 0.96 (0.16) | 6.35 (1.21) |
| P value | .87 | .87 | .71 | .87 | .52 |
| AST | |||||
| Low (<Q1) | 6.04 (1.97) | 0.19 (0.09) | 0.27 (0.06) | 1.06 (0.26) | 5.71 (1.50) |
| High (>Q3) | 5.66 (1.40) | 0.19 (0.06) | 0.23 (0.05) | 0.85 (0.13) | 6.38 (1.81) |
| P value | .46 | .46 | .09 | .14 | .44 |
ALT, alanine transaminase; AST, aspartate transaminase.
Data presented for CYP2C19 non-PMs.
PK parameters are given as mean (SD).
AUC0-∞ corrected for LBW normalized dose.
Cl/F and Vd/F normalized by LBW.
Pantoprazole Vd/F, normalized by body weight, was significantly higher in female children (0.28 [0.08] L/kg LBW, n = 18) than male children (0.22 [0.06] L/kg LBW, n = 17); P < .05. No other differences in PK were observed between the sexes.
Eleven children had mild elevation in serum transaminases, 9 with alanine transaminase and 2 with aspartate transaminase above norms for age (Table I). Pantoprazole PK variables were comparable between children with transaminase elevation and those without (Table III).
Intergroup comparisons of the PK variables, including CL/F, Vd/F, t1/2, and AUC0-∞ of pantoprazole and pantoprazole-sulfone, were conducted in relation to CYP2C19 genotype. CL/F and Vd/F, normalized by LBW, in participants with 2 CYP2C19 functional alleles (EM; n = 20) were significantly higher compared with participants with 1 CYP2C19 functional allele (IM; n = 15); P < .01.AUC0-∞ of pantoprazole was significantly lower in CYP2C19 EM participants compared with IM participants; P < .01.
AUClast of pantoprazole in the only PM in this study was, expectedly, greater than 2-fold higher (13.44 mcg*h/mL/1 mg/kg LBW) than all the non-PMs in the study (6.34 [1.86–12.81] mcg*h/mL/1 mg/kg LBW). Pantoprazole-sulfone Cmax and AUClast, corrected for weight-normalized pantoprazole dose, in the only CYP2C19 PM were also higher than the corresponding values in all the non-PMs. No other significant differences in pantoprazole-sulfone disposition were noted among CYP2C19 genotypes, or between CYP2C19 phenotype groups (ie, EM vs IM).
Nine AEs were reported in 7 (17%) of the 41 study participants. All AEs were mild and the majority (>85%) occurred in adolescents, with only 1 AE reported in a child <12 years of age. Two AEs (5%) were considered related to study drug (headache and hiccups). No serious AEs were reported.
Discussion
Our study demonstrates that the PK of pantoprazole in children is affected by obesity, with higher exposures and slower drug clearance observed in obese children and adolescents relative to nonobese, age-matched, historical controls (Table II). These findings argue against empiric dose escalation of PPIs in obese pediatric patients and suggest that dose reduction may be warranted in this patient population if using TBW for dosing. In addition to noncompartmental PK analysis in this study, in another study, population PK analyses are being performed, using the same dataset, to evaluate the FDA-approved weight-tiered dosing specifically for obese children. The pharmacokinetic knowledge generated by these investigations can be used to interpret and compare PPI efficacy (eg, pharmacodynamics) between obese and nonobese children.
Ninety-nine percent of metabolic processes, including drug clearance, take place in lean body tissues.21 Although, compared with nonobese counterparts, obese patients have a greater absolute TBW and LBW, their overall LBW-to-TBW ratio is decreased,24 potentially explaining our observation of decreased pantoprazole clearance in obese vs nonobese pediatric patients. Our observations agree with published conclusions that dosing schemes using traditional body size measurements (ie, TBW) and drug physiochemical properties (eg, lipophilicity) are insufficient and unreliable in predicting dose-exposure relationships for obese children.5,25 In addition, there is now growing evidence to suggest that obesity per se may impact the activity of drug metabolizing enzymes (eg, CYP2C19, CYP2D6).25,26 Consequently, the observed differences in PK between obese and nonobese pediatric patients may not be simply a matter of size or allometric scaling.
