Abstract
Background
To estimate a pooled association between hearing impairment and risk of mild cognitive impairment and dementia.
Methods
PubMed, Embase, and Web of Science were searched for prospective cohort studies that examined the association between hearing impairment and risk of mild cognitive impairment and/or dementia. Random-effects models were fitted to estimate the summary risk ratios (RRs) and 95% confidence interval (CIs), which represents the pooled association between hearing impairment with risk of mild cognitive impairment and dementia, compared to subjects free of hearing impairment.
Results
Four studies on hearing impairment with mild cognitive impairment and 7 studies on hearing impairment with dementia were included in the meta-analysis. A total of 15,521 subjects were studied with follow-up periods between 2 and 16.8 years. Hearing impairment was associated with a greater risk of mild cognitive impairment (RR = 1.30, 95% CI: 1.12, 1.51) and dementia (RR = 2.39, 95% CI: 1.58, 3.61).
Conclusions
The meta-analysis showed that hearing impairment is associated with a higher risk of mild cognitive impairment and dementia among older adults.
Keywords: Hearing impairment, Dementia, Mild cognitive impairment, Meta-analysis
Introduction
Dementia, featured with loss of intellectual abilities severe enough to interfere with occupational or social functioning, has become a major public health burden in the US. The prevalence of dementia in 2012 was 8.8% among the population of ≥65-year-olds in the US [1]. The Framingham Heart Study indicated that the remaining lifetime risk of developing any type of dementia was 10.9 and 19% for a 65-year-old man and woman, respectively [2]. It is expected that the number of people living with dementia worldwide will exceed 135 million by 2050 [3]. According to the Alzheimer's Association, the aggregate cost of care for Americans aged 65 and older with dementia will reach USD 259 billion in 2017 [4]. Currently, no effective therapies are available to cure dementia or slow its progression. Mild cognitive impairment (MCI) is an intermediate state between normal cognition and dementia, with a published prevalence between 5.0 and 36.7% worldwide [5]. MCI contributes to disease burden and increases the risk of dementia [6].
Hearing impairment, or hearing loss often occurs with aging and is common among older adults - approximately one-third of people over 65 years of age are affected by disabling hearing loss [7]. Hearing loss is the third most common chronic condition and the fourth most detrimental condition that affects quality of life in older adults [8]. Evidence has shown that hearing impairment is more common in patients with dementia than in healthy older adults [9]. Hearing impairment was hypothesized to be associated with cognitive decline decades ago [10, 11]; however, population-based research on associations between hearing impairment and MCI and dementia has not been frequently conducted until recently. A number of prospective cohort studies during the last decade suggested that hearing im pairment is associated with a higher risk of MCI and dementia. However, these studies used varied diagnostic criteria for hearing impairment and MCI/dementia. It is not known whether these differences affect the association between hearing impairment and risk of MCI and dementia. Clarifying the role of hearing impairment in MCI and dementia is fundamental, since if its role is established, hearing assessments might be clinically applied for assessing the risk of MCI/dementia, and hearing aids may be used to delay dementia among older adults with hearing impairment. Moreover, routine hearing care may be administered among the general population to maintain cognitive function and further reduce the public health burden caused by MCI/dementia.
A recent meta-analysis examined the pooled association between hearing loss and risk of Alzheimer disease, a major type of dementia [12]. However, no study has summarized the association between hearing impairment and risk of overall dementia. Therefore, we conducted a systematic review and meta-analysis with the primary objective of summarizing the existing evidence of the association between hearing impairment and risk of MCI and dementia among older adults.
Methods
Search Strategy
We followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guideline for the meta-analysis [13]. We systematically searched PubMed, Embase, and Web of Science for epidemiological studies on the association between hearing impairment and MCI and/or dementia. Search terms included “hearing impairment,” “hearing loss,” “cognitive impairment,” “mild cognitive impairment,” “dementia,” and “Alzheimer's disease.” We also searched for studies listed in review papers, in case there were potential studies not captured by the database search strategy. The search was limited to articles written in English.
Data Selection and Extraction
We included original full-text studies that: (a) were prospective cohort studies published in peer-reviewed journals from January 1966 to June 2017, (b) included adult participants free from MCI or all-cause dementia as outcomes at baseline and receiving standardized hearing examination, (c) assessed primary outcomes including MCI and/or dementia. We further excluded studies that included only cognitive function and cognitive change as outcomes without assessing cognitive status of MCI and/or dementia, as well as studies without testing hearing ability.
Study selection was conducted in 3 steps. First, the titles of studies identified in our literature search were independently evaluated by 2 reviewers. Second, the abstracts of studies that remained after the initial screening were independently evaluated by 2 reviewers, and disagreements were reconciled. Third, information of studies that met inclusion criteria were extracted independently by 2 reviewers, including sample size, regions, follow-up time, age, sex, race/ethnicity, assessment and prevalence of hearing impairment, ascertainment of MCI/dementia, results of studies and covariates included in the analysis.
Quality Assessment
Quality assessment was conducted using the Newcastle-Ottawa Scale [14]. The Newcastle-Ottawa Scale evaluates the quality of cohort studies in 3 domains: selection of exposed and unexposed cohorts (representativeness of the exposed cohort, selection of the unexposed cohort, ascertainment of exposure, and demonstration of absence of outcome at the beginning of studies), comparability of exposed and unexposed cohorts (analysis appropriately adjusted for potential confounding factors, including the most important factors and additional ones), and outcome ascertainment (adequacy of outcome assessment, length of follow-up, and adequacy of follow-up). A study was awarded 1 point for each variable within domains of selection and outcome and 2 points for comparability for a possible maximum total score of 9. The quality assessment was conducted independently by 2 reviewers, and the results were reconciled until a consensus was reached.
Statistical Analysis
The analyses of MCI and dementia were conducted separately. Risk ratios (RRs) and 95% confidence intervals (CIs) were derived from included studies after full adjustment. Other effect measures (odds ratios, incidence rate ratios, and hazards ratios) were considered equivalent to RR. Data were pooled across studies using random-effects meta-analysis models and weighted by the inverse of the estimated variance, comparing the participants with hearing impairment with those free of hearing impairment as indicated in each study. If studies had multiple comparisons (e.g., mild/moderate/severe hearing impairment) and showed a general linear trend, then the comparisons between groups with moderate hearing impairment and normal hearing were chosen for meta-analysis, since it is more likely to represent the relative risk comparing individuals with hearing impairment and those without. The pooled RR was considered statistically significant if the 95% CI did not contain 1. Forest plots were created to illustrate individual and pooled risk estimates. I2 statistic was calculated to quantify the proportion of between-study heterogeneity attributable to variability in the association rather than sampling variation, and significant heterogeneity was considered if the p values corresponding to I2 statistic were smaller than 0.05 [15]. Begg's test was conducted to examine potential publication bias. A p value corresponding to the z value smaller than 0.05 indicates a significant publication bias. Subgroup analysis on study characteristics, including regions of studies (North America/Asia/Europe), mean age of subjects (≥65 years or not), assessment of hearing impairment and assessment of MCI/dementia were planned but ultimately not conducted due to the limited number of studies included. Sensitivity analysis was conducted by excluding studies with extreme results, defined as larger than twice or smaller than half of the pooled results. All analyses were conducted using Stata 14 (Stata Corp, College Station, TX, USA).
Results
Literature Search
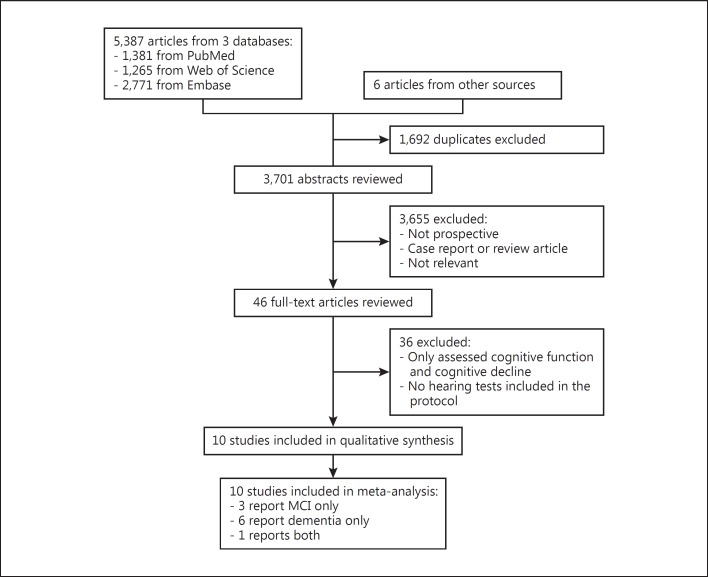
The database search yielded a total of 5,387 citations (Fig. 1). An additional 6 studies were identified from the bibliography of relevant reviews. After eliminating duplicates, 3,701 remained. Of these, 3,655 studies were not relevant or not prospective cohort studies and were therefore excluded. The 46 remaining studies were retrieved in full text to be examined in more detail. Of these, 36 were ultimately excluded for not meeting other inclusion criteria. A total of 10 studies met the inclusion criteria and were included in the meta-analysis [16, 17, 18, 19, 20, 21, 22, 23, 24, 25], including 4 studies reporting MCI and 7 studies reporting dementia as outcomes (1 study reported both).