In our study, using LBW-based dosing of pantoprazole in obese adolescents led to pantoprazole exposures, and clearance, comparable to nonobese adolescents (Table II). However, although LBW dosing helped reduce the observed difference in pantoprazole exposure between obese and nonobese children (6–11 years of age), it did not fully account for the PK differences observed in the younger age group, suggesting an age effect, as well as an obesity effect. Previous studies of pantoprazole,22,27,28 and other PPIs,28–30 support our observation of an age effect on pantoprazole PK, with a trend toward higher apparent clearance, lower systemic exposure, and shorter pantoprazole t1/2 observed in obese children compared with obese adolescents (Table III). A potential confounding factor contributing to the observed age-effect in our study is the use of the Janmahasatian equation to calculate LBW, which may overestimate LBW for younger children, as the equation is based on anthropometric measures derived from individuals 18–82 years of age.23 However, using a more recently developed equation by Al-Salami et al, suggested to perform better for younger children,31 did not offer any pharmacokinetic advantage over the Janmahasatian equation in our patient population (data not presented). Using either formula, LBW-based dosing in young obese patients (<12 years of age) was superior to traditional TBW-based dosing in approximating targeted pantoprazole exposures, previously reported as therapeutic32 in nonobese peers.
Although the AEs associated with a 1-time dose of pantoprazole were mild and infrequent in our study, there is rising concern regarding potential adverse effects associated with long-term high-dose PPI exposure (eg, osteopenic fractures, gastrointestinal infections, pneumonia).12–15 LBW-based dosing may be a strategy for reducing unintentional and unnecessary PPI overexposure for obese children who, compared with nonobese peers, are at greater risk for GERD7 and, as demonstrated by our data, PPI overexposure.
As summarized in Table III, our data also suggest that pantoprazole dose reduction may be warranted in pediatric patients with 1 or 2 nonfunctional CYP2C19 alleles (ie, CYP2C19 IMs and PMs), who demonstrate higher exposure to, and slower clearance of, pantoprazole than individuals with 2 functional alleles (ie, CYP2C19 EM). Similarities in parent:metabolite AUC0-∞ ratios of pantoprazole and pantoprazole-sulfone (Table III) highlight how minor the contribution of hepatic CYP3A4 appears to the systemic clearance of pantoprazole. Previous observations of significantly higher exposure to the CYP2C19 substrate voriconazole in an obese adolescent with the CYP2C19 PM phenotype33 further highlight the necessity of dose reduction for certain CYP2C19 substrates in obese pediatric patients and support our observation that minor pathways of hepatic clearance may not sufficiently compensate for decreased CYP2C19 activity. Studies of other PPIs in obese pediatric patients are needed to determine whether the observed changes in pantoprazole PK in obese children and adolescents are drug-specific or extend to other PPIs.
Our study is limited to pharmacokinetic data and represents the first step in assessing appropriate drug dosing for obese children. Historical pediatric pantoprazole data were used for PK comparisons between obese and nonobese children; however, subjects were age- and sex-matched, as well as genotyped for CYP2C19, to limit confounding factors other than obesity.
Pantoprazole PK is affected by obesity, with higher pantoprazole exposures observed in obese children and adolescents, relative to nonobese peers. LBW-based dosing of pantoprazole led to pantoprazole exposures in obese adolescents similar to nonobese peers, and higher pantoprazole exposures were observed in obese children 6–11 years of age relative to nonobese peers. Therefore, pantoprazole may be dosed based on LBW in obese children, 6–17 years of age, when body weight-based dosing is needed. Based on these pharmacokinetic data, empiric dose escalation of pantoprazole is not appropriate for obese children. Pharmacodynamic studies that achieve comparable systemic PPI exposures between obese and nonobese children are needed to assess whether there is any clinical benefit to dose escalation for obese pediatric patients. Dose reduction should be considered for CYP2C19 PMs and IMs.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Pediatric Trials Network (NICHD-2012-PAN01). P.S. receives research support from the National Institutes of Health (NIH-1R21HD080606-01A1) and the National Institute of Child Health and Human Development (HHSN275201000003I).
Glossary
- AE
Adverse event
- AUC
Area under the concentration time curve
- AUCinf
AUC from time zero to infinity
- AUClast
AUC from time zero to the time of the last measurable concentration
- BMI
Body mass index
- CL/F
Apparent oral clearance
- Cmax
Peak plasma concentration
- CYP
Cytochrome
- CYP2C19
CYP P450 2C19 enzyme
- CYP2C19
Gene that encodes the CYP P450 2C19 enzyme
- EMs
Extensive metabolizers
- FDA
Food and Drug Administration
- GERD
Gastroesophageal reflux disease
- IMs
Intermediate metabolizers
- LBW
Lean body weight
- PK
Pharmacokinetics
- PM
Poor metabolizer
- PPIs
Proton pump inhibitors
- t1/2
Half-life
- TBW
Total body weight
- Tmax
Time to Cmax
- Vd/F
Apparent terminal-phase volume of distribution
Appendix
Additional members of the Pediatric Trials Network (PTN) Members The Best Pharmaceuticals for Children Act – PTN Steering Committee
Daniel K. Benjamin Jr, MD, PhD, Katherine Y. Berezny, BSMT, MPH, P., Michael CohenWolkowiez, MD, PhD, Duke Clinical Research Institute, Durham, NC; Matthew M. Laughon, MD, MPH, University of North Carolina, Chapel Hill, NC; Ian M. Paul, MD, MSc, Penn State College of Medicine, Hershey, PA; Michael J. Smith, MD, MSCE, University of Louisville, Louisville, KY; John van den Anker, MD, PhD, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, MD, Children’sHospital of Philadelphia, Philadelphia, PA.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, MD, Perdita Taylor-Zapata, MD, Anne Zajicek, PharmD, Zhaoxia Ren, MD, PhD, Ekaterini Tsilou, MD, Alice Pagan, BBA.
The EMMES Corporation (Data Coordinating Center): Ravinder Anand, PhD, Traci Clemons, PhD, Gina Simone, BS.
PTN Study Team Investigators and Study Coordinators Arkansas Children’s Hospital, Little Rock, AR (enrolled 13 subjects): Lee Howard, RN, CCRC (Site Coordinator, SC); The Children’s Mercy Hospital, Kansas City, MO (enrolled 23 subjects): Jaylene Weigel, RN, MSN, MBA-HCM, CPN, CCRC (SC); East Carolina University, Greenville, NC (enrolled 5 subjects): Nancy DardenSaad, BS, RN, CCRC (SC).
Footnotes
Trial Registration ClinicalTrials.gov NCT02186652
The other authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States. JAMA. 2013;311:6–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spivak H, Hewitt MF, Onn A, Half EE. Weight loss and improvement of obesity-related illness in 500 US patients following laparoscopic adjustable gastric banding procedure. Am J Surg. 2005;189:27–32. doi: 10.1016/j.amjsurg.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–50. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 5.Harskamp-van Ginkel MW, Hill KD, Becker K, Testoni D, Cohen-Wolkowiez M, Gonzalez D, et al. Drug dosing in obese children: a systematic review of current pharmacokinetic data. JAMA Pediatr. 2015;169:678–85. doi: 10.1001/jamapediatrics.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe S, Siegel D, Benjamin DK., Jr Gaps in drug dosing for obese children: a systematic review of the commonly prescribed acute care medications. Clin Ther. 2015;37:1924–32. doi: 10.1016/j.clinthera.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebnick C, Getahun D, Smith N, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Extreme childhood obesity is associated with increased risk for gastroesophageal reflux disease in a large population-based study. Int J Pediatr Obes. 2011;6:e267–3. doi: 10.3109/17477166.2010.491118. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons TE, Gold BD. The use of proton pump inhibitors in children: a comprehensive review. Paediatr Drugs. 2003;5:25–40. doi: 10.2165/00128072-200305010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130:23–31. doi: 10.1542/peds.2011-2879. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention/National Center for Health Statistics. National ambulatory medical care survey: 2012 state and national summary tables. [Accessed October 17, 2016]; cdc.gov/nchs/fastats/drug-use-therapeutic.htm. Published March 29, 2014. Updated October 7, 2016.