Fig. 1.
Flowchart showing selection of study reports for the meta-analysis.
Systematic Review
Among the 10 cohort studies included, 8 were from the US [16, 17, 18, 20, 21, 22, 23, 24], 1 was from UK [19], and 1 was from Singapore [25]. The pooled sample included 15,521 participants with 1,044 cases of incident MCI and 886 cases of dementia. Table 1 summarizes the main study characteristics. The number of participants ranged from 274 to 4,463. The mean age ranged from 56.1 to 77.4 years. There were more females than males. The average follow-up period ranged from 2 years to 16.8 years.
Table 1.
Characteristics of cohort studies included in the systematic review and meta-analysis (n = 10)
| Study | Country | Sample size, n | Follow-up, years | Age, years | Female, % | Assessment of HI | Definition of MCI/dementia | Results | Covariates |
|---|---|---|---|---|---|---|---|---|---|
| Framingham Heart Study Gates, 2002 [16] | USA | 740 | Mean: 8.4 (range: 3–12) | Normal hearing: 71.7±4.9 HI: 77.8±5.2 |
Normal hearing: 61.4 HI: 53.3 | A central auditory speech-processing deficit was defined as a score of 50% or less correct on the Synthetic Sentence Identification with Ipsilateral Competing Message Test in at least one ear with normal word recognition ability in both ears Prevalence: 2.0% | The diagnosis of probable Alzheimer disease was made prospectively using the NINCDS-ADRDA criteria | Alzheimer's dementia (n = 40): RR = 23.3, 95% CI: 6.6, 82.7 | Age, gender, education level, apolipoprotein allele E4 presence, and hearing level |
| Baltimore Longitudinal Study of Aging Lin, 2011 [17] | USA | 639 | Median: 11.9 | By HL status: Normal: 59.9±12.2 Mild: 71.1±8.6 Moderate: 77.0±8.4 Severe: 77.7±4.8 |
By HL status: Normal: 50.5 Mild: 24.8 Moderate: 32.1 Severe: 16.7 |
HL was defined by a pure-tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better-hearing ear (normal, <25 dB; mild loss, 25–40 dB; moderate loss, 41–70 dB; and severe loss, >70 dB) Prevalence: 28.8% | Dementia: by consensus diagnostic conference | All-cause dementia (n = 58): Mild HI: HR = 1.89, 95% CI: 1.00, 3.58; Moderate HI: HR = 3.00, 95% CI: 1.43, 6.30; Severe HI: HR = 4.94, 95% CI: 1.09, 22.40 Alzheimer's dementia (n = 37): per 10 dB of HL: HR = 1.27, 95% CI: 1.06, 1.50 |
Sex, age, race, education, diabetes mellitus, smoking, and hypertension |
| Adult Changes in Thought Gates, 2011 [18] | USA | 274 | Mean: 2.2 (range: 0.8–4) | Mean: 80 (range: 71–96) | 62.8 | With severe CAD: SSI-ICM: <50% correct DSI: <50% correct DDT: <50% correct With moderate CAD: SSI-ICM: <80% correct DSI: <80% correct DDT: <80% correct Prevalence: 16.1% |
Alzheimer's dementia: physician diagnosis according to NINCDS-ADRDA criteria | Alzheimer's dementia (n = 21): With severe CAD: By DSI <50%: HR =9.9, 95% CI: 3.6, 26.7 By DDT <50%: HR= 2.2, 95% CI: 0.7, 7.0 By SSI-CCM <50%: HR = 2.1, 95% CI: 0.7, 6.6 With moderate CAD: By DSI <80%: HR = 6.8, 95% CI: 1.9, 24.1; By DDT <80%: HR = 7.0, 95% CI: 1.6, 31.0; By SSI-CCM <80%: HR = 2.5, 95% CI: 0.9, 7.5 |
Education |
| Caerphilly Prospective Study Gallacher, 2012 [19] | UK | 1,057 | Mean: 16.8 (range: 14.5–19.8) | Mean: 56.1±4.4 | 0 | Pure-tone unaided audiometric threshold assessed at 0.5, 1, 2, and 4 KHz at baseline and after 9 years Prevalence: 43.2% | Dementia (Alzheimer's and vascular) DSM-IV criteria | All-cause dementia (n = 79): OR = 2.67, 95% CI: 1.38, 5.18 Vascular dementia (n = 38): OR = 2.40, 95% CI: 0.99, 5.83 Nonvascular dementia (n = 41): OR = 2.96, 95% CI: 1.21, 7.22 MCI (n = 146): OR = 1.24, 95% CI: 0.77, 2.01 |
Age, social class, anxiety, premorbid intelligence |
| Health ABC study Lin, 2013 [20] | USA | 1,984 | 6 | Mean: 77.4 | By hearing status: Normal: 62.3 HL: 44.8 | Pure-tone average: mild loss, 25–40 dB; moderate loss, 41–70 dB; and severe loss, >70 dB Prevalence: 58.6% | 3MS score of less than 80 or a decline in 3MS score of more than 5 points from baseline | MCI (n = 609): Overall: HR = 1.24, 95% CI: 1.05, 1.48 Mild HL: HR = 1.19, 95% CI: 0.99, 1.44 Moderate HL or above: HR = 1.36, 95% CI: 1.08, 1.70 Per 10 dB of HL: HR = 1.07, 95% CI: 1.01, 1.14 |
Age, sex, race/ethnicity, education, study site, smoking status, hypertension, diabetes mellitus, and stroke history |