- 11.Chen WY, Chang WL, Tsai YC, Cheng HC, Lu CC, Sheu BS. Double-dosed pantoprazole accelerates the sustained symptomatic response in overweight and obese patients with reflux esophagitis in Los Angeles grades A and B. Am J Gastroenterol. 2010;105:1046–52. doi: 10.1038/ajg.2009.632. [DOI] [PubMed] [Google Scholar]
- 12.Kwok CS, Yeong JKY, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medications. Bone. 2011;48:768–76. doi: 10.1016/j.bone.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Freedberg DE, Haynes K, Denburg MR, Zemel BS, Leonard MB, Abrams JA, et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int. 2015;26:250–7. doi: 10.1007/s00198-015-3168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark CM, Cade MN. Side effects and complications of proton pump inhibitors: a pediatric perspective. J Pediatr. 2016;168:16–22. doi: 10.1016/j.jpeds.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Chung EY, Yardley J. Are there risks associated with empiric acid suppression treatment of infants and children suspected of having gastroesophageal reflux disease? Hosp Pediatr. 2013;3:16–23. doi: 10.1542/hpeds.2012-0077. [DOI] [PubMed] [Google Scholar]
- 16.Shin JM, Nayoung K. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward RM, Kearns GL. Proton pump inhibitors in pediatrics. Paediatr Drugs. 2013;15:119–31. doi: 10.1007/s40272-013-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearns GL, Blumer J, Schexnayder S, James LP, Adcock KG, Reed MD. Single-dose pharmacokinetics of oral and intravenous pantoprazole in children and adolescents. J Clin Pharmacol. 2008;48:1356–65. doi: 10.1177/0091270008321811. [DOI] [PubMed] [Google Scholar]
- 19.Kearns GL, Leeder JS, Gaedigk A. Impact of the CYP2C19*17 allele on the pharmacokinetics of omeprazole and pantoprazole in children: evidence for a differential effect. Drug Metab Dispos. 2010;38:894–7. doi: 10.1124/dmd.109.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearns GL, Winter HS. Proton pump inhibitors in pediatrics: relevant pharmacokinetics and pharmacodynamics. J Pediatr Gastroenterol Nutr. 2003;37:S52–9. doi: 10.1097/00005176-200311001-00011. [DOI] [PubMed] [Google Scholar]
- 21.Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49:163–75. doi: 10.1111/j.1753-4887.1991.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 22.Ward RM, Kearns GL, Tammara B, Bishop P, O’Gorman MA, James LP, et al. A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease. J Clin Pharmacol. 2011;51:876–87. doi: 10.1177/0091270010377501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 24.Forbes GB, Welle SL. Lean body mass in obesity. Int J Obes. 1983;7:99–107. [PubMed] [Google Scholar]
- 25.Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Knibbe CAJ, Brill MJE, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol. 2015;55:149–67. doi: 10.1146/annurev-pharmtox-010814-124354. [DOI] [PubMed] [Google Scholar]
- 27.Pettersen G, Moukassi MS, Theoret Y, Labbé L, Faure C, Nguyen B. Population pharmacokinetics of intravenous pantoprazole in paediatric intensive care patients. Br J Clin Pharmacol. 2009;67:216–27. doi: 10.1111/j.1365-2125.2008.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litalien C, Theoret Y, Faure C. Pharmacokinetics of proton pump inhibitors in children. Clin Pharmacokinet. 2005;44:441–66. doi: 10.2165/00003088-200544050-00001. [DOI] [PubMed] [Google Scholar]
- 29.Andersson T, Hassall E, Lundborg P, Shepherd R, Radke M, Marcon M, et al. Pharmacokinetics of orally administered omeprazole in children. Am J Gastroenterol. 2000;95:3101–6. doi: 10.1111/j.1572-0241.2000.03256.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Li J, Hamer-Maansson JE, Andersson T, Fulmer R, Illueca M, et al. Pharmacokinetic properties of esomeprazole in children aged 1 to 11 years with symptoms of gastroesophageal reflux disease: a randomized, open-label study. Clin Ther. 2006;28:1868–76. doi: 10.1016/j.clinthera.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Al-Salami HS, Goulding A, Grant A, Taylor R, Holfod N, Duffull SB. Prediction of fat-free mass in children. Clin Pharmacokinet. 2015;54:1169–78. doi: 10.1007/s40262-015-0277-z. [DOI] [PubMed] [Google Scholar]
- 32.Katashima M, Yamamoto K, Tokuma Y, Hata T, Sawada Y, Iga T. Comparative pharmacokinetics/pharmacodynamic analysis of proton pump inhibitors omeprazole, lansoprazle, and pantoprazole, in humans. Eur J Drug Metab Pharmacokinet. 1998;23:19–26. doi: 10.1007/BF03189822. [DOI] [PubMed] [Google Scholar]
- 33.Moriyama B, Jarosinski PF, Figg WD, Henning SA, Danner RL, Penzak SR, et al. Pharmacokinetics of intravenous voriconazole in obese patients: implications of CYP2C19 homozygous poor metabolizer genotype. Pharmacotherapy. 2013;33:e19–22. doi: 10.1002/phar.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]