| Deal, 2017 [21] | USA | 1,889 | 9 | Mean: 75.5±3 (range: 70–79) | 53 | A pure-tone average in decibels hearing level (dBHL) was calculated in the better hearing ear using thresholds from 0.5 to 4 kHz, and HI was defined as normal hearing (≤25 dBHL), mild (26–40 dBHL), and moderate/severe (>40 dBHL) Prevalence: 58.4% | Dementia was defined using a prespecified algorithm incorporating medication use, hospital records, and neurocognitive test scores | Dementia (n = 229): Mild HI: HR = 1.02, 95% CI: 0.75, 1.40 Moderate/severe HI: HR = 1.55, 95% CI: 1.10, 2.19 Per 10 dB increase: HR = 1.14, 95% CI: 1.03, 1.26 |
Age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke |
| Cache County Study on Memory, Health, and Aging Gurgel, 2014 [22] | USA | 4,463 | By hearing status: Normal: 6.08 HL: 4.32 |
Mean: 75.4 (range: 65.3–102.4) | 57.0 | Subjects with at least one testing item coded with auditory impairment were then coded in a dichotomous indicator as HL present or absent Prevalence: 18.7% | Diagnoses of dementia were based on DSM-III-R criteria | Dementia (n = 72): HR = 1.27, 95% CI: 1.03, 1.56 | Sex, presence of APOE-4 allele, education, baseline age, cardiovascular risk factors |
| Epidemiology of Hearing Loss Study Fischer, 2016 [23] | USA | 1,884 | 10 | Mean: 66.7±8.4 | 59.1 | HI was a pure-tone average of hearing thresholds (0.5, 1, 2, 4 kHz) of >25 dB hearing level in either ear Prevalence: 43.8% | Cognitive impairment was defined as a Mini-Mental State Examination score of <24 or history of dementia or Alzheimer disease | MCI (n = 145): HR = 1.90, 95% CI: 1.11, 3.26 | Age, sex, education, smoking, BMI, exercise, alcohol consumption, hypertension, diabetes, high inflammatory markers, non-HDL, intima-media thickness, frailty score, longest held job, cold or stuffy nose, nasal polyps, deviated septum, allergies, head injury, stroke or TIA, epilepsy |
| Washington Heights-Inwood Columbia Aging Golub, 2017 [24] | USA | 1,881 | Mean: 7.4±4.6 (range: 0.9–22) | Mean: 75.8±6.3 | 70 | Hearing normal vs. abnormal, hearing normal versus abnormal (repeat of first variable occurring later during the visit), no impairment in hearing versus some difficulty hearing versus must speak loudly versus must shout versus cannot rate, auditory ability adequate versus inadequate, has hearing aid; if any HL was noticed among any of the variables that assessed HL, or if the subject wore hearing aids, OHL was considered present Prevalence: 10.8% | A neuropsychological battery was administered at baseline and all follow-up visits; dementia was then diagnosed using previously defined cutoff scores decided in a consensus panel of neurologists, neuropsychologists, and psychiatrists | Dementia (n = 377): HR = 1.69, 95% CI: 1.3, 2.3 Alzheimer disease: HR = 1.85, 95% CI: 1.3, 2.6 Vascular dementia: HR = 1.34, 95% CI: 0.5, 3.5 |
Age, sex, ethnicity, race, years of education, diabetes mellitus, hypertension, heart disease, smoking, APOE4 genotype, and stroke |
| Singapore Longitudinal Ageing Study Heywood, 2017 [25] | Singapore | 2,599 | Median: 3.8 | ≥55 | 63.8 | Hearing was tested at a study site by a research nurse using the whispered voice test in a standard manner Prevalence: 2.7% | MMSE ≤23 or neuropsychological domain scores below 2 SD of age- and education-adjusted mean and evidence of social or occupational function impairment (dependency in 1 or more basic ADL or CDR score ≥1) | MCI (n = 144): HR = 1.85, 95% CI: 0.78, 4.40 | Sex, age, ethnicity, education, central obesity, hypertension, diabetes, dyslipidemia, smoking, alcohol, leisure time activity, cardiac diseases, depressive symptoms |
MCI, mild cognitive impairment; RR, risk ratio; HR, hazard ratio; OR, odds ratio; HI, hearing impairment; HL, hearing loss; OHL, observed hearing loss; NINCDS-ADRDA, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association; 3MS score, Modified Mini-Mental State score; MMSE, Mini-Mental State Examination; DSM, Diagnostic and Statistical Manual; SSI-ICM, Synthetic Sentence Identification with Ipsilateral Competing Message; DSI, Dichotic Sentence Identification Test; DDT, Dichotic Digits Test; CAD, central auditory dysfunction; BMI, body mass index; HDL, high-density lipoprotein; TIA, transient ischemic attack.
Except for 1 study that used a combination of voice test and self-reported hearing aid, all the included studies used a standardized hearing assessment, including pure-tone average of hearing thresholds, central auditory speech (including Synthetic Sentence Identification test with Ipsilateral Competing Message, Dichotic Sentence Identification test, Dichotic Digits Test) and whispered voice test. The cutoff points of hearing tests also varied. The prevalence of hearing impairment varied substantially across studies, from 2.0 to 58.6% (Table 1). The assessment of MCI and dementia included physician diagnosis and cutoff points of specific cognition scales. Clinical outcomes also differed across studies (Table 1).
Quantitative Analysis
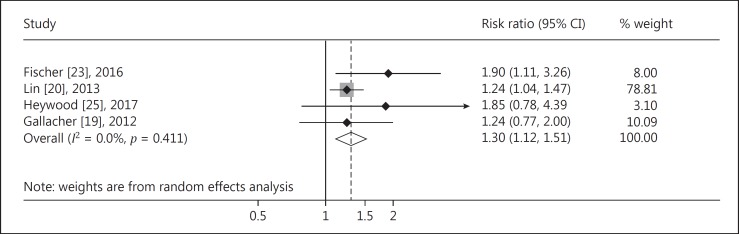
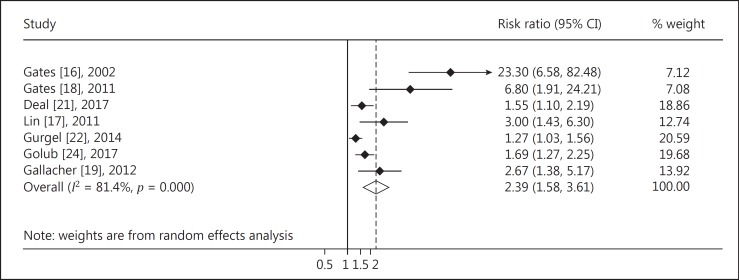
The meta-analysis of 4 cohort studies indicated that there was a significantly higher risk of MCI among subjects with hearing impairment (RR = 1.30, 95% CI: 1.12, 1.51; Fig. 2) compared to those with normal hearing. The pooled analysis of 7 cohort studies indicated that there was a significantly higher risk of dementia among subjects with hearing impairment (RR = 2.39, 95% CI: 1.58–3.61; Fig. 3). No significant heterogeneity of estimates was found among the 4 studies focused on MCI (I2 = 0.0%, p = 0.411), while there was significant heterogeneity across studies on hearing impairment and the risk of dementia (I2 = 81.4%, p < 0.001).
Fig. 2.
Forest plot of the associations between hearing impairment and risk of MCI.
Fig. 3.
Forest plot of the associations between hearing impairment and risk of dementia.
The Begg's test of the association between hearing impairment and risk of MCI showed good symmetry (z = 1.02, p = 0.222), indicating that publication bias was not likely. Studies on the associations between hearing impairment and risk of dementia showed significant publication bias (z = 2.40, p = 0.016), but the publication bias no longer existed after excluding 2 studies with extreme results (z = 1.71, p = 0.086).
Quality Assessment
The methodologic quality of the 10 studies included in the meta-analysis, as scored with the Newcastle-Ottawa Scale, showed that the mean total score was 8.6 out of a maximum score of 9 (range 5–9), which indicated that, overall, the methodologic quality was good for the included studies (Table 2). The 10 studies generally received good scores for the criteria on selection, comparability, and outcome.
Table 2.
Quality assessment of included studies according to the Newcastle-Ottawa Scale
| Study | Selection |
Comparability |
Outcome |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed cohort representative | selection of nonexposed cohort | ascertainment of exposure | outcome not present at baseline | analysis adjusted for confounding factors | assessment of outcome | length of follow-up | Adequacy of follow-up | ||
| Gates, 2002 [16] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Lin, 2011 [17] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Gates, 2011 [18] | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 5 |
| Gallacher, 2012 [19] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Lin, 2013 [20] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Gurgel, 2014 [22] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Fischer, 2016 [23] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Deal, 2017 [21] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Golub, 2017 [24] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Heywood, 2017 [25] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Discussion
The present meta-analysis identified 10 prospective cohort studies that have investigated hearing impairment as a predictor of MCI and dementia among older adults. In particular, results from all studies on hearing impairment and dementia indicated positive associations despite variations in hearing test protocols, assessment methods, and outcome measures. Our pooled analysis confirms a strong association between hearing impairment and adverse cognitive status, including MCI and dementia among older adults. To our knowledge, this is the first study to summarize the existing literature on the prospective association between hearing impairment and risk of MCI and dementia in older adults.
Similar systematic reviews and meta-analyses on hearing impairment and dementia have been published recently. One systematic review summarized 17 studies on hearing loss as a risk factor of dementia and cognitive decline and demonstrated that hearing loss is associated with higher incidence of dementia in older adults; however, the assessments of hearing ability and cognition varied among studies [26]. Zheng et al. [12] conducted a meta-analysis on hearing impairment and risk of Alzheimer disease, a major type of dementia, using cohort studies and found that hearing impairment significantly increases the risk of Alzheimer's disease (RR = 2.82, 95% CI: 1.47–5.42; p = 0.002). The results of our analysis are consistent with these studies.
One feature of our included studies is that all subjects had in-person evaluations of hearing ability in order to accurately assess hearing impairment. Consequently, we excluded studies that used other methods for ascertainment of hearing impairment. Fritze et al. [27] used the International Classification of Diseases 10th Revision (ICD-10) definition of hearing impairment and found that patients with hearing impairment of either side had a higher risk of dementia incidence than patients without hearing impairment (hazards ratio = 1.20, p < 0.001). This is consistent with our finding.
While the precise mechanisms through which hearing impairment brings about adverse cognitive status are not clear, several potential mechanisms have been proposed. First, auditory deprivation may cause decreased socialization and increased depression, as well as a decline in cognitive function, which can result in MCI or dementia [28]. Additionally, it is possible that hearing impairment causes cognitive resources to be diverted from memory function into auditory processing, which creates an excessive cognitive load on higher cortical functions, leading to cognitive decline and dementia [29]. Hearing impairment may also modify the auditory pathway and the brain, which causes cognitive decline and dementia [30].
Alternatively, studies suggest that hearing impairment and cognitive decline share a common neurodegenerative process, which leads to both hearing impairment and MCI/dementia [31]. If this is true, then hearing impairment does not cause MCI and dementia, and improvement of hearing would not improve cognition or reduce the risk of MCI and dementia. If this theory was true, cognition could only be improved through a neuroregenerative process. This is unlikely to be true, since all of our included studies consistently showed hearing impairment precedes the occurrence of MCI and dementia, and a previous study has indicated that use of hearing aids is associated with better cognition, independently of social isolation and depression [32]. While further efforts are required to explore the exact mechanisms to achieve an optimal effect in prevention of MCI/dementia, our findings suggest a causal relationship between hearing impairment and MCI and dementia.
If there is a true causal relationship between hearing impairment and MCI/dementia, it would provide insights for prevention of dementia in clinical settings. Hearing aid use appears to reduce adverse health outcomes due to loneliness, which is positively associated with dementia [33]. Therefore, use of hearing aids is posited to reduce the risk of dementia. Future intervention studies are needed to assess whether treatment of hearing impairment can reduce the risk of MCI and dementia in older adults. Very few studies have tested the relationship between using auditory amplification and cognitive change. Acar et al. [34] showed that after 3 months of using hearing aids, all subjects with hearing impairment showed a significant improvement of their psychosocial and cognitive conditions. MacDonald et al. [35] showed in a randomized controlled trial that hearing augmentation significantly improved performance on Mini-Mental State Examination scores compared to the control group.
Although the results are promising, hearing aid use is rare in the US. Only 18% of older adults with impactful hearing loss actually use custom-fit amplification [8]. This may be due to cost barriers and low coverage by health insurance and Medicaid, so that hearing aids are only accessible to individuals with a high socioeconomic status [36]. Given the benefits of hearing aid use in reducing the risk of dementia, we call for affordable approaches to auditory care. This remains a great challenge for the “high-risk strategy,” which is to identify and treat high-risk individuals; however, even if hearing aids become more accessible, such high-risk strategy may not be sufficient to fundamentally prevent the risk of dementia associated with hearing impairment. Several studies included in our meta-analysis indicated monotonically linear associations between hearing impairment and dementia [17, 20, 21], in addition to using cutoff points for indicator of hearing impairment. This indicated that there is no specific threshold of hearing impairment associated with significantly increasing risk of dementia. Instead, each incremental unit of hearing impairment may be associated with a higher risk of dementia. In this case, the “population strategy,” defined by Geoffrey Rose as “a public health-oriented approach to preventive medicine and public health which predicts that shifting the population distribution of a risk factor prevents more burden of disease than targeting people at high risk” should also be considered in this case [37]. Improving hearing ability of the whole population may fundamentally reduce the prevalence of hearing impairment and reduce the risk of dementia. The implementation of population strategy, however, is challenging, since the public awareness of hearing impairment as an important risk factor of MCI and dementia is low among older adults compared to others, such as physical activity [38]. Therefore, it is imperative to educate the population on the importance of hearing impairment and MCI and dementia. In addition to health education, it is suggested that routine hearing care be conducted in primary care and community settings. Currently, hearing is not frequently tested as a potential cause of cognitive decline in primary care clinics [39], though it has a great potential for dementia prediction with a relatively low cost. A combination of population strategy and high-risk strategy may potentially achieve the largest effect in preventing dementia.
This meta-analysis is limited by the fact that the number of studies that met our inclusion criteria is relatively small (4 for MCI, 7 for dementia). However, our included studies were all recently published population-based studies with a standardized procedure of the hearing test, which reflects the latest trend of current research on hearing impairment and MCI/dementia and ensures the quality of the studies. In addition, the generalizability of our study is a potential issue in interpretation, since 8 out of 10 studies included were from the US. This calls for more studies to be conducted in other regions. Another important limitation is that the definition of MCI varied among different studies, and only 1 out of the 4 studies used clinical standards for diagnosis. We encourage future studies to implement standardized assessments that reduce the influence of hearing on MCI evaluation, through alternative nonverbal modalities, in order to reduce any potential false-positive results. However, our analysis suggested that no significant heterogeneity was detected, so the result was not significantly affected.
In conclusion, our meta-analysis showed that hearing impairment is associated with a higher risk of MCI and dementia among older adults. Future intervention studies are needed to assess whether treatment of hearing impairment can reduce the risk of MCI and dementia in older adults. Measures should also be taken to improve hearing ability among older adults for further prevention of MCI and dementia.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Funding Sources
This study has received no funding.
Author Contributions
J.W. and E.K.C. designed the study. J.W. and Y.H. conducted the literature search and data analysis. L.Z. and Q.H. performed the data extraction. J.W. drafted the manuscript. All authors contributed to and approved the final manuscript.
References
- 1.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D'Agostino RB. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Guerchet M, Prina M. Geneva: World Health Organization; 2015. The Epidemiology and Impact of Dementia: Current State and Future Trends. [Google Scholar]
- 4.Alzheimer's Association 2017 Alzheimer's disease facts and figures. Alzheimer Dementia. 2017;13:325–373. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Thalamuthu A, Andrews G, Brayne C, Matthews FE, Stephan BC, Lipton RB, Katz MJ, Ritchie K, Carriere I, Ancelin ML, Lam LC, Wong CH, Fung AW, Guaita A, Vaccaro R, Davin A, Ganguli M, Dodge H, Hughes T, Anstey KJ, Cherbuin N, Butterworth P, Ng TP, Gao Q, Reppermund S, Brodaty H, Schupf N, Manly J, Stern Y, Lobo A, Lopez-Anton R, Santabarbara J. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: The COSMIC Collaboration. PLoS One. 2015;10:e0142388. doi: 10.1371/journal.pone.0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RO, Cha RH, Mielke MM, Geda YE, Boeve BF, Machulda MM, Knopman DS, Petersen RC. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84:1854–1861. doi: 10.1212/WNL.0000000000001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Fact sheet. Geneva: WHO; 2015. Deafness and hearing loss. [Google Scholar]
- 8.Palmer CV, Mulla R, Dervin E, Coyan KC. HearCARE: Hearing and communication assistance for resident engagement. Semin Hear. 2017;38:184–197. doi: 10.1055/s-0037-1601574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gates GA, Cobb JL, Linn RT, Rees T, Wolf PA, D'Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- 10.Uhlmann RF, Larson EB, Koepsell TD. Hearing impairment and cognitive decline in senile dementia of the Alzheimer's type. J Am Geriatr Soc. 1986;34:207–210. doi: 10.1111/j.1532-5415.1986.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas PD, Hunt WC, Garry PJ, Hood RB, Goodwin JM, Goodwin JS. Hearing acuity in a healthy elderly population: effects on emotional, cognitive, and social status. J Gerontol. 1983;38:321–325. doi: 10.1093/geronj/38.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Fan S, Liao W, Fang W, Xiao S, Liu J. Hearing impairment and risk of Alzheimer's disease: a meta-analysis of prospective cohort studies. Neurol Sci. 2017;38:233–239. doi: 10.1007/s10072-016-2779-3. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. Ottawa: Ottawa Hospital Research Institute; 2014. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gates GA, Beiser A, Rees TS, D'Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer's disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, Elwood P. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- 20.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deal JA, Betz J, Yaffe K, Harris T, Purchase-Helzner E, Satterfield S, Pratt S, Govil N, Simonsick EM, Lin FR. Hearing impairment and incident dementia and cognitive decline in older adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72:703–709. doi: 10.1093/gerona/glw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35:775–781. doi: 10.1097/MAO.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BE, Klein R, Tweed TS. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. 2016;64:1981–1987. doi: 10.1111/jgs.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golub JS, Luchsinger JA, Manly JJ, Stern Y, Mayeux R, Schupf N. Observed hearing loss and incident dementia in a multiethnic cohort. J Am Geriatr Soc. 2017;65:1691–1697. doi: 10.1111/jgs.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heywood R, Gao Q, Nyunt MSZ, Feng L, Chong MS, Lim WS, Yap P, Lee TS, Yap KB, Wee SL, Ng TP. Hearing loss and risk of mild cognitive impairment and dementia: findings from the Singapore longitudinal ageing study. Dement Geriatr Cogn Disord. 2017;43:259–268. doi: 10.1159/000464281. [DOI] [PubMed] [Google Scholar]
- 26.Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:69–79. doi: 10.1002/lio2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritze T, Teipel S, Ovari A, Kilimann I, Witt G, Doblhammer G. Hearing impairment affects dementia incidence. An analysis based on longitudinal health claims data in Germany. PLoS One. 2016;11:e0156876. doi: 10.1371/journal.pone.0156876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150:378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- 29.Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL, Wingfield A. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL) Ear Hear. 2016;37((suppl 1)):5s–27s. doi: 10.1097/AUD.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 30.Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, Resnick SM. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis A, McMahon CM, Pichora-Fuller KM, Russ S, Lin F, Olusanya BO, Chadha S, Tremblay KL. Aging and hearing health: the life-course approach. Gerontologist. 2016;56((suppl 2)):S256–S267. doi: 10.1093/geront/gnw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, McCormack A, Munro KJ. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10:e0119616. doi: 10.1371/journal.pone.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein BE, Sirow LW, Moser S. Relating hearing aid use to social and emotional loneliness in older adults. Am J Audiol. 2016;25:54–61. doi: 10.1044/2015_AJA-15-0055. [DOI] [PubMed] [Google Scholar]
- 34.Acar B, Yurekli MF, Babademez MA, Karabulut H, Karasen RM. Effects of hearing aids on cognitive functions and depressive signs in elderly people. Arch Gerontol Geriatr. 2011;52:250–252. doi: 10.1016/j.archger.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald AA, Joyson A, Lee R, Seymour DG, Soiza RL. The effect of hearing augmentation on cognitive assessment scales at admission to hospital. Am J Geriatr Psychiatry. 2012;20:355–361. doi: 10.1097/JGP.0b013e3182107e88. [DOI] [PubMed] [Google Scholar]
- 36.Stahl SM. Does treating hearing loss prevent or slow the progress of dementia? Hearing is not all in the ears, but who's listening? CNS Spectr. 2017;22:247–250. doi: 10.1017/S1092852917000268. [DOI] [PubMed] [Google Scholar]
- 37.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 38.Lin FR, Albert M. Hearing loss and dementia - who is listening? Aging Ment Health. 2014;18:671–673. doi: 10.1080/13607863.2014.915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou R, Dana T, Bougatsos C, Fleming C, Beil T. Rockville: Agency for Healthcare Research and Quality (US); 2011. Screening for Hearing Loss in Adults Ages 50 Years and Older: A Review of the Evidence for the US Preventive Services Task Force. [PubMed] [Google Scholar